Ecological Evidence for the Fitness Trade-Off in Triazine Resistant Chenopodium Album L.: Can We Exploit the Cost of Resistance?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Growth Analysis Experiment
2.3. Neighbouring Plant Competition Experiment
2.4. Statistical Analysis
3. Results
3.1. Growth Analysis
3.2. Neighbour Competition Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arntzen, C.J.; Pfister, K.; Steinback, K.E. The mechanism of chloroplast triazine resistance: Alteration in the site of herbicide action. In Herbicide Resistance in Plants; LeBaron, H.M., Gressel, J., Eds.; J. Wiley and Sons Inc.: New York, NY, USA, 1982; pp. 185–214. [Google Scholar]
- LeBaron, H.M.; McFarland, J.E.; Burnside, O.C. The triazine herbicides: A milestone in the development of weed control technology. In The Triazine Herbicides; LeBaron, H.M., McFarland, J.E., Burnside, O.C., Eds.; Elsevier: San Diego, CA, USA, 2008; pp. 1–12. [Google Scholar]
- Ryan, G.F. Resistance of common groundsel to simazine and atrazine. Weed Sci. 1970, 18, 614–616. [Google Scholar] [CrossRef]
- Heap, I. The International Survey of Herbicide Resistant Weeds. Available online: http://www.weedscience.com (accessed on 14 March 2019).
- Devine, M.D.; Shukla, A. Altered target sites as a mechanism of herbicide resistance. Crop Prot. 2000, 19, 881–889. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Harrington, K.C. Non-target site mechanisms of resistance to herbicides. Critic. Rev. Plant Sci. 2017, 36, 24–34. [Google Scholar] [CrossRef]
- Anderson, M.P.; Gronwald, J.W. Atrazine resistance in a velvetleaf (Abutilon theophrasti) biotype due to enhanced glutathione S-transferase activity. Plant Physiol. 1991, 96, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Patzoldt, W.L.; Dixon, B.S.; Tranel, P.J. Triazine resistance in Amaranthus tuberculatus (Moq) Sauer that is not site-of-action mediated. Pest Manag. Sci. 2003, 59, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Jasieniuk, M.; Brule-Babel, A.L.; Morrison, I.N. The evolution and genetics of herbicide resistance in weeds. Weed Sci. 1996, 44, 176–193. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Neve, P.; Powles, S.B. Fitness costs associated with evolved herbicide resistance alleles in plants. New Phytol. 2009, 184, 751–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanizadeh, H.; Harrington, K.C. Perspectives on non-target site mechanisms of herbicide resistance in weedy plant species using evolutionary physiology. AoB Plants 2017, 9, plx035. [Google Scholar] [CrossRef]
