Killing Weed Seeds with Exhaust Gas from a Combine Harvester
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiments
2.2. Statistical Analysis
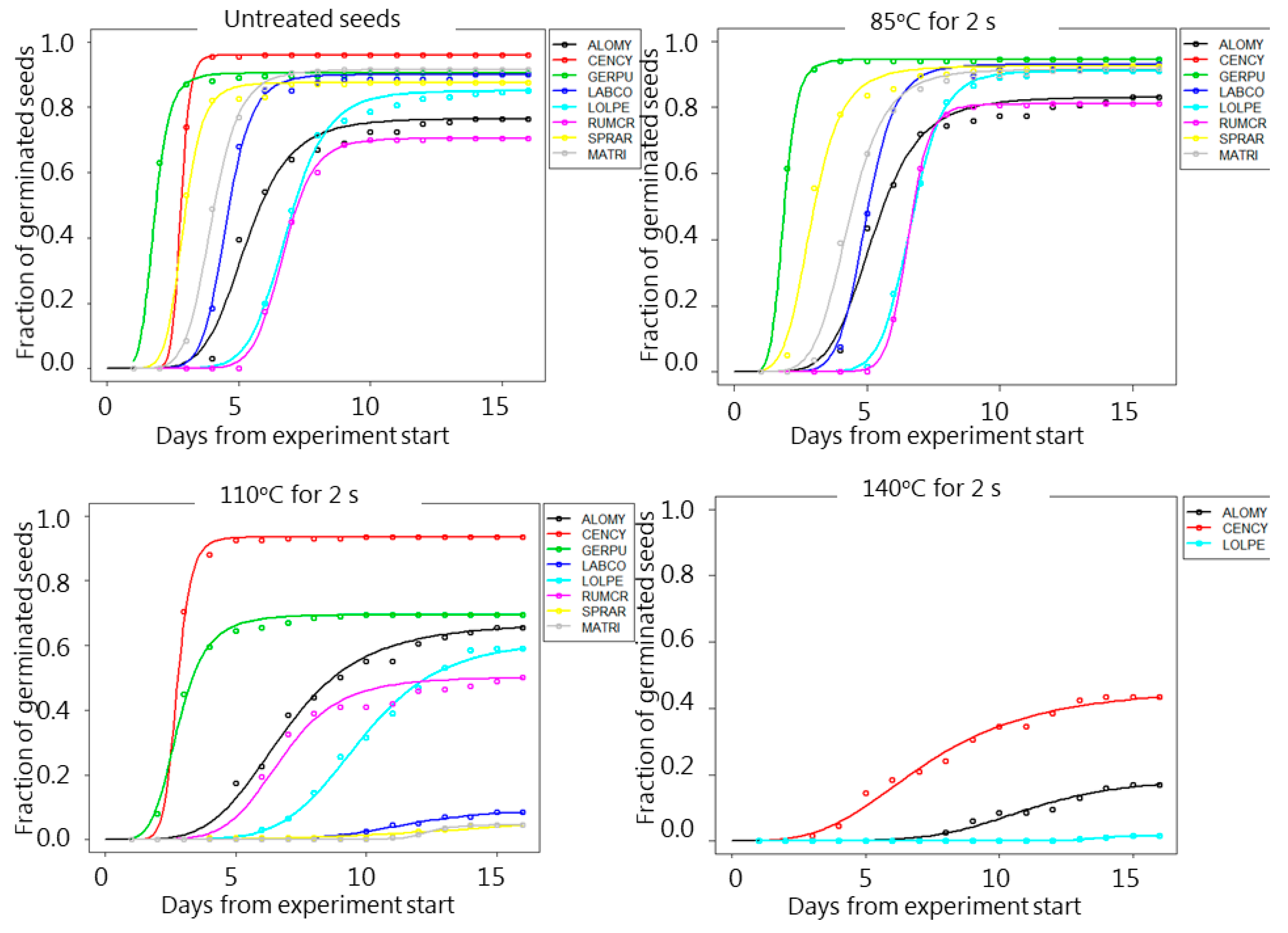
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. AGP—Weeds. 2017. Available online: http://www.fao.org/agriculture/crops/thematic-sitemap/theme/biodiversity/weeds/en/ (accessed on 12 September 2019).
