Metabolic Changes and Increased Levels of Bioactive Compounds in White Radish (Raphanus sativus L. cv. 01) Sprouts Elicited by Oligochitosan
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Oligochitosan (O-80) Preparation
2.3. Plant Material and Sample Preparation
2.4. Total Phenolic Content Assay
2.5. Antioxidant Activity Assays
2.5.1. Ferric Ion Reducing Antioxidant Power (FRAP) Assay
2.5.2. (2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) Radical Scavenging Activity
2.5.3. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.6. Metabolomics Study Using Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis
2.7. Data Processing Using Chemometric Tools
2.8. Glucoraphasatin Content Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Non-Targeted Metabolome Analysis of WRS Treated with Oligochitosan O-80
3.2. Hierarchical Clustering Analysis (HCA)
3.3. Identification of Sinapoyl Derivatives by LC-DAD-MS Chromatography
3.4. Antioxidant Content
3.5. Total Phenolic Content
3.6. Effect of Oligochitosan O-80 Treatment on the Glucoraphasatin Content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, S.O.; Lee, I.S. Induction of quinone reductase, the phase 2 anticarcinogenic marker enzyme, in Hepa1c1c7 cells by radish sprouts, Raphanus sativus L. J. Food Sci. 2006, 71, 144–148. [Google Scholar] [CrossRef]