- Tian, D.; Traw, M.B.; Chen, J.Q.; Kreitman, M.; Bergelson, J. Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature 2003, 423, 74–77. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Neve, P.; Powles, S.B. Resistance cost of a cytochrome P450 herbicide metabolism mechanism but not an ACCase target site mutation in a multiple resistant Lolium rigidum population. New Phytol. 2005, 167, 787–796. [Google Scholar] [CrossRef]
- Fernández-Moreno, P.T.; Alcántara-de la Cruz, R.; Smeda, R.J.; De Prado, R. Differential resistance mechanisms to glyphosate result in fitness cost for Lolium perenne and L. multiflorum. Front. Plant Sci. 2017, 8, 1796. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh, H.; Harrington, K.C. Fitness costs associated with multiple resistance to dicamba and atrazine in Chenopodium album. Planta 2019, 249, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Babineau, M.; Mathiassen, S.K.; Kristensen, M.; Kudsk, P. Fitness of ALS-inhibitors herbicide resistant population of loose silky bentgrass (Apera spica-venti). Front. Plant Sci. 2017, 8, 1660. [Google Scholar] [CrossRef] [PubMed]
- Conard, S.G.; Radosevich, S.R. Ecological fitness of Senecio vulgaris and Amaranthus retroflexus biotypes susciptible or resistant to atrazine. J. App. Ecol. 1979, 16, 171–177. [Google Scholar] [CrossRef]
- Holt, J.S.; Stemler, A.J.; Radosevich, S.R. Differential light responses of photosynthesis by triazine-resistant and triazine-susceptible Senecio vulgaris Biotypes. Plant Physiol. 1981, 67, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.E.; Warwick, S.I. Competitive relationships between atrazine resistant and susceptible populations of Amaranthus retroflexus and A. powellii from southern Ontario. New Phytol. 1982, 92, 131–139. [Google Scholar] [CrossRef]
- Holt, J.S. Reduced growth, competitiveness, and photosynthetic efficiency of triazine-resistant Senecio vulgaris from California. J. App. Ecol. 1988, 25, 307–318. [Google Scholar] [CrossRef]
- Kremer, E.; Kropff, M.J. Comparative growth of triazine-susceptible and -resistant biotypes of Solanum nigrum at different light levels. Ann. Bot. 1999, 83, 637–644. [Google Scholar] [CrossRef]
- Frenkel, E.; Matzrafi, M.; Rubin, B.; Peleg, Z. Effects of environmental conditions on the fitness penalty in herbicide resistant Brachypodium hybridum. Front. Plant Sci. 2017, 8, 94. [Google Scholar] [CrossRef]
- Conn, J.S.; Thomas, D.L. Common lambsquarters (Chenopodium album) interference in spring barley. Weed Technol. 1987, 1, 312–313. [Google Scholar] [CrossRef]
- Schweizer, E.E. Common lambsquarters (Chenopodium album) interference in sugarbeets (Beta vulgaris). Weed Sci. 1983, 31, 5–8. [Google Scholar] [CrossRef]