- Heap, I. Herbicide resistant weeds. In Integrated Weed Management; Pimental, D., Peshin, E., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 281–301. [Google Scholar]
- Duke, S. Why have no new herbicide modes of action appeared in recent years? Pest Manag. Sci. 2011, 68, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Andreasen, C.; Streibig, J.C. Evaluation of changes in weed flora in arable fields of Nordic countries—Based on Danish long-term surveys. Weed Res. 2011, 51, 214–226. [Google Scholar] [CrossRef]
- Chauvel, B.; Guillemin, J.-P.; Gasquez, J.; Gauvrit, C. History of chemical weeding from 1944 to 2011 in France: Changes and evolution of herbicide molecules. Crop Prot. 2012, 42, 320–326. [Google Scholar] [CrossRef]
- Funk, C.; Kennedy, B. The New Food Fights: U.S. Public Divides over Food Science, Differing Views on Benefits and Risks of Organic Foods, GMOs as Americans Report Higher Priority for Healthy Eating; Pew Research Center: Washington, DC, USA, 2016. [Google Scholar]
- Liebmann, M.; Dyck, E. Crop rotation and intercropping strategies for weed management. Ecol. Appl. 1993, 3, 92–122. [Google Scholar] [CrossRef]
- Melander, B.; Liebman, M.; Davis, A.S.; Gallandt, E.R.; Bàrberi, P.; Moon, A.; Rasmussen, J.; van der Weide, R.; Vidotto, F. Non-chemical weed management. In Weed Research: Expanding Horizons; Hatcher, P.E., Froud-Williams, R.J., Eds.; John Wiley &Sons Ltd.: West Sussex, UK, 2017; pp. 245–270. [Google Scholar]
- Robinson, R.A.; Sutherland, W.J. Post-war changes in arable farming and biodiversity in Great Britain. J. Appl. Ecol. 2002, 39, 157–176. [Google Scholar] [CrossRef] [Green Version]
- Andreasen, C.; Jensen, H.A.; Jensen, S.M. Decreasing diversity in the soil seed bank after 50 years in Danish arable fields. Agric. Ecosyst. Environ. 2017, 259, 61–71. [Google Scholar] [CrossRef]
- Osteen, C.D.; Fernandez-Cornejo, J. Herbicide use trends: A backgrounder. Choices 2016, 31, 31–37. [Google Scholar]
- Roberts, H.A.; Feast, P.M. Fate of seeds of some annual weeds in different depths of cultivated and undisturbed soil. Weed Res. 1972, 12, 316–324. [Google Scholar] [CrossRef]
- Roberts, H.A.; Feast, P.M. Emergence and longevity of seeds of annual weeds in cultivated and undisturbed soil. J. Appl. Ecol. 1973, 10, 133–143. [Google Scholar] [CrossRef]
- Walsh, M.; Newman, P.; Powles, S. Targeting weed seeds in-crop: A new weed control paradigm for global agriculture. Weed Technol. 2013, 27, 431–436. [Google Scholar] [CrossRef]
- Lewis, J. Longevity of crop and weed seeds: Survival after 20 years in the soil. Weed Res. 1973, 13, 179–191. [Google Scholar] [CrossRef]
- Jensen, H.A. Seed Burial Experiment at the Danish State Seed Testing Station, 1934–1983. Seed Test. Int. 2016, 151. Available online: https://www.seedtest.org/upload/cms/user/Danish-Seed-Burial-Experiment.pdf (accessed on 12 September 2019).
- Walsh, M.J.; Harrington, R.B.; Powles, S.B. Harrington seed destructor: A new nonchemical weed control tool for global grain crops. Crop Sci. 2012, 52, 1343–1347. [Google Scholar] [CrossRef]
- StedmanTM. Stedman Cage Mill Primer. Available online: https://www.stedman-machine.com/ (accessed on 12 September 2019).
