Soil–plant interactions are highly complex as the soil with its edaphic and biological factors provides most of the essential nutrients and water to the plant [
13]. In the changing climate, plants are continuously subjected to abiotic stress, for instance, water deficit, which is a main restricting factor for agricultural production; it hinders plant growth and diminishes productivity [
32]. There is a dire need to ameliorate water deficit by the use of various nutrients, which is also cost-effective. Therefore, in this study, the potential roles of seed inoculation by plant growth promoting rhizobacteria (PGPR), foliar application of salicylic acid (SA) and their combination in regulating water deficit tolerance in wheat were discussed. Our results showed that the integrative use of PGPR in association with SA achieved an efficacious strategy to attenuate the harmful effects of water deficit as well as the amelioration of productivity and nutrient uptake of wheat higher than the individual application of SA or PGPR under water-deficient conditions. Thus, an understanding of interactions between plants and both PGPR and SA having an impact on plant development and water deficit tolerance is required.
It was found that inoculation with PGPR enhanced microbial activity, leading to improved soil physical and chemical properties as well as soil moisture constants [
15]. The application of PGPR can produce an exo-polysaccharide, which can play a critical role in the improvement of soil structure, soil aggregation, and therefore, improve the soil permeability and composition. This can enhance a beneficial impact to hold more water in the soil and consequently, can maintain plant growth under water stress conditions. In addition, the application of PGPR can increase soil microbial liveliness, which can subsequently lead to a decline in the soil bulk density and an increment in the soil water holding capacity under water stress conditions [
33]. Salicylic acid exhibited a slight impact on soil moisture constants because it was added in our examination as a foliar application to improve the physiological processes, yield-related traits, and productivity [
18,
34]. The combination treatment of soil application of PGPR before planting and foliar application of SA at 45 and 60 days after sowing led to improved soil moisture constants owing to the diverse processes and systems for each substance. Seed inoculation with PGPR enhanced soil properties as well as soil moisture constants owing to the effective microbial activity and available nutrients in the soil, which resulted in enhanced root and plant growth [
35]. Plants inoculated with PGPR had greater formation of a more effective root system for water absorption [
14]. Foliar application of salicylic acid was stimulatory to root and shoot growth and further strengthened the efficacy of PGPR on root and shoot growth [
36]. Additionally, these findings were consistent with the observations in [
