Net Enclosure of ‘Honeycrisp’ and ‘Gala’ Apple Trees at Different Bloom Stages Affects Fruit Set and Alters Seed Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
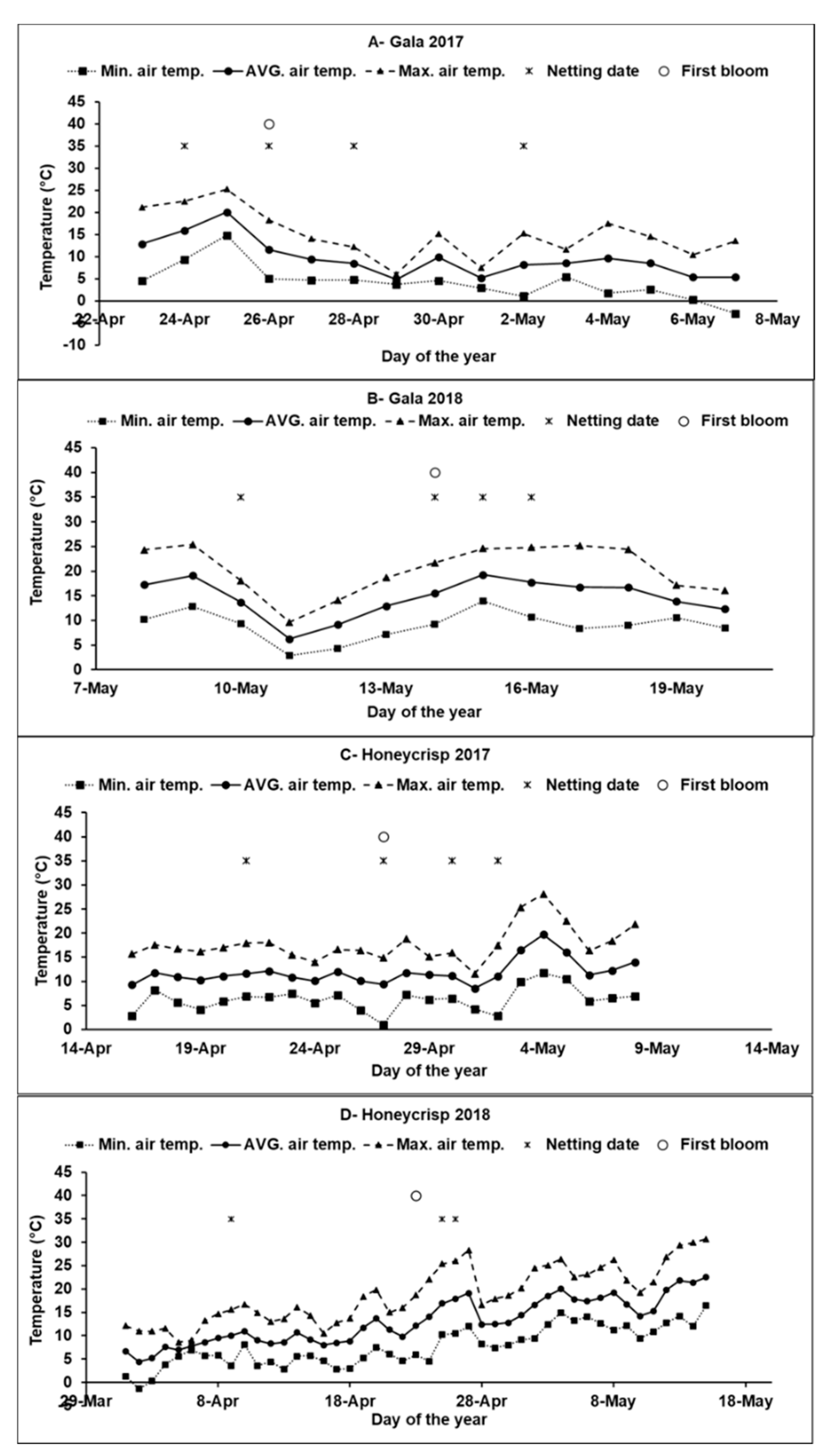
2.2. Experimental Design and Treatments
2.3. Preharvest Measurements and Yield Components
2.4. Postharvest Measurements
2.5. Statistical Analysis
3. Results
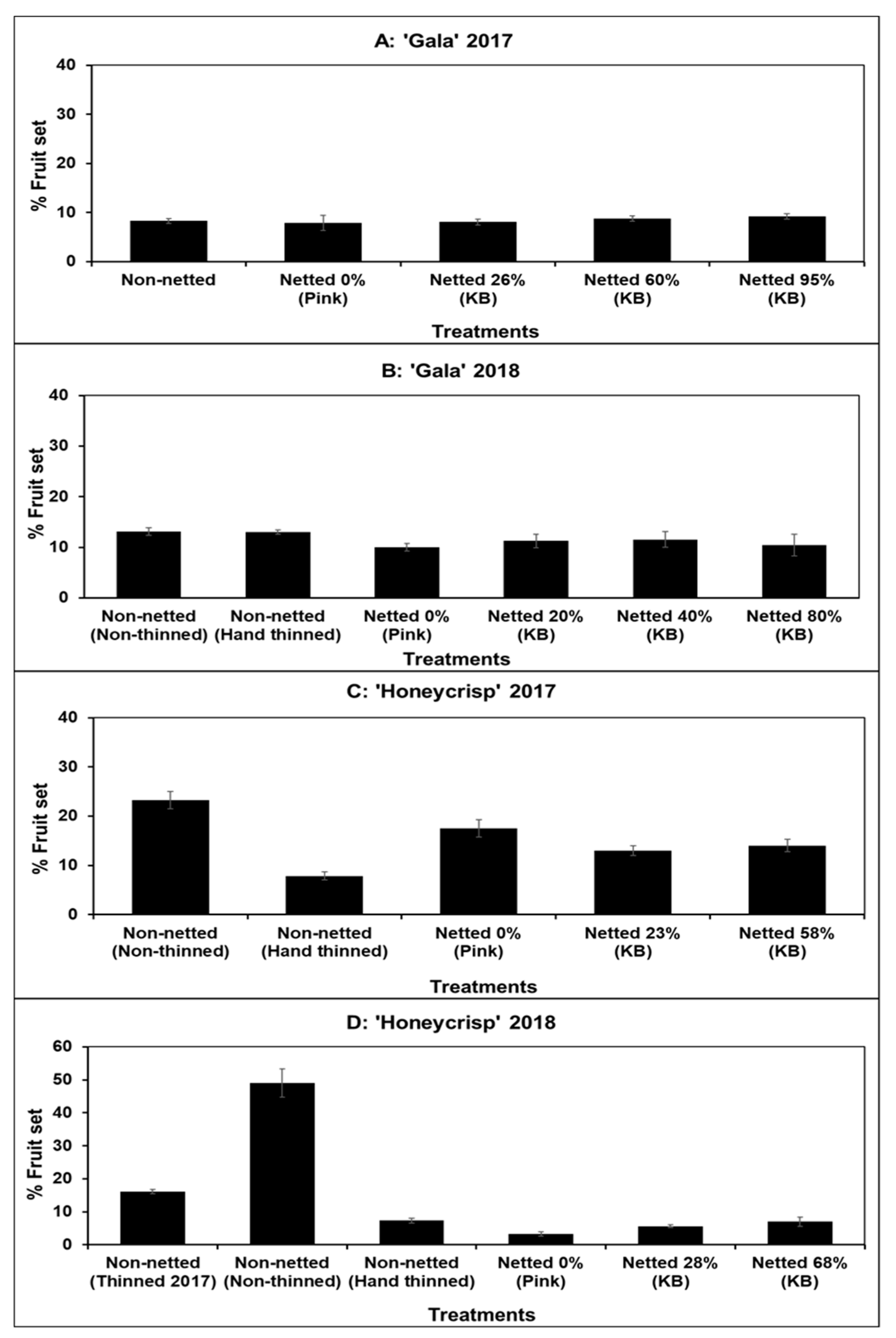
3.1. Fruit Set
3.2. Seed Number
3.3. Yield and Fruit Weight
3.4. Fruit Quality Attributes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Granatstein, D.; Kirby, E.; Ostenson, H.; Willer, H. Global situation for organic tree fruits. Sci. Hortic. (Amst.) 2016, 208, 3–12. [Google Scholar] [CrossRef]
- Dennis, F.G. The history of fruit thinning. Plant Growth Regul. 2000, 31, 1–16. [Google Scholar] [CrossRef]
- Link, H. Significance of flower and fruit thinning on fruit quality. Plant Growth Regul. 2000, 31, 17–26. [Google Scholar] [CrossRef]
- Musacchi, S.; Green, D. Innovations in apple tree cultivation to manage crop load and ripening. In Achieving Sustainable Cultivation of Apples; Evans, K., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; pp. 195–237. ISBN 9781786760326. [Google Scholar]
- Reighard, G.L.; Henderson, W.G. Mechanical blossom thinning in South Carolina peach orchards. Acta Hortic. 2012, 965, 117–122. [Google Scholar] [CrossRef]
- Schupp, J.R.; Auxt Baugher, T.; Miller, S.S.; Harsh, R.M.; Lesser, K.M. Mechanical thinning of peach and apple trees reduces labor input and increases fruit size. HortTechnology 2008, 18, 660–670. [Google Scholar] [CrossRef]
- Ngugi, H.K.; Schupp, J.R. Evaluation of the Risk of Spreading Fire Blight in Apple Orchards with a Mechanical String Blossom Thinner. HortScience 2009, 44, 862–865. [Google Scholar] [CrossRef] [Green Version]
- Bangerth, F. Abscission and thinning of young fruit and their regulation by plant hormones and bioregulators. Plant Growth Regul. 2000, 31, 43–59. [Google Scholar] [CrossRef]
- Williams, M.W. Chemical thinning of apples. Hortic. Rev. Am. Soc. Hortic. Sci. 1979, 1, 270–300. [Google Scholar] [CrossRef]