- Vieregge, C. Personal Information. CLAAS Selbstfahrende Erntemaschinen GmbH; Vorentwicklung-Funktionstechnik: Harsewinkel, Germany, 2018. [Google Scholar]
- Korsmo, E.; Vidme, T.; Fykse, H. Kosmos Ugras Pjansjer; Norsk Landbruk/Landbruksforlaget: Olso Norge, Norway, 1981. [Google Scholar]
- Holm-Nielsen, C. Frø Fra de Dyrkede Land; Forskningscenter Flakkebjerg, Frederiksberg Bogtrykkeri: Copenhagen, Denmark, 1989. [Google Scholar]
- Mossberg, B.; Stenberg, L. Den Nye Nordiske Flora; Gyldendal: Copenhagen, Denmark, 2003. [Google Scholar]
- Andreasen, C.; Bitarafan, Z.; Fenselau, J.; Glasner, C. Exploiting waste heat from combine harvesters to damage harvested weed seeds and reduce weed infestation. Agriculture 2018, 8, 42. [Google Scholar] [CrossRef]
- Ritz, C.; Streibig, J.C. Bioassay analysis using R. J. Stat. Softw. 2005, 12, 1–22. [Google Scholar] [CrossRef]
- Ritz, C.; Pipper, C.B.; Streibig, J.C. Analysis of germination data from agricultural experiments. Eur. J. Agron. 2013, 45, 1–6. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, B.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Fidelis, A.; Daibes, L.F.; Martins, A.R. To resist or to germinate? The effect of fire on legume seeds in Brazilian subtropical grasslands. Acta Bot. Bras. 2016, 30, 1. [Google Scholar] [CrossRef]
- Moreira, B.; Pausas, J.G. Tanned or Burned: The Role of Fire in Shaping Physical Seed Dormancy. PLoS ONE 2012, 7, e51523. [Google Scholar] [CrossRef]
- Arcamone, J.; Jaureguiberry, P. Germination response of common annual and perennial forbs to heat shock and smoke treatments in the Chaco Serrano, central Argentina. Austral Ecol. 2018, 43, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Keeley, J.E.; Bond, W.J. Convergent seed germination in South African fynbos and Californian chaparral. Plant Ecol. 1997, 133, 153. [Google Scholar] [CrossRef]
- Hanley, M.E.; Fenner, M.; Neuman, G. Pre-germination heat shock and seedling growth of fire following Fabaceae from four Mediterranean-climate regions. Acta Oecol. 2001, 22, 315–320. [Google Scholar] [CrossRef]
- Hanley, M.E.; Unna, J.E.; Darvill, B. Seed size and germination response: A relationship for fire-following plan species exposed to thermal shock. Oecologia 2003, 134, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D.; Black, M. Seeds. Physiology of Development and Germination, 2nd ed.; Plenum: New York, NY, USA; London, UK, 1994. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. Does seed dormancy play a role in germination? Ecology of Rumex crispus. Weed Res. 1985, 33, 340–343. [Google Scholar] [CrossRef]
- ISTA. International rules for seed Testing; The International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2011. [Google Scholar]