13], who reported that PGPR-induction can cause alterations in root architecture as well as boost root surface area and subsequently increase nutrient and water absorption. Likewise, a positive correlation between water deficit and membrane damage were witnessed by [
37], and the bacterial inoculation decreased the membrane damage compared to uninoculated plants under water deficit [
32,
38]. Furthermore, foliar application of SA acts as a cofactor in regulating the physiological traits and therefore, ameliorating leaf water content and photosynthetic processes which resulted in the improvement in the root hairs efficiency to uptake water and enhance plant growth. Consequently, it was found that using the combination of PGPR side-by-side with SA was efficient in mitigating water deficit conditions and improving the nutritional status of plants. The results of the present study are consistent with the results of [
39], which showed that foliar spraying of salicylic acid has the capability to produce enzymatic and non-enzymatic metabolites to detoxify the detrimental impacts of reactive oxygen species, produce of antioxidant compounds, and seed inoculation by microbes that can fix the nitrogen in the soil under harsh conditions.
It is noteworthy in the present examination that the integration of PGPR (soil application) alongside SA (foliar application) greatly improved the yield-related traits and wheat productivity possibly through collaboration with auxin and/or cytokinin synthesis, the increment of cell division and photosynthesis [
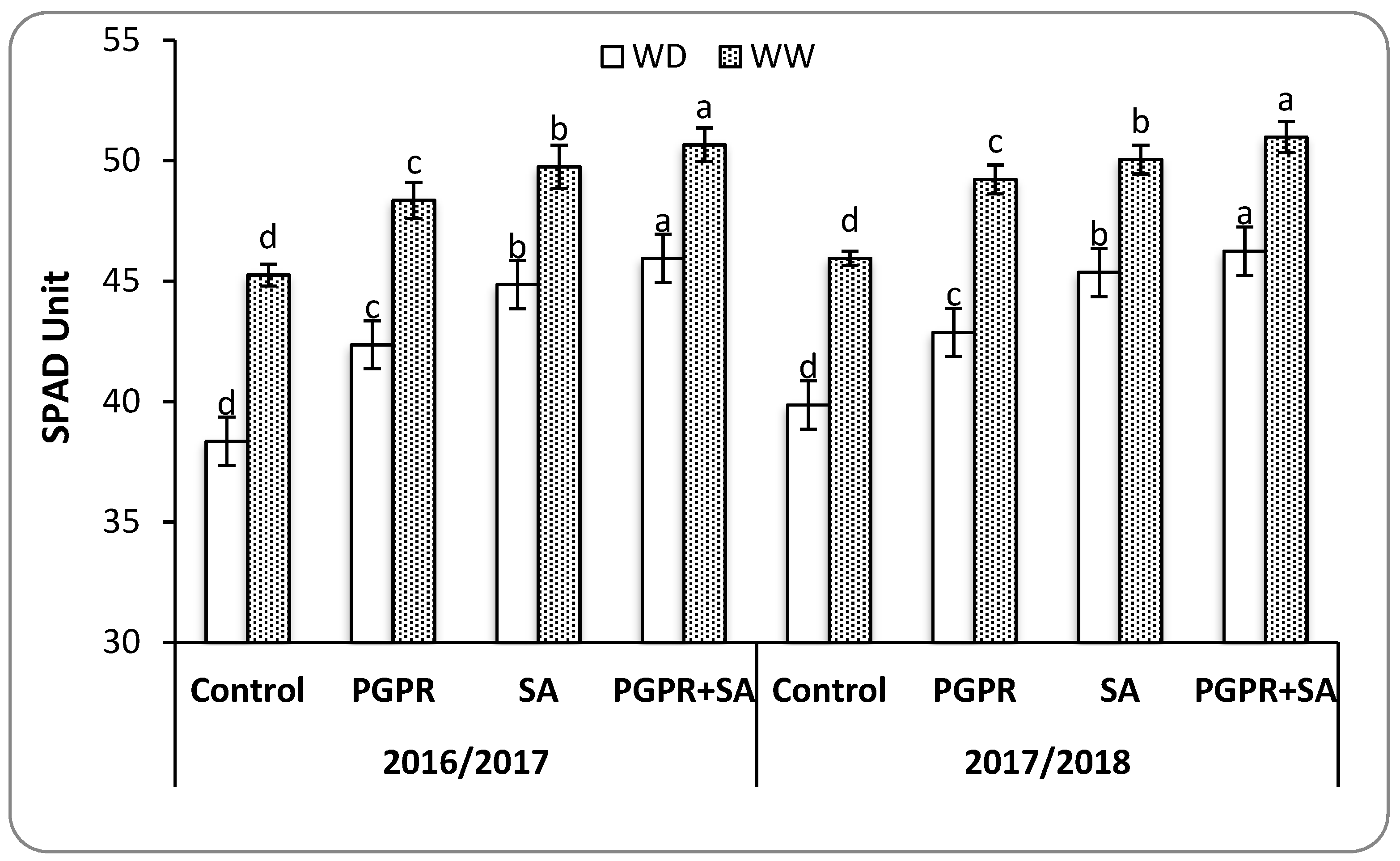
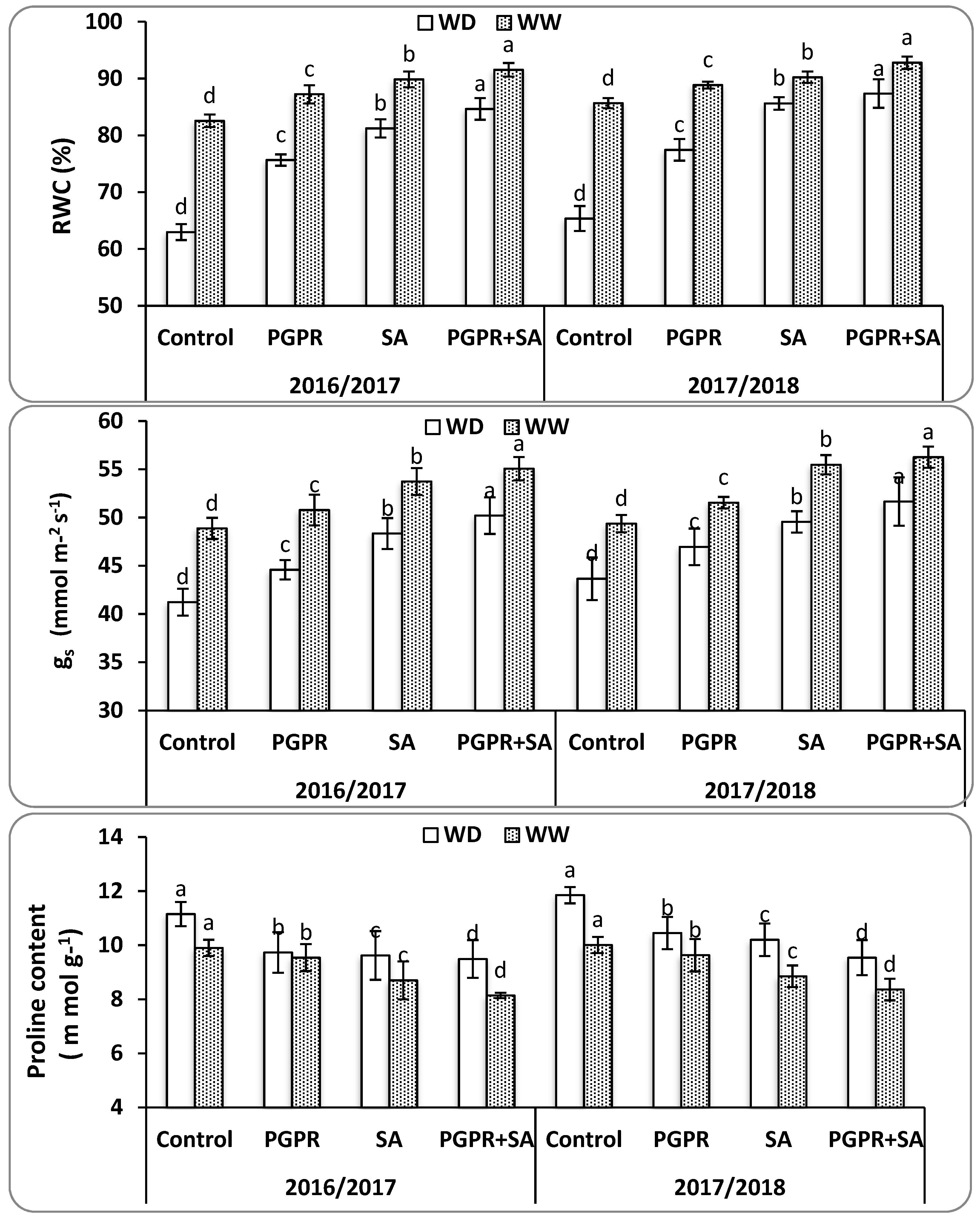
40]. The SA further helped the PGPR inoculated plants to improve the physiological traits and photosynthetic activity through enhanced CO
2 assimilation rates due to increased stomatal conductance, relative water content, and chlorophyll content under water deficit [
9,
41]. Furthermore, the integration between PGPR and SA reduced the proline content that accumulates under water deficit conditions resulting in osmotic adjustment, free radical scavenging, and stabilization of subcellular structures in plant cells and mitigation of the harmful impacts of water deficit [
42]. Moreover, compatible solutes, such as proline, which are produced under water deficit may help plants in osmoregulation processes. A noteworthy result is the integrated treatment of PGPR and SA were more stimulatory for physiological traits than the PGPR or SA treatment alone. These studies are in line with [
13], who reported the contributory role of the integration between bacterium inoculum and salicylic acid as foliar spraying in increasing cell elongation, which owing to water flow from the xylem to the adjacent elongation cells, results in strengthened mitosis and cell enlargement leading to an increase in yield-related traits and productivity. It could be summarized that PGPR and its combination with SA increased chlorophyll content in leaves could be attributed to higher availability of nutrients and increased organic matter in the rhizosphere leading to increases in 1000-grain weight, number of grains spike