- Yoder, K.; Yuan, R.; Combs, L.; Byers, R.; McFerson, J.; Schmidt, T. Effects of temperature and the combination of liquid lime sulfur and fish oil on pollen germination, pollen tube growth, and fruit set in apples. HortScience 2009, 44, 1277–1283. [Google Scholar] [CrossRef]
- Broothaerts, W.; Van Neram, I.; Keulemans, J. Update on and review of the incompatibility (S-) genotypes of apple cultivars. HortScience 2004, 39, 943–947. [Google Scholar] [CrossRef]
- Dzhangaliev, A.D. The Wild Apple Tree of Kazakhstan. Hortic. Rev. 2010, 29, 63–303. [Google Scholar] [CrossRef]
- Sheick, R.; Serra, S.; De Franceschi, P.; Dondini, L.; Musacchi, S. Characterization of a novel self-incompatibility allele in Malus and S-genotyping of select crabapple cultivars. Sci. Hortic. 2018, 240, 186–195. [Google Scholar] [CrossRef]
- Matsumoto, S.; Komori, S.; Kitahara, K.; Imazu, S.; Soejima, J. S-genotypes of 15 Apple Cultivars and Self-compatibility of “Megumi”. J. Jpn. Soc. Hortic. Sci. 1999, 68, 236–241. [Google Scholar] [CrossRef]
- De Witte, K.; Vercammen, J.; Van Daele, G.; Keulemans, J. Fruit set, seed set and fruit weight in apple as influenced by emasculation, self-pollination and cross-pollination. Acta Hortic. 1996, 423, 177–183. [Google Scholar] [CrossRef]
- Broothaerts, W.; Keulemans, J.; Van Nerum, I. Self-fertile apple resulting from S-RNase gene silencing. Plant Cell Rep. 2004, 22, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Yuda, E.; Utsunomiya, N.; Kubota, N. Seed formation by self-pollination of ‘Rome Beauty’ apple in East Java. Jpn. J. Trop. Agric. 1991, 35, 289–293. [Google Scholar]
- Schwabe, W.W.; Mills, J.J. Hormones and parthenocarpic fruit set: A literature survey. Hortic. Abstr. 1981, 51, 661–698. [Google Scholar]
- Spena, A.; Rotino, G.L. Parthenocarpy. In Current Trends in the Embryology of Angiosperms; Bhojwani, S.S., Soh, W.Y., Eds.; Springer Netherlands: Dordrech, The Netherlands, 2001; pp. 435–450. [Google Scholar] [CrossRef]
- Westwood, M.N. Temperate Zone Pomology, Physiology and Culture; Timber Press Inc.: Portland, OR, USA, 1993; 523p. [Google Scholar]