- Copeland, L.O.; McDonald, M.B. Principles of Seed Science and Technology, 4th ed.; Kluwer Academic Publishers: Norwell, MA, USA, 2001; p. 488. [Google Scholar]
- Yasin, M.; Andreasen, C. Breaking seed dormancy of Alliaria petiolata with phytohormones. Plant Growth Reg. 2015, 77, 307–315. [Google Scholar] [CrossRef]
- Bitarafan, Z.; Andreasen, C. Seed Production and Seed Shattering of Black Grass (Alopecurus mysuroides) in Winther Wheat. In Proceedings of the 18th European Weed Research Society Symposium (EWRS 2018), Ljubjana, Slovenien, 17–21 June 2018; p. 181. [Google Scholar]
- Glasner, C.; Vieregge, C.; Robert, J.; Fenselau, J.; Bitarafan, Z.; Andreasen, C. Evaluation of new harvesting methods to reduce weeds on arable fields and collect a new feedstock. Energies 2019, 12, 1688. [Google Scholar] [CrossRef]
- Walsh, M.J.; Powles, S.B. High seed retention at maturity of annual weeds infesting crop fields highlights the potential for harvest weed seed control. Weed Technol. 2014, 28, 486–493. [Google Scholar] [CrossRef]
- Westerman, P.R.; Gerowitt, B. The probability of maize biomass contamination with weed seeds. J. Plant Dis. Prot. 2012, 119, 68. [Google Scholar] [CrossRef]
- Yasin, M.; Rosenqvist, E.; Andreasen, C. The effect of reduced light intensities on grass weeds species. Weed Sci. 2017, 65, 603–613. [Google Scholar] [CrossRef]


| Weed Species | Short Description | Thousand Seed Weight (g) | Size | Plant Height |
|---|---|---|---|---|
| (mm) | (cm) | |||
| Alopecurus myosuroides Huds. | Spikelet with one seed and with a 5–6 mm awn | 2.0 | 5.7 × 1.9 × 0.9 | 20−60 |
| Centaurea cyanus L. | Seed with stiff hairs (4 mm) at the apex | 4.5 | 2.4 × 1.7 × 1.2 | 20−80 |
| Geranium pusillum L. | Cross-section round | 1.1 | 1.1 × 1.1 × 1.1 | 5−30 |
| Lapsana communis L. | Cross-section oval to elliptic | 1.1 | 3.7 × 0.9 × 0.6 | 20−120 |
| Lolium perenne L. | Cross-section c-shaped | 2.0 | 6.6 × 1.4 × 1.0 | 20−-60 |
| Rumex × crispus L. | Cross-section triangular | 1.4 | 2.2 × 1.5 × 1.5 | 40−100 |
| Spergula arvensis L. | Cross-section oval | 0.5 | 1.2 × 1.2 × 0.8 | 10−30 |
| Tripleurospermum inodorum (L) Sch. Bip. | Cross-section triangular to square | 0.4 | 1.8 × 0.7 × 0.7 | 20−80 |
| Engine Setting | |||||
|---|---|---|---|---|---|
| Unit | Idle | 30% | 50% | 100% | |
| Temperature | °C | 75 or 85 | 110 | 140 | 220 |
| Air speed | m s−1 | 21.4 | 23.8 | 24.0 | 38.0 |
| Air flow | L min−1 | 508 | 565 | 570 | 902 |
| Treatment at 75 °C in Series 1 and 85 °C in Series 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 2 s | 4 s | 6 s | ||||||
| Species | Series | d | t50 | d | t50 | d | t50 | d | t50 |