- Fischer, D.W.; Harvey, R.G.; Bauman, T.T.; Phillips, S.; Hart, S.E.; Johnson, G.A.; Kells, J.J.; Westra, P.; Lindquist, J. Common lambsquarters (Chenopodium album) interference with corn across the northcentral United States. Weed Sci. 2004, 52, 1034–1038. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Harrington, K.C. Cross-resistance to auxinic herbicides in dicamba-resistant Chenopodium album. N. Z. J. Agric. Res. 2017, 60, 45–53. [Google Scholar] [CrossRef]
- Rahman, A. Current status of herbicide resistance in New Zealand weeds. In Proceedings of the 9th Australian Weeds Conference, Adelaide, Australia, 6–10 August 1990; pp. 196–200. [Google Scholar]
- Ghanizadeh, H.; Harrington, K.C.; James, T.K. A comparison of dicamba absorption, translocation and metabolism in Chenopodium album populations resistant and susceptible to dicamba. Crop Prot. 2018, 110, 112–116. [Google Scholar] [CrossRef]
- Rahman, A.; James, T.; Trolove, M. Characteristics and control of dicamba-resistant common lambsquarters (Chenopodium album). Weed Biol. Manag. 2014, 14, 88–98. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Harrington, K.C.; James, T.K.; Woolley, D.J. A quick test using seeds for detecting dicamba resistance in fathen (Chenopodium album). Aus. J. Crop. Sci. 2015, 9, 337–343. [Google Scholar]
- Warwick, S.I.; Black, L. The relative competitiveness of atrazine susceptible and resistant populations of Chenopodium album and C. strictum. Can. J. Bot. 1981, 59, 689–693. [Google Scholar] [CrossRef]
- Parks, R.J.; Curran, W.S.; Roth, G.W.; Hartwig, N.L.; Calvin, D.D. Herbicide susceptibility and biological fitness of triazine-resistant and susceptible common lambsquarters (Chenopodium album). Weed Sci. 1996, 44, 517–522. [Google Scholar] [CrossRef]
- Ghanizadeh, H. Aspects of Herbicide Resistance in Three New Zealand Weed Species. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2015; p. 233. [Google Scholar]
- Hunt, R.; Causton, D.R.; Shipley, B.; Askew, A.P. A modern tool for classical plant growth analysis. Ann. Bot. 2002, 90, 485–488. [Google Scholar] [CrossRef]
- Weiner, J. A neighborhood model of annual-plant interference. Ecology 1982, 63, 1237–1241. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Neve, P.; Powles, S.B. Evidence for an ecological cost of enhanced herbicide metabolism in Lolium rigidum. J. Ecol. 2009, 97, 772–780. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Orr, H.A. Fitness and its role in evolutionary genetics. Nat. Rev. Genet. 2009, 10, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purrington, C.B. Costs of resistance. Curr. Opin. Plant Biol. 2000, 3, 305–308. [Google Scholar] [CrossRef]
- Bergelson, J.; Purrington, C.B.; Palm, C.J.; Lopez-Gutierrez, J.C. Cost of resistance: A test using transgenic Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 263, 1659–1663. [Google Scholar]