- Tsao, T. Growth substances: Role in fertilization and sex expression. In Plant Growth Substances; Skoog, F., Ed.; Spring: New York, NY, USA, 1980; pp. 345–348. [Google Scholar]
- Gustafson, F.G. The Cause of Natural Parthenocarpy. Am. J. Bot. 1939, 26, 135–138. [Google Scholar] [CrossRef]
- Bangerth, F. A Role for Auxin and Auxin Transport Inhibitors on the Ca Content of Artificially Induced Parthenocarpic Fruits. Physiol. Plant 1976, 37, 191–194. [Google Scholar] [CrossRef]
- Goldwin, K.G.; Schwabe, W.W. Partheno-carpic fruit in Cox’s Orange Pippin apples, obtained without hormones. J. Hortic. Sci. 1975, 50, 175–178. [Google Scholar] [CrossRef]
- Brault, A.M.; de Oliveira, D. Seed number and an asymmetry index of McIntosh’ apples. HortScience 1995, 30, 44–46. [Google Scholar] [CrossRef]
- Keulemans, J.; Brusselle, A.; Eyssen, R.; Vercammen, J.; Van Daele, G. Fruit weight in apple as influenced by seed number and pollinizer. Acta Hortic. 1996, 423, 201–210. [Google Scholar] [CrossRef]
- Dennis, F.G. Flowering, Pollination and Fruit Set and Development. Apples: Botany, Porduction and Uses; Ferree, D.C., Ed.; CAB International: Cambridge, UK, 2003; pp. 153–166. [Google Scholar]
- Manja, K.; Aoun, M. The use of nets for tree fruit crops and their impact on the production: A review. Sci. Hortic. 2019, 246, 110–122. [Google Scholar] [CrossRef]
- Sauphanor, B.; Severac, G.; Maugin, S.; Toubon, J.F.; Capowiez, Y. Exclusion netting may alter reproduction of the codling moth (Cydia pomonella) and prevent associated fruit damage to apple orchards. Entomol. Exp. Appl. 2012, 145, 134–142. [Google Scholar] [CrossRef]
- Dorigoni, A.; Micheli, F. Reti multifunzionali in frutteto:dirado, antigrandine e difesa. L’Informatore Agrar. 2015, 4, 51–55. [Google Scholar]
- Chouinard, G.; Firlej, A.; Cormier, D. Going beyond sprays and killing agents: Exclusion, sterilization and disruption for insect pest control in pome and stone fruit orchards. Sci. Hortic. (Amst.) 2016, 208, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, I.; Alegre, S. The effect of anti-hail nets on fruit protection, radiation, temperature, quality and profitability of ‘Mondial Gala’ apples. J. Appl. Hortic. 2006, 8, 91–100. [Google Scholar] [CrossRef]