- Takaya, Y.; Kondo, Y.; Furukawa, T.; Niwa, M. Antioxidant constituents of radish sprout (Kaiware-daikon), Raphanus sativus L. J. Agric. Food Chem. 2003, 51, 8061–8066. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, G.R.; Bagatta, M.; Pagnotta, E.; Angelino, D.; Gennari, L.; Ninfali, P.; Rollin, P.; Iori, R. Comparison of bioactive phytochemical content and release of isothiocyanates in selected Brassica sprouts. Food Chem. 2013, 141, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Kobayashi-Hattori, K.; Tenmyo, C.; Kamei, T.; Uda, Y.; Sugita-Konishi, Y.; Oishi, Y.; Takita, T. Effect of Japanese radish (Raphanus sativus) sprout (Kaiware-daikon) on carbohydrate and lipid metabolisms in normal and streptozotocin-induced diabetic rats. Phytother. Res. 2006, 20, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Umeda, T.; Higuchi, O.; Tsuzuki, T.; Suzuki, T.; Miyazawa, Y. Evaporative light-scattering analysis of sulforaphane in broccoli samples: Quality of broccoli products regarding sulforaphane contents. J. Agric. Food Chem. 2006, 54, 2479–2483. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef]
- Hanlon, P.R.; Barnes, D.M. Phytochemical composition and biological activity of 8 varieties of radish (Raphanus sativus L.) sprouts and mature taproots. J. Food Sci. 2011, 76, 185–192. [Google Scholar] [CrossRef]