- Vila-Aiub, M.M.; Gundel, P.E.; Preston, C. Experimental methods for estimation of plant fitness costs associated with herbicide-resistance genes. Weed Sci. 2015, 63, 203–216. [Google Scholar] [CrossRef]
- Darmency, H.; Gasquez, J. Appearance and spread of triazine resistance in common lambsquarters (Chenopodium album). Weed Technol. 1990, 4, 173–177. [Google Scholar] [CrossRef]
- Williams, M.M., II; Jordan, N.; Yerkes, C. The fitness cost of triazine resistance in jimsonweed (Datura stramonium L.). Am. Midl. Nat. 1995, 133, 131–137. [Google Scholar]
- Warwick, S.I.; Black, L.D. Relative fitness of herbicide-resistant and susceptible biotypes of weeds. Phytoprotection 1994, 75, 37–49. [Google Scholar] [CrossRef]
- Poorter, H. Interspecific variation in relative growth rate: On ecological causes and physiological consequences. In Causes and Consequences of Variation in Growth Rate and Productivity of Higher Plants; Lambers, H., Ed.; SPB Academic Publishing: The Hague, The Netherlands, 1989; pp. 45–69. [Google Scholar]
- Sun, S.; Frelich, L.E. Flowering phenology and height growth pattern are associated with maximum plant height, relative growth rate and stem tissue mass density in herbaceous grassland species. J. Ecol. 2011, 99, 991–1000. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Harrington, K.C. Weed management in New Zealand pastures. Agronomy 2019, 9, 448. [Google Scholar] [CrossRef]
- Jordan, N.; Kelrick, M.; Brooks, J.; Kinerk, W. Biorational management tactics to select against triazine-resistant Amaranthus hybridus: A field trial. J. App. Ecol. 1999, 36, 123–132. [Google Scholar] [CrossRef]
- Goldberg, D.E. Components of resource competition in plant communities. In Perspectives in Plant Competition; Grace, J.B., Tilman, D., Eds.; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
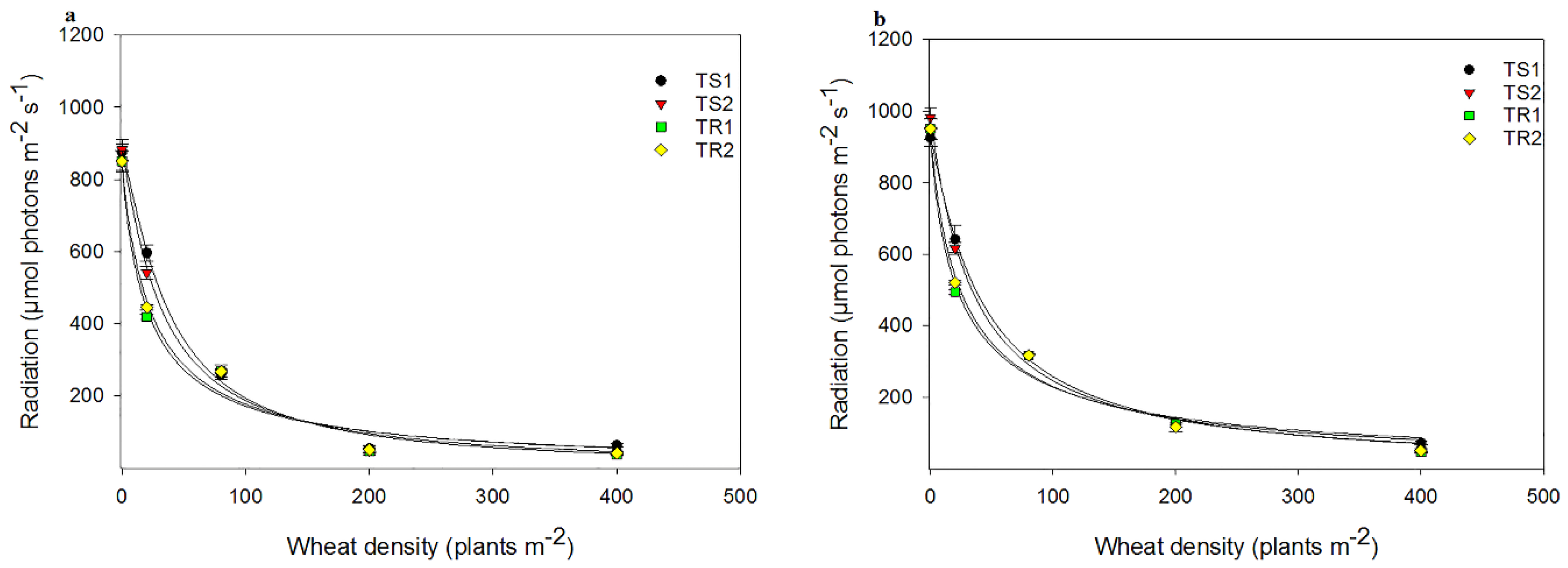
| First Experiment | |||||
| TS1 | TS2 | TR1 | TR2 | ||
| p values | |||||
| Dry weight (mg plant−1) * | 2709.2 a | 2638.5 a | 1416.7 b | 1456.9 b | 0.0001 |
| Leaf area (cm−2 plant−1) * | 232.7 a | 196.3 a | 128.0 b | 122.2 b | 0.0001 |
| RGR (mg mg−1 d−1) * | 0.149 a | 0.147 a | 0.120 b | 0.124 b | 0.0001 |
| NAR (mg cm−2 d−1) * | 1.311 ab | 1.446 a | 1.044 b | 1.146 b | 0.0480 |
| LAR (cm2 mg−1) * | 0.162 | 0.153 | 0.149 | 0.145 | 0.8150 |
| Second Experiment | |||||
| TS1 | TS2 | TR1 | TR2 | ||
| p values | |||||
| Dry weight (mg plant−1) ‡ | 3076.9 a | 2738.5 a | 1567.8 b | 1517.0 b | 0.0001 |
| Leaf area (cm−2 plant−1) * | 177.9 a | 173.2 a | 131.5 b | 137.0 b | 0.012 |
| RGR (mg mg−1 d−1) ‡ | 0.147 a | 0.144 a | 0.131 b | 0.127 b | 0.004 |
| NAR (mg cm−2 d−1) ‡ | 1.736 a | 1.636 a | 1.164 b | 1.108 b | 0.0001 |