- Do Amarante, C.V.T.; Steffens, C.A.; Argenta, L.C. Yield and fruit quality of “Gala” and “Fuji” apple trees protected by white anti-hail net. Sci. Hortic. (Amst.) 2011, 129, 79–85. [Google Scholar] [CrossRef]
- Kalcsits, L.A.; Mupambi, G.; Serra, A.; Musacchi, S.; Layne, D.R.; Schmidt, T.; Mendoza, M.; Asteggiano, L.; Jarolmasjed, S.; Sindhuja, S.; et al. Above and below-ground environmental changes associated with the use of photoselective protective netting to reduce sunburn in apple. Agric. For. Meteorol. 2017, 237, 9–17. [Google Scholar] [CrossRef]
- Alaphilippe, A.; Capowiez, Y.; Simon, S.; Saudreau, M.; Caruso, S.; Vergani, S. Codling moth exclusion netting: An overview of French and Italian experiences. IOBC-WPRS Bull 2016, 112, 31–35. [Google Scholar]
- McCaskill, M.R.; McClymont, L.; Goodwin, I.; Green, S.; Partington, D.L. How hail netting reduces apple fruit surface temperature: A microclimate and modelling study. Agric. For. Meteorol. 2016, 226–227, 148–160. [Google Scholar] [CrossRef]
- Kelderer, M.; Lardschneider, E.; Rainer, A. Crop regulation with single row netting structures and their influence on crop quality. In Proceedings of the Ecofruit—16th International Conference on Organic-Fruit Growing, Hohenheim, Germany, 17–19 February 2014; pp. 127–131. [Google Scholar]
- Chouinard, G.; Veilleux, J.; Pelletier, F.; Larose, M.; Philion, V.; Joubert, V.; Cormier, D. Impact of exclusion netting row covers on ‘Honeycrisp’ apple trees grown under northeastern North American conditions: Effects on photosynthesis and fruit quality. Insects 2019, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Stampar, F.; Hudina, M.; Usenik, V.; Sturm, K.; Zadravec, P. Influence of Black and White Nets on Photosynthesis, Yield and Fruit Quality of Apple (Malus Domestica Borkh.). Acta Hortic. 2001, 557, 357–361. [Google Scholar] [CrossRef]
- Mupambi, G.; Anthony, B.M.; Layne, D.R.; Musacchi, S.; Serra, S.; Schmidt, T.; Kalcsits, L.A. The influence of protective netting on tree physiology and fruit quality of apple: A review. Sci. Hortic. (Amst.) 2018, 236, 60–72. [Google Scholar] [CrossRef]