- Abdull Razis, A.F.; De Nicola, G.R.; Pagnotta, E.; Iori, R.; Ioannides, C. A glucosinolate-rich extract of Japanese Daikon perturbs carcinogen-metabolizing enzyme systems in rat, being a potent inducer of hepatic glutathione S-transferase. Eur. J. Nutr. 2013, 52, 1279–1285. [Google Scholar] [CrossRef]
- Okamura, T.; Umemura, T.; Inoue, T.; Tasaki, M.; Ishii, Y.; Nakamura, Y.; Park, E.Y.; Sato, K.; Matsuo, T.; Okamoto, S.; et al. Chemopreventive effects of 4-methylthio-3-butenyl isothiocyanate (Raphasatin) but not curcumin against pancreatic carcinogenesis in hamsters. J. Agric. Food Chem. 2013, 61, 2103–2108. [Google Scholar] [CrossRef]
- Bhaskara Reddy, M.V.; Arul, J.; Angers, P.; Couture, L. Treatment of wheat seeds induces resistance to Fusarium graminearum and improves seed quality. J. Agric. Food Chem. 1999, 47, 1208–1216. [Google Scholar] [CrossRef]
- Meng, X.; Tang, Y.; Zhang, A.; Huang, X.; Zhang, Z. Effect of oligochitosan on development of Colletotrichum musae in vitro and in situ and its role in protection of banana fruits. Fruits 2012, 67, 147–155. [Google Scholar]
- Limpanavech, P.; Chaiyasuta, S.; Vongpromek, R.; Pichyangkura, R.; Khunwasi, C.; Chadchawan, S.; Lotrakul, P.; Bunjongrat, R.; Chaidee, A.; Bangyeekhun, T. Chitosan effects on floral production, gene expression, and anatomical changes in the Dendrobium orchid. Sci. Hort. 2008, 116, 65–72. [Google Scholar] [CrossRef]