| LAR (cm2 mg−1) ‡ | 0.134 | 0.130 | 0.152 | 0.146 | 0.335 |
| First Experiment | |||||
| TS1 | TS2 | TR1 | TR2 | ||
| p values | |||||
| Dry weight (mg plant−1) ‡ | 6517.5 a | 6315.5 a | 5261.1 b | 5321.2 b | 0.006 |
| Leaf area (cm−2 plant−1) ‡ | 188.4 | 183.2 | 199.2 | 198.3 | 0.897 |
| RGR (mg mg−1 d−1) * | 0.044 b | 0.045 b | 0.057 a | 0.056 a | 0.014 |
| NAR (mg cm−2 d−1) ‡ | 0. 865 b | 1.087 ab | 1.096 ab | 1.154 a | 0.12 |
| LAR (cm2 mg−1) ‡ | 0.064 | 0.050 | 0.062 | 0.058 | 0.179 |
| Second Experiment | |||||
| TS1 | TS2 | TR1 | TR2 | ||
| p values | |||||
| Dry weight (mg plant −1) ‡ | 6753.6 a | 6817.3 a | 5255.6 b | 5370.1 b | 0.0001 |
| Leaf area (cm−2 plant−1) * | 184.2 | 191.8 | 215.1 | 207.1 | 0.49 |
| RGR (mg mg−1 d−1) ‡ | 0.048 bc | 0.046 c | 0.061 a | 0.057 ab | 0.012 |
| NAR (mg cm−2 d−1) ‡ | 1.145 | 1.131 | 1.046 | 1.015 | 0.577 |
| LAR (cm2 mg−1) ‡ | 0.047 b | 0.048 b | 0.070 a | 0.066 a | 0.0001 |
| Height (cm) | ||||||
|---|---|---|---|---|---|---|
| First Experiment | Second Experiment | |||||
| 35 DAT ‡ | 55 DAT | Final Height * | 35 DAT | 55 DAT | Final Height * | |
| TS1 | 55.8 a | 126.5 a | 140.6 | 51.9 a | 122.8 a | 138.8 |
| TS2 | 51.8 b | 138.4 a | 136.7 | 54.3 a | 127.0 a | 134.7 |
| TR1 | 29.7 c | 87.0 b | 135.3 | 27.4 b | 90.9 b | 133.1 |
| TR2 | 28.5 c | 85.8 b | 135.6 | 25.0 b | 88.7 b | 134.9 |
| p values | 0.0001 | 0.0001 | 0.051 | 0.0001 | 0.0001 | 0.062 |
| Population | First Experiment | Second Experiment | ||||
|---|---|---|---|---|---|---|
| Biomass | Height | Leaf Area | Biomass | Height | Leaf Area | |
| TS1 | 0.0064 b | 0.0036 b | 0.0052 b | 0.0046 b | 0.0039 bc | 0.0075 c |
| TS2 | 0.0059 c | 0.0045 b | 0.0041 b | 0.0047 b | 0.0035 c | 0.0088 bc |
| TR1 | 0.0131 a | 0.0093 a | 0.0111 a | 0.0093 a | 0.0056 ab | 0.0111 ab |
| TR2 | 0.0128 a | 0.0074 a | 0.0137 a | 0.0103 a | 0.0073 a | 0.0122 a |
| p values | 0.0001 | 0.0034 | 0.0015 | 0.0006 | 0.0049 | 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanizadeh, H.; Harrington, K.C. Ecological Evidence for the Fitness Trade-Off in Triazine Resistant Chenopodium Album L.: Can We Exploit the Cost of Resistance? Agronomy 2019, 9, 523. https://doi.org/10.3390/agronomy9090523
Ghanizadeh H, Harrington KC. Ecological Evidence for the Fitness Trade-Off in Triazine Resistant Chenopodium Album L.: Can We Exploit the Cost of Resistance? Agronomy. 2019; 9(9):523. https://doi.org/10.3390/agronomy9090523
Chicago/Turabian StyleGhanizadeh, Hossein, and Kerry C. Harrington. 2019. "Ecological Evidence for the Fitness Trade-Off in Triazine Resistant Chenopodium Album L.: Can We Exploit the Cost of Resistance?" Agronomy 9, no. 9: 523. https://doi.org/10.3390/agronomy9090523