- Blanpied, G.D.; Slisby, K.J. Predicting harvest date windows for apple. Cornell Coop. Ext. Bull. 1992, 221, 1–12. [Google Scholar]
- Ziosi, V.; Noferini, M.; Fiori, G.; Tadiello, A.; Trainotti, L.; Casadoro, G.; Costa, G. A new index based on vis spectroscopy to characterize the progression of ripening in peach fruit. Postharvest Biol. Technol. 2008, 49, 319–329. [Google Scholar] [CrossRef]
- Elkins, R.B.; Van Den Ende, B.; Beutel, J. Vegetative growth and fruit development. Pear Production and Handling Manual; Mticham, E.J., Elkins, N.R., Eds.; UC-ANR. Publication: Oakland, CA, USA, 2007; Volume 3483, pp. 51–58. [Google Scholar]
- Ramírez, F.; Davenport, T.L. Apple pollination: A review. Sci. Hortic. 2013, 162, 188–203. [Google Scholar] [CrossRef]
- Free, J.B. Comparison of the Importance of Insect and Wind Pollination of Apple Trees. Nature 1964, 201, 726–727. [Google Scholar] [CrossRef]
- Palmer-Jones, T.; Clinch, P.G. Observations on the pollination of apple trees (Malus sylvestris Mill.). N. Z. J. Agric. Res. 1966, 9, 191–196. [Google Scholar] [CrossRef]
- Bowker, G.E.; Crenshaw, H.C. Electrostatic forces in wind-pollination-Part 2: Stimulations of pollen capture. Atmos. Environ. 2007, 41, 1596–1603. [Google Scholar] [CrossRef]
- Vakin, Y.; Gan-Mor, S.; Bechar, A.; Romen, B.; Eisikowitch, D. The role of electrostatic forces in pollination. Plant Syst. Evol. 2000, 222, 133–142. [Google Scholar] [CrossRef]
- Rotino, G.L.; Perri, E.; Zottini, M.; Sommer, H.; Spena, A. Genetic engineering of parthenocarpic plants. Nat. Biotechnol. 1997, 15, 1398. [Google Scholar] [CrossRef] [PubMed]
- Sanzol, J.; Herrero, M. The “effective pollination period” in fruit trees. Sci. Hortic. 2001, 90, 1–17. [Google Scholar] [CrossRef]
- Corollaro, M.; Manfrini, L.; Endrizzi, I.; Aprea, E.; Demattè, M.; Charles, M.; Grappadelli, L. The effect of two orchard light management practices on the sensory quality of apple: Fruit thinning by shading or photo-selective nets. J. Hortic. Sci. Biotechnol. 2015, 90, 99–108. [Google Scholar] [CrossRef]