−1, and number of spikes m
−2 due to the pivotal impact of chlorophyll on photosynthetic activity [
41]. Foliar application of SA demonstrated, in the present examination, a better capability of increasing grain and straw yield than soil application of PGPR. Various reports depicted that PGPR can assist plants in tolerating water deficit, as indicated by the incremental soil aggregation and preserving of higher water potential around the roots, which in turn increased uptake of nutrients in plants exposed to water deficit [
43]. These findings are in congruence with earlier reports on the impact of PGPR on water deficit tolerance in maize, sunflower [
44], and wheat [
45]. Furthermore, PGPR-inoculated plants were successful in increasing the nitrogen, phosphorus, and potassium uptake into plant biomass [
44,
46]. Under water deficit, nutrient content (N-P-K) of plant biomass was improved when plants were treated with PGPR, perhaps owing to incremental root growth and root number that exploited more soil volume for efficient absorption of available essential nutrients [
47], thus leading to higher biomass production [
1]. These results agree with those obtained in [
48], who found that plants inoculated with PGPR in soils with a low availability of the essential elements led to increased N, P, and K uptake due to their increased content in the soil after inoculation compared to non-inoculated plants because of the pivotal impact of root morphology and expansion of the root hairs. The findings in [
49], are also in accordance with our results. Similar results were documented in [
33], who stated that salicylic acid assists in translocation and uptake of essential elements in plant biomass. It is inferred from the results obtained that foliar application of SA at stem elongation and booting stage has increased the yield-related traits of wheat, whereas it has been stated that SA has a positive role in increasing relative water content, stomatal conductance, and decreasing proline content, leading to metabolic transport of photosynthetic assimilates to wheat grains through the impact on phloem flow [
17]. Furthermore, this might be attributed to an increment of nitrogen uptake, phosphorus uptake, and potassium uptake, subsequently increasing the assimilation and dry matter accumulation in wheat plants. The highest grain yield, straw yield, and harvest index were achieved when the combination of PGPR and SA was applied. Our findings showed that the physiological, yield-related traits and yield of wheat were significantly higher with the integration between PGPR and SA followed by individual application of SA and PGPR compared to the untreated (control) treatment. The integrative use of PGPR with SA was found to be an effective strategy for the improvement of crop development and productivity under water deficit conditions.