- Barrientos Carvacho, H.; Perez, C.; Zuniga, G.; Mahn, A. Effect of methyl jasmonate, sodium selenate and chitosan as exogenous elicitors on the phenolic compounds profile of broccoli sprouts. J. Sci. Food Agric. 2014, 94, 2555–2561. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lehmann, M.; Schwarzlander, M.; Obata, T.; Sirikantaramas, S.; Burow, M.; Olsen, C.E.; Tohge, T.; Fricker, M.D.; Moller, B.L.; Fernie, A.R.; et al. The metabolic response of Arabidopsis roots to oxidative stress is distinct from that of heterotrophic cells in culture and highlights a complex relationship between the levels of transcripts, metabolites, and flux. Mol. Plant 2009, 2, 390–406. [Google Scholar] [CrossRef]
- Lommen, A. MetAlign: Interface-driven, versatile metabolomics tool for hyphenated full-scan mass spectrometry data preprocessing. Anal. Chem. 2009, 15, 3079–3086. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, 486–494. [Google Scholar] [CrossRef]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef]
- Kim, H.-J.; Chen, F.; Wang, X.; Choi, J.-H. Effect of methyl jasmonate on phenolics, isothiocyanate, and metabolic enzymes in radish sprout (Raphanus sativus L.). J. Agric. Food Chem. 2006, 54, 7263–7269. [Google Scholar] [CrossRef]
- Nićiforović, N.; Abramovič, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Pajak, P.; Socha, R.; Galkowska, D.; Roznowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Jahangir, M.; van den Hondel, C.A.M.J.J.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Fungal infection-induced metabolites in Brassica rapa. Plant Sci. 2009, 176, 608–615. [Google Scholar] [CrossRef]
- Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Madala, N.E.; Dubery, I.A. Phenylpropanoid defenses in Nicotiana tabacum cells: Overlapping metabolomes indicate common aspects to priming responses induced by lipopolysaccharides. PLoS ONE 2016, 11, e0151350. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Radojčić Redovniković, I.; Glivetić, T.; Delonga, K.; Vorkapić-Furač, J. Glucosinolates and their potential role in plant. Period. Biol. 2008, 110, 297–309. [Google Scholar]
- Coqueiro, D.S.O.; Maraschin, M.; Piero, R.M.D. Chitosan reduces bacterial spot severity and acts in phenylpropanoid metabolism in tomato plants. J. Phytopathol. 2011, 159, 488–494. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem. 2011, 129, 35–44. [Google Scholar] [CrossRef]
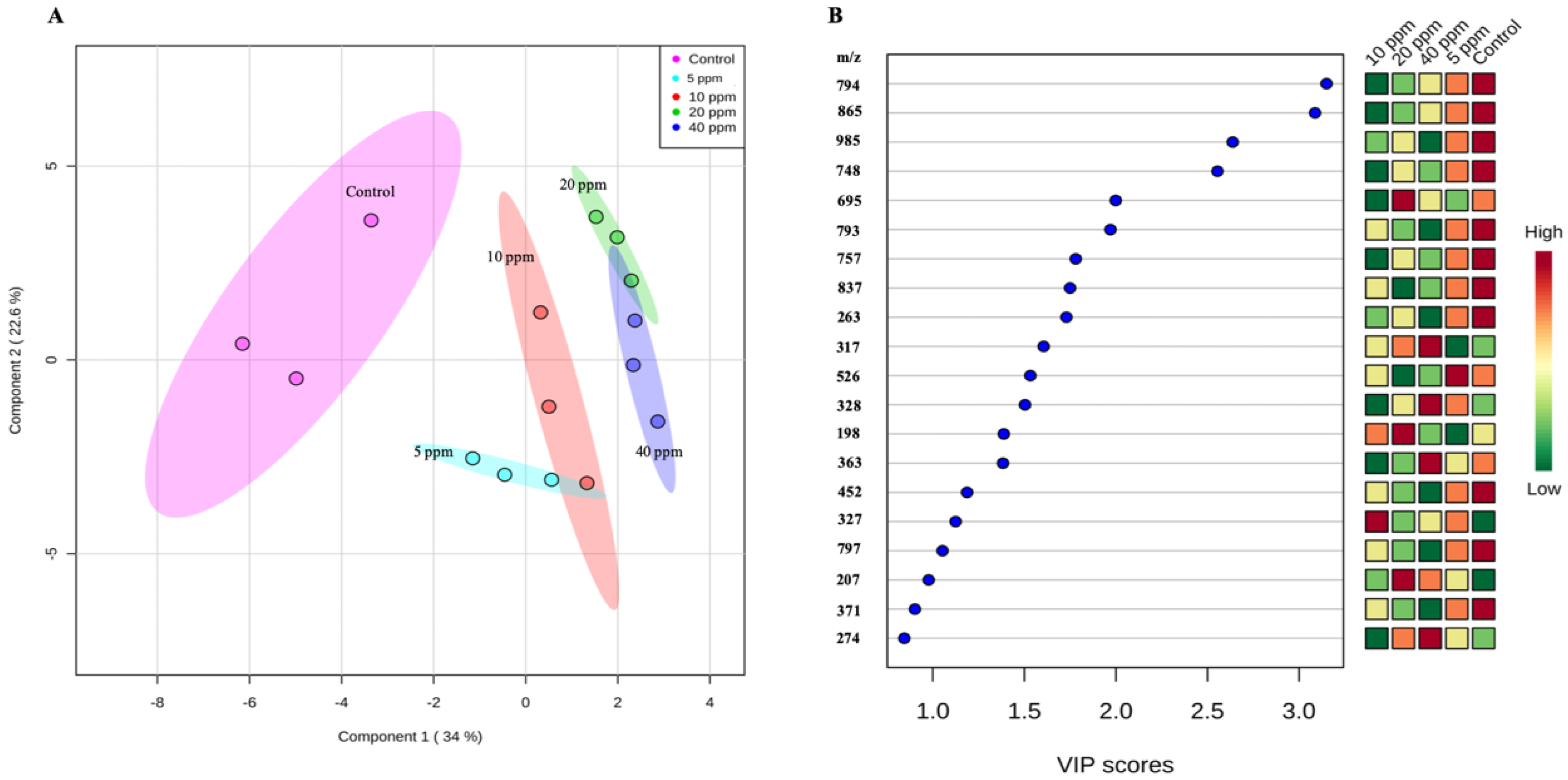
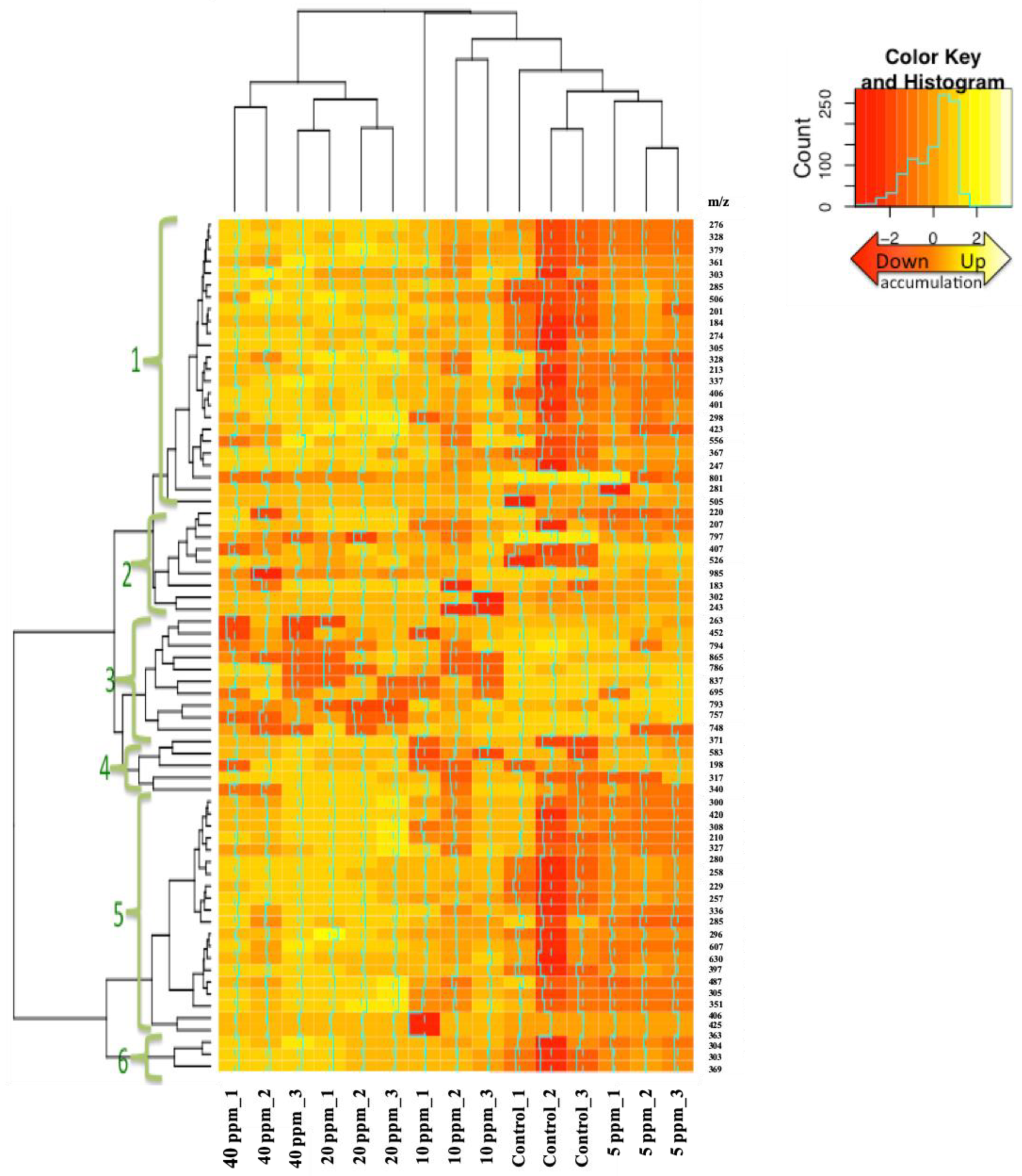
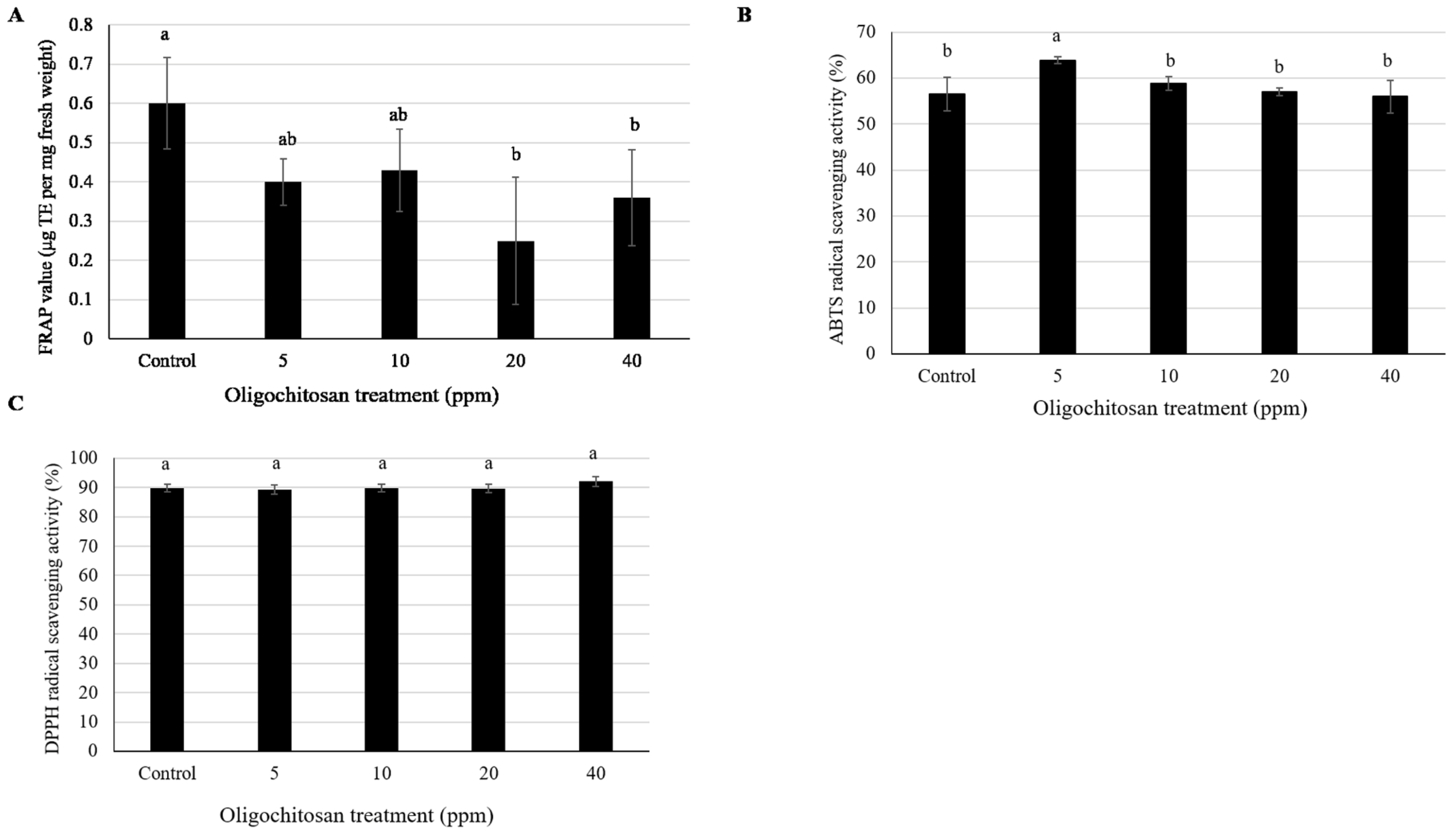
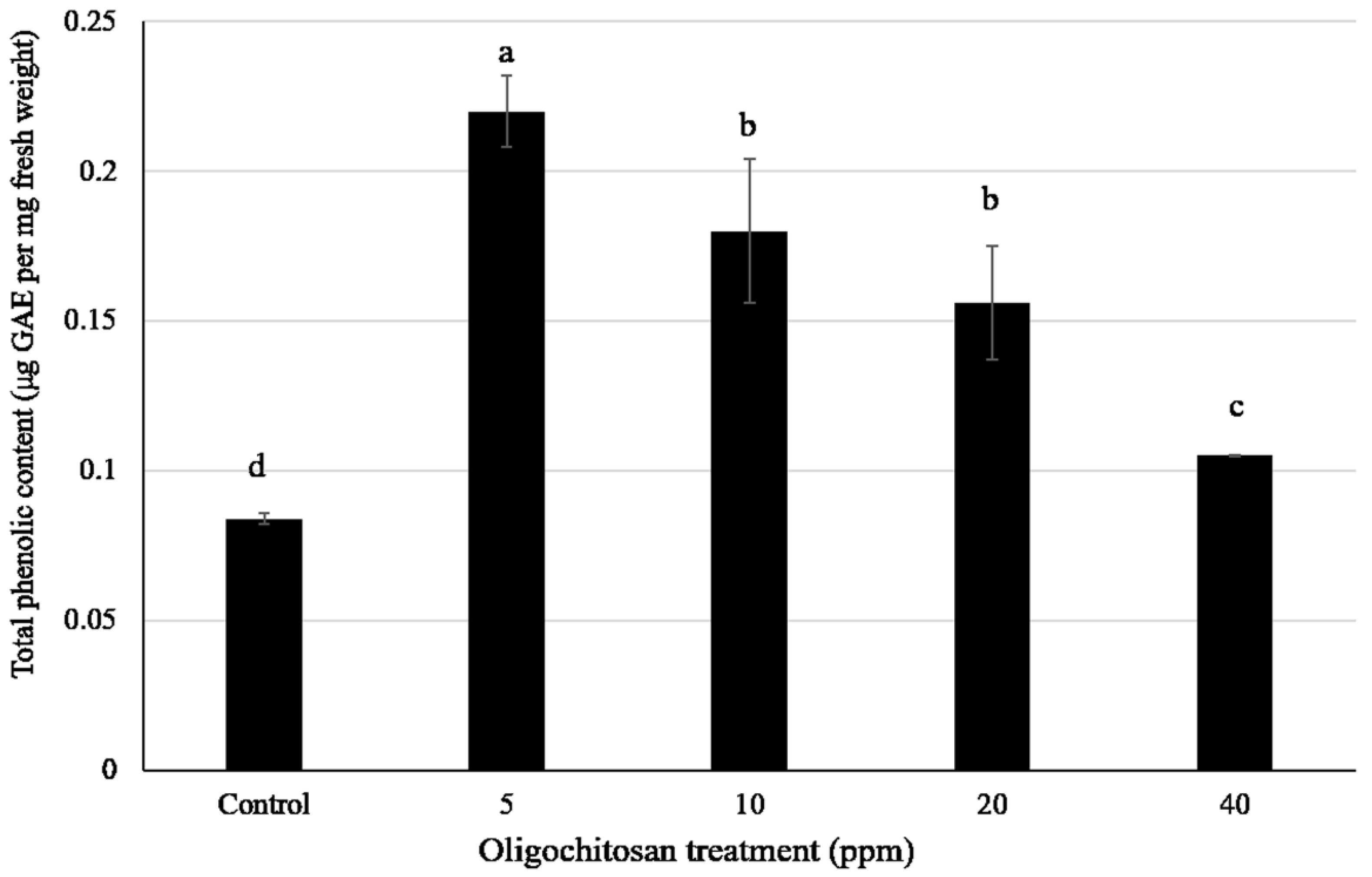

| Mass-To-Charge (m/z) Ratio | Retention Time (min) | HCA * | Fold Change ** (Mean ± Standard Error of Mean (SEM), n = 3) at Oligochitosan Concentration of: | |||
|---|---|---|---|---|---|---|
| 5 ppm | 10 ppm | 20 ppm | 40 ppm | |||
| 274 | 11.8 | 1 | 2.29 ± 0.23 d | 3.54 ± 0.24 c | 4.41 ± 0.10 b | 5.06 ± 0.06 a |