| Alopecurus myosuroides | 1 | 66 (3) a | 4.2 (0.1) a | 85 (3) b | 4.9 (0.1) b | 86 (3) b | 4.8 (0.1) b | 82 (3) b | 5.1 (0.1) c |
| Alopecurus myosuroides | 2 | 77 (3) a | 5.3 (0.1) a | 83 (3) ab | 5.2 (0.1) a | 85 (3) b | 5.2 (0.1) a | 81 (3) ab | 5.4 (0.1) a |
| Centaurea cyanus | 1 | 93 (2) a | 1.3 (0.1) a | 96 (1) a | 1.9 (0.0) b | 96 (1) a | 2.1 (0.0) c | 98 (1) b | 2.5 (0.1) d |
| Centaurea cyanus | 2 | 96 (1) a | 2.8 (0.0) a | 95 (2) a | 2.6 (0.1) b | 92 (2) a | 2.6 (0.0) b | 93 (2) a | 2.8 (0.0) a |
| Geranium pusillum | 1 | 92 (2) a | 1.5 (0.1) a | 95 (2) a | 1.3 (0.0) b | 95 (2) a | 1.4 (0.0) a | 86 (3) b | 1.9 (0.1) c |
| Geranium pusillum | 2 | 90 (2) a | 1.8 (0.0) a | 94 (2) a | 1.8 (0.0) a | 90 (2) a | 2.1 (0.0) b | 82 (3) b | 3.0 (0.1) c |
| Lapsana communis | 1 | 95 (2) a | 4.1 (0.1) a | 98 (1) a | 4.5 (0.1) b | 96 (1) a | 6.5 (0.1) c | 71 (3) b | 9.1 (0.2) d |
| Lapsana communis | 2 | 90 (2) a | 4.5 (0.1) a | 93 (2) a | 4.9 (0.1) b | 84 (3) ab | 9.3 (0.2) c | 47 (4) c | 12.3 (0.2) d |
| Lolium perenne | 1 | 89 (2) a | 6.7 (0.1) a | 93 (2) a | 6.6 (0.1) a | 90 (2) a | 6.6 (0.1) a | 89 (2) a | 7.7 (0.1) b |
| Lolium perenne | 2 | 85 (3) a | 6.8 (0.1) a | 91 (2) ab | 6.8 (0.1) a | 92 (2) b | 6.9 (0.1) a | 83 (3) ab | 7.7 (0.1) b |
| Tripleurospermum inodorum | 1 | 94 (2) a | 2.5 (0.1) a | 97 (1) a | 1.9 (0.1) b | 96 (1) a | 3.6 (0.1) c | 83 (3) b | 6.9 (0.2) d |
| Tripleurospermum inodorum | 2 | 92 (2) a | 3.9 (0.1) a | 92 (2) a | 4.3 (0.1) b | 80 (3) b | 9.8 (0.2) c | 64 (3) c | 10.8 (0.1) d |
| Rumex crispus | 1 | 89 (2) a | 4.9 (0.1) a | 95 (2) b | 4.8 (0.1) a | 91 (2) ab | 5.5 (0.1) b | 78 (4) c | 8.5 (0.3) c |
| Rumex crispus | 2 | 71 (3) a | 6.7 (0.1) a | 81 (3) b | 6.5 (0.1) a | 83 (3) b | 6.9 (0.1) b | 46 (4) c | 9.2 (0.3) c |
| Spergula arvensis | 1 | 90 (2) a | 1.4 (0.1) a | 91 (2) a | 1.4 (0.0) a | 94 (2) ab | 1.5 (0.1) a | 70 (3) c | 4.2 (0.2) b |
| Spergula arvensis | 2 | 87 (0) a | 2.9 (0.1) a | 92 (2) a | 2.9 (0.1) a | 66 (3) b | 5.8 (0.2) b | 52 (2) bc | 14.4 (3.3) c |
| Treatment at 110 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 2 s | 4 s | 6 s | ||||||
| Species | Series | d | t50 | d | t50 | d | t50 | d | t50 |
| Alopecurus myosuroides | 1 | 66 (3) a | 4.2 (0.1) a | 72 (3) a | 5.6 (0.2) b | 15 (120) a | 19 (18.0) ab | - | - |
| Alopecurus myosuroides | 2 | 77 (3) a | 5.3 (0.1) a | 67 (5) b | 6.9 (0.2) b | - | - | - | - |
| Centaurea cyanus | 1 | 93 (2) a | 1.3 (0.1) a | 98 (1) b | 1.9 (0.0) b | 50 (161) ab | 24 (31.4) ab | - | - |
| Centaurea cyanus | 2 | 96 (1) a | 2.8 (0.0) a | 93 (2) a | 2.7 (0.0) a | 18 (33) b | 19 (19.7) a | - | - |
| Geranium pusillum | 1 | 92 (2) a | 1.5 (0.1) a | 89 (2) a | 1.6 (0.1) b | - | - | - | - |
| Geranium pusillum | 2 | 90 (2) a | 1.8 (0.1) a | 70 (3) b | 2.8 (0.1) b | - | - | - | - |