- Devoghalaere, F.; Doucen, T.; Guitton, B.; Keeling, J.; Payne, W.; Ling, T.J.; Ross, J.J.; Hallett, I.C.; Gunaseelan, K.; Dayatilake, G.A.; et al. A genomics approach to understanding the role of auxin in apple (Malus x domestica) fruit size control. BMC Plant Biol. 2012, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Kelderer, M.; Casera, C.; Lardschneider, E.; Rainer, A. Controlling codling moth with different netting structures and their influence on crop yield and quality. In Proceedings of the Ecofruit—14th International Conference on Cultivation Technique and Phytopathological Problems in Organic Fruit-Growing, Fördergemeinschaft Ökologischer Obstbau e. V., Weinsberg, Germany, 22–24 February 2010; Volume 14, pp. 183–190. [Google Scholar]


| Targeted King Bloom% | Actual King Bloom % | |||
|---|---|---|---|---|
| ‘Gala’ | ‘Honeycrisp’ | |||
| 2017 | 2018 | 2017 | 2018 | |
| Non-netted | (Nonthinned) | (Nonthinned) | (Nonthinned) | (Thinned 2017) |
| Non-netted | (Hand thinned) | (Hand thinned) | (Nonthinned) | |
| Non-netted | (Hand thinned) | |||
| 0% | 0% | 0% | 0% | 0% |
| 20% | 26% | 20% | 23% | 28% |
| 40% | 60% | 40% | 58% | 68% |
| 80% | 95% | 80% | ||
| Treatments | Avg. Tree Yield | YE | Cropload | Fruit Weight | Red Color | Firmness | SSC | Shape z | |
|---|---|---|---|---|---|---|---|---|---|
| (kg) | (Fruit No.) | (kg·cm−2) | (Fruit No./cm2) | (g) | (%) | (kg) | (%) | Height/Width | |
| ‘Gala’ 2017 | |||||||||
| Non-netted (Nonthinned) | 23 | 181.1 | 0.64 | 4.9 | 127.2 | 39 | 3.8 a y | 12.1 | 1.1 |
| Netted 0% (Pink w) | 20.6 | 160.3 | 0.66 | 5.1 | 128.2 | 41 | 3.7 ab | 12 | 1.1 |
| Netted 26% (KB) | 21.5 | 164.5 | 0.6 | 4.9 | 130.5 | 36 | 3.6 bc | 11.9 | 1.1 |
| Netted 60% (KB) | 21.5 | 159 | 0.6 | 4.6 | 135.5 | 33 | 3.5 c | 11.8 | 1.5 |
| Netted 95% (KB) | 20.9 | 162.6 | 0.6 | 4.9 | 128.4 | 35 | 3.8 a | 11.8 | 1.1 |
| ‘Gala’ 2018 | |||||||||
| Non-netted (Nonthinned) | 29.8 | 212.1 | 0.82 | 5.8 | 139.9 | 43.5 | 6 | 12.4 | 0.93 |
| Non-netted (Hand thinned) | 26.8 | 190.6 | 0.74 | 5.3 | 140.4 | 54.1 | 5.2 | 11.6 | 0.94 |
| Netted 0% (Pink) | 26.6 | 215.1 | 0.86 | 6.9 | 126 | 48 | 5.5 | 11.3 | 0.97 |