| 305 | 18.0 | 1 | 1.82 ± 0.18 d | 3.60 ± 0.28 c | 4.50 ± 0.36 b | 5.45 ± 0.22 a |
| 184 | 7.4 | 1 | 2.21 ± 0.21 c | 3.42 ± 0.34 b | 4.42 ± 0.09 a | 3.88 ± 0.09 ab |
| 243 | 12.9 | 2 | 1.67 ± 0.05 c | 1.80 ± 0.90 bc | 3.35 ± 0.04 ab | 4.12 ± 0.36 a |
| 985 | 8.0 | 2 | 0.49 ± 0.10 a | 0.47 ± 0.14 a | 0.22 ± 0.04 ab | 0.13 ± 0.06 b |
| 748 | 1.9 | 3 | 0.59 ± 0.12 a | 0.45 ± 0.12 a | 0.03 ± 0.02 b | 0.08 ± 0.07 b |
| 317 | 13.0 | 4 | 0.55 ± 0.45 b | 2.71 ± 1.60 b | 7.40 ± 0.64 a | 9.89 ± 0.16 a |
| 198 | 3.1 | 4 | 0.80 ± 0.17 c | 1.23 ± 0.80 c | 6.99 ± 0.76 a | 4.71 ± 0.20 b |
| 257 | 11.8 | 5 | 2.02 ± 0.18 c | 3.01 ± 0.10 b | 3.68 ± 0.26 a | 4.01 ± 0.08 a |
| 285 | 11.8 | 5 | 1.87 ± 0.15 d | 2.65 ± 0.17 c | 3.52 ± 0.09 b | 4.01 ± 0.11 a |
| Peak No. | Retention Time (min) | Molecular Ion [M + H]+ (m/z) | Fragment [M + H]+ (m/z) | Proposed Bioactive Compound | Fold Change * (Mean ± SEM, n = 3) at Oligochitosan Concentration of: | |||
|---|---|---|---|---|---|---|---|---|
| 5 ppm | 10 ppm | 20 ppm | 40 ppm | |||||
| 1 | 6.9 | 487 | 207, 369, 487 | Sinapic acid conjugate | 1.17 ± 0.05 a | 1.18 ± 0.07 a | 1.35 ± 0.05 b | 1.34 ± 0.09 a |
| 2 | 13.0 | 363 | 207, 363 | Sinapoyl malate | 1.11 ± 0.08 a | 1.27 ± 0.14 a | 1.55 ± 0.02 a | 1.26 ± 0.06 a |
| 3 | 14.4 | 777 | 207, 369 | Sinapic acid conjugate | 0.94 ± 0.05 b | 1.09 ± 0.11 a | 1.31 ± 0.08 b | 1.26 ± 0.07 a |
| 4 | 15.4 | 358 | 207, 336 | Sinapic acid conjugate | 1.09 ± 0.38 ab | 0.82 ± 0.49 a | 0.21 ± 0.03 c | 0.44 ± 0.15 b |