| Lapsana communis | 1 | 95 (2) a | 4.1 (0.1) a | 15 (5) b | 13.7 (1.7) b | 13 (30) b | 25 (12.2) ab | - | - |
| Lapsana communis | 2 | 90 (2) a | 4.5 (0.1) a | 9 (2) b | 11.5 (0.9) b | - | - | - | - |
| Lolium perenne | 1 | 89 (2) a | 6.7 (0.1) a | 67 (4) b | 9.6 (0.3) b | - | - | - | - |
| Lolium perenne | 2 | 85 (3) a | 6.8 (0.1) a | 62 (4) b | 9.8 (0.3) b | - | - | - | - |
| Tripleurospermum inodorum | 1 | 94 (2) a | 2.5 (0.1) a | 42 (4) b | 8.4 (0.2) b | - | - | - | - |
| Tripleurospermum inodorum | 2 | 92 (2) a | 3.9 (0.1) a | 5 (1) b | 12.3 (0.3) b | - | - | - | - |
| Rumex crispus | 1 | 89 (2) a | 4.9 (0.1) a | 75 (3) b | 7.2 (0.3) b | - | - | - | - |
| Rumex crispus | 2 | 71 (3) a | 6.7 (0.1) a | 50 (4) b | 6.7 (0.2) a | - | - | - | - |
| Spergula arvensis | 1 | 90 (2) a | 1.4 (0.1) a | 13 (5) b | 12.8 (2.6) b | - | - | 15 (351) a | 17 (7.5) b |
| Spergula arvensis | 2 | 87 (0) a | 2.9 (0.1) a | 12 (2) b | 19 (18.5) a | - | - | - | - |
| Treatment at 140 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 2 s | 4 s | 6 s | ||||||
| Species | Series | d | t50 | d | t50 | d | t50 | d | t50 |
| Alopecurus myosuroides | 1 | 66 (3) a | 4.2 (0.1) a | 15 (10) b | 15.0 (3.7) b | - | - | - | - |
| Alopecurus myosuroides | 2 | 77 (3) a | 5.3 (0.1) a | 19 (3) b | 10.9 (0.7) b | - | - | - | - |
| Centaurea cyanus | 1 | 93 (3) a | 1.3 (0.1) a | 63 (7) b | 9.5 (1.1) b | 2.5 c | 12.5 c | - | - |
| Centaurea cyanus | 2 | 96 (1) a | 2.8 (0.0) a | 47 (4) b | 7.3 (0.5) b | - | - | - | - |
| Geranium pusillum | 1 | 92 (2) a | 1.5 (4.8) a | 13 (3) b | 16.0 (16.0) a | - | - | - | - |
| Lolium perenne | 1 | 89 (2) a | 6.7 (8.0) a | 29 (108) a | 31.1 (31.1) a | - | - | - | - |
| Lolium perenne | 2 | 85 (3) a | 6.8 (10.0) a | 1 (1) b | 13.5 (0.5) b | - | - | - | - |
| Spergula arvensis | 1 | 90 (2) a | 1.4 (4.6) a | 41.2 (3.8) b | 6.8 (0.5) b | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakobsen, K.; Jensen, J.A.; Bitarafan, Z.; Andreasen, C. Killing Weed Seeds with Exhaust Gas from a Combine Harvester. Agronomy 2019, 9, 544. https://doi.org/10.3390/agronomy9090544
Jakobsen K, Jensen JA, Bitarafan Z, Andreasen C. Killing Weed Seeds with Exhaust Gas from a Combine Harvester. Agronomy. 2019; 9(9):544. https://doi.org/10.3390/agronomy9090544
Chicago/Turabian StyleJakobsen, Klaus, Jakob A. Jensen, Zahra Bitarafan, and Christian Andreasen. 2019. "Killing Weed Seeds with Exhaust Gas from a Combine Harvester" Agronomy 9, no. 9: 544. https://doi.org/10.3390/agronomy9090544
APA StyleJakobsen, K., Jensen, J. A., Bitarafan, Z., & Andreasen, C. (2019). Killing Weed Seeds with Exhaust Gas from a Combine Harvester. Agronomy, 9(9), 544. https://doi.org/10.3390/agronomy9090544