| Netted 20% (KB) | 27.9 | 233 | 0.84 | 6.9 | 119.5 | 40.1 | 5.6 | 11.1 | 0.98 |
| Netted 40% (KB) | 25.2 | 209.8 | 0.73 | 6.1 | 121.1 | 42.6 | 5.9 | 11.6 | 0.96 |
| Netted 80% (KB) | 25.7 | 218.8 | 0.80 | 6.8 | 120.7 | 45.6 | 5.4 | 11.4 | 0.92 |
| Treatments | Avg. Tree Yield | YE | Cropload | Fruit Weight | Red Color | Firmness | Dry Matter | SSC | Shape z | |
|---|---|---|---|---|---|---|---|---|---|---|
| (kg) | (Fruit no.) | (kg·cm−2) | (Fruit no./cm2) | (g) | (%) | (kg) | (%) | (%) | % Misshapen | |
| ‘Honeycrisp’ 2017 | ||||||||||
| Non-netted (Nonthinned) | 35.1 a y | 366 a | 1.4 a | 14.5 a | 96 c | 53 a | 6.5 | 14.5 d | 11.1 b | 47.6 |
| Non-netted (Hand thinned) | 27.8 ab | 139 b | 1 b | 5 c | 204 a | 46 a | 6.6 | 15.2 bc | 11.4 ab | 33.8 |
| Netted 0% (Pink w) | 22.3 b | 148 b | 0.9 b | 6.3 bc | 155 b | 44 ab | 6.7 | 15.8 a | 12 a | 45.1 |
| Netted 23% (KB) | 31.7 ab | 213 b | 1.1 b | 7.1 bc | 151 b | 32 b | 6.5 | 15.6 ab | 11.5 ab | 47.7 |
| Netted 58% (KB) | 31.6 ab | 217 b | 1.2 ab | 8.4 b | 146 b | 31 b | 6.3 | 14.8 cd | 11 b | 48.4 |
| ‘Honeycrisp’ 2018 | ||||||||||
| Non-netted (Thinned 2017) | 31.7 a | 338 a | 1 a | 11.1 a | 94 c | 55 ab | 7.1 c | 15.1 b | 12.1 d | 45.1 |
| Non-netted (Nonthinned) | 5.1 c | 35 b | 0.18 c | 1.2 b | 152 b | 68 a | 8.3 a | 17.6 a | 14.7 a | 32.9 |
| Non-netted (Hand thinned) | 16.9 b | 74 b | 0.58 b | 2.5 b | 234 a | 52 bc | 7.6 b | 17.3 a | 14.2 a | 48 |
| Netted 0% (Pink) | 12.1 bc | 50 b | 0.47 bc | 2 b | 240 a | 40 c | 7.6 b | 17.3 a | 13.6 b | 54.5 |
| Netted 28% (KB) | 14.9 bc | 59 b | 0.46 bc | 1.8 b | 257 a | 45 bc | 7.5 bc | 17.1 a | 13 c | 56.4 |
| Netted 68% (KB) | 16.9 b | 71 b | 0.58 b | 2.4 b | 242 a | 49 bc | 7.6 b | 17.2 a | 13.3 bc | 51.6 |
| Treatments | Fruit Weight | Firmness | SSC | Starch | Acidity |
|---|---|---|---|---|---|
| (g) | (kg) | (%) | (1–10) | (%) | |
| Non-netted (Nonthinned) | 115.1 | 6.3 | 12.4 | 7.1 | 0.27 |
| Non-netted (Hand thinned) | 137.1 | 6.5 | 11.6 | 7.7 | 0.27 |
| Netted 0% (Pink z) | 123.9 | 6.5 | 11.3 | 7.8 | 0.28 |
| Netted 20% (KB) | 116.8 | 6.2 | 11.1 | 7.7 | 0.23 |