| 5 | 16.9 | 615 | 207, 351, 369 | Sinapic acid conjugate | 0.82 ± 0.20 b | 1.42 ± 0.42 a | 1.35 ± 0.15 b | 1.12 ± 0.09 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakpenthai, A.; Khaksar, G.; Burow, M.; Olsen, C.E.; Sirikantaramas, S. Metabolic Changes and Increased Levels of Bioactive Compounds in White Radish (Raphanus sativus L. cv. 01) Sprouts Elicited by Oligochitosan. Agronomy 2019, 9, 467. https://doi.org/10.3390/agronomy9080467
Rakpenthai A, Khaksar G, Burow M, Olsen CE, Sirikantaramas S. Metabolic Changes and Increased Levels of Bioactive Compounds in White Radish (Raphanus sativus L. cv. 01) Sprouts Elicited by Oligochitosan. Agronomy. 2019; 9(8):467. https://doi.org/10.3390/agronomy9080467
Chicago/Turabian StyleRakpenthai, Apidet, Gholamreza Khaksar, Meike Burow, Carl Erik Olsen, and Supaart Sirikantaramas. 2019. "Metabolic Changes and Increased Levels of Bioactive Compounds in White Radish (Raphanus sativus L. cv. 01) Sprouts Elicited by Oligochitosan" Agronomy 9, no. 8: 467. https://doi.org/10.3390/agronomy9080467
APA StyleRakpenthai, A., Khaksar, G., Burow, M., Olsen, C. E., & Sirikantaramas, S. (2019). Metabolic Changes and Increased Levels of Bioactive Compounds in White Radish (Raphanus sativus L. cv. 01) Sprouts Elicited by Oligochitosan. Agronomy, 9(8), 467. https://doi.org/10.3390/agronomy9080467