| Netted 40 (KB) | 124.8 | 6.2 | 11.6 | 8 | 0.24 |
| Netted 80% (KB) | 135.4 | 6.3 | 11.4 | 7.6 | 0.25 |
| Treatments | Avg. Weight Loss | Red Color | Firmness | Dry Matter | SSC |
|---|---|---|---|---|---|
| (g) | (%) | (kg) | (%) | (%) | |
| ‘Honeycrisp’ 2017 | |||||
| Non-netted (Nonthinned) | 2.8 c z | 49 a | 6.3 b | 14.2 c | 10.8 c |
| Non-netted (Hand thinned) | 4 a | 44 a | 6.5 ab | 15.4 ab | 11.6 ab |
| Netted 0% (Pink) y | 3.8 ab | 42 a | 6.7 a | 15.7 a | 12 a |
| Netted 23% (KB) | 3.4 b | 23 b | 6.5 ab | 15.3 ab | 11.1 bc |
| Netted 58% (KB) | 3.7 ab | 31 b | 6.5 ab | 15.1 b | 11.1 bc |
| ‘Honeycrisp’ 2018 | |||||
| Non-netted (Thinned 2017) | 2.9 c | 55 b | 7.2 c | 15.4 c | 12 e |
| Non-netted (Nonthinned) | 4.7 a | 67 a | 8.1 a | 17.5 ab | 14.5 a |
| Non-netted (Hand thinned) | 4 b | 53 b | 7.6 b | 17.5 ab | 14.1 a |
| Netted 0% (Pink) | 4.3 ab | 45 bc | 7.5 bc | 17 ab | 13.4 b |
| Netted 28% (KB) | 4.7 a | 47 b | 7.6 b | 17.6 a | 13.3 c |
| Netted 68% (KB) | 4.4 ab | 51 b | 7.5 bc | 16.7 b | 12.6 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsysy, M.; Serra, S.; Schwallier, P.; Musacchi, S.; Einhorn, T. Net Enclosure of ‘Honeycrisp’ and ‘Gala’ Apple Trees at Different Bloom Stages Affects Fruit Set and Alters Seed Production. Agronomy 2019, 9, 478. https://doi.org/10.3390/agronomy9090478
Elsysy M, Serra S, Schwallier P, Musacchi S, Einhorn T. Net Enclosure of ‘Honeycrisp’ and ‘Gala’ Apple Trees at Different Bloom Stages Affects Fruit Set and Alters Seed Production. Agronomy. 2019; 9(9):478. https://doi.org/10.3390/agronomy9090478
Chicago/Turabian StyleElsysy, Mokhles, Sara Serra, Phil Schwallier, Stefano Musacchi, and Todd Einhorn. 2019. "Net Enclosure of ‘Honeycrisp’ and ‘Gala’ Apple Trees at Different Bloom Stages Affects Fruit Set and Alters Seed Production" Agronomy 9, no. 9: 478. https://doi.org/10.3390/agronomy9090478
APA StyleElsysy, M., Serra, S., Schwallier, P., Musacchi, S., & Einhorn, T. (2019). Net Enclosure of ‘Honeycrisp’ and ‘Gala’ Apple Trees at Different Bloom Stages Affects Fruit Set and Alters Seed Production. Agronomy, 9(9), 478. https://doi.org/10.3390/agronomy9090478








