Contrasting Effects of NaCl and NaHCO3 Stresses on Seed Germination, Seedling Growth, Photosynthesis, and Osmoregulators of the Common Bean (Phaseolus vulgaris L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Salt- and Alkaline Stress Treatment
2.2. Seedling Emergence and Survival Investigations
2.3. Measurement of Leaf Gas Exchange Parameters and Physiological Indices
2.4. Determination of Inorganic Ions and Organic Solutes
2.5. Statistical Data Analysis
3. Results
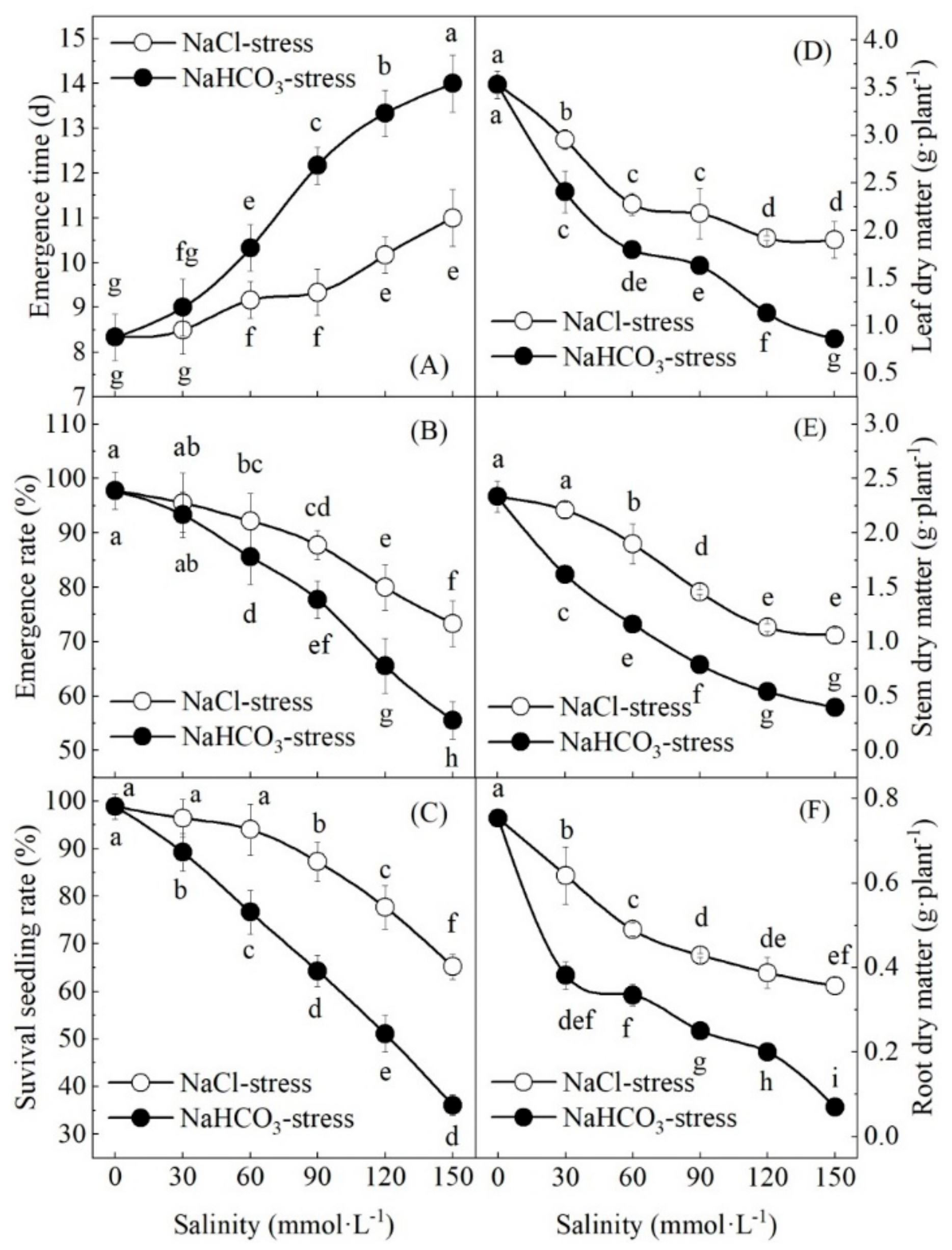
3.1. Effect of Neutral Salt and Alkaline Salt Stresses on Seedling Emergence and Survival
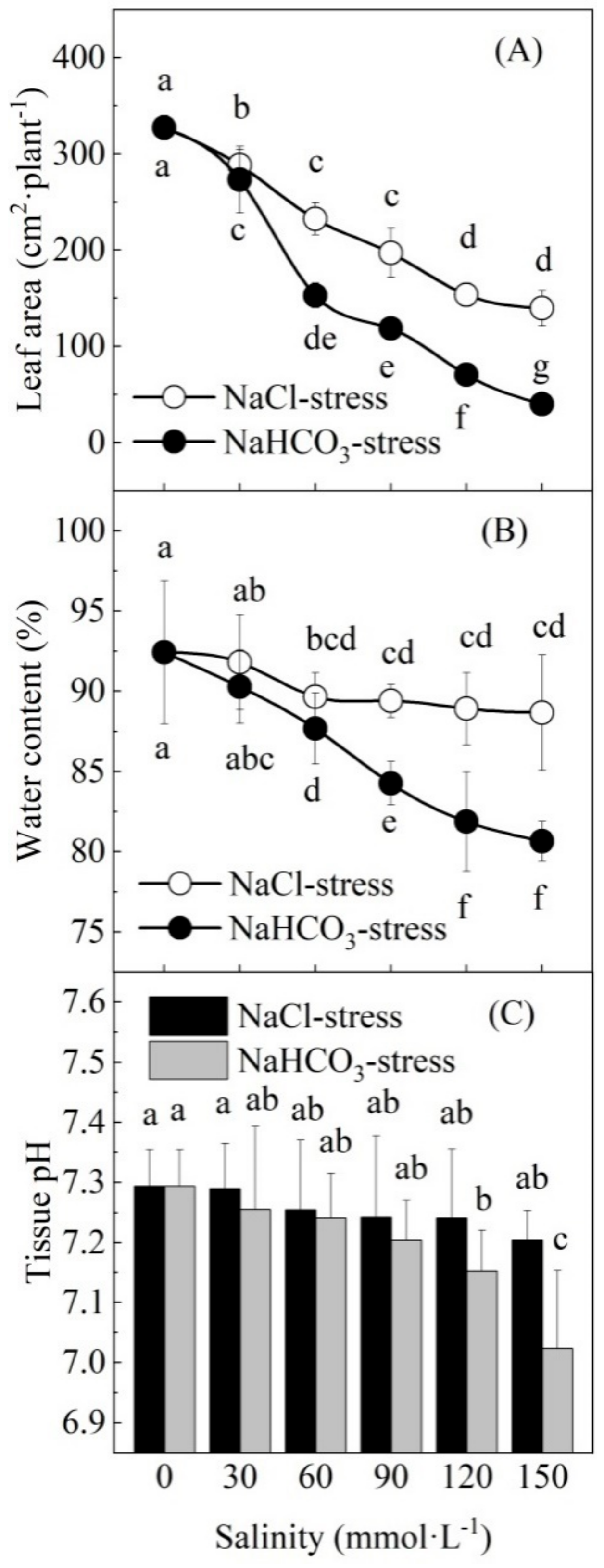
3.2. Effect of Neutral Salt and Alkaline Salt Stresses on Growth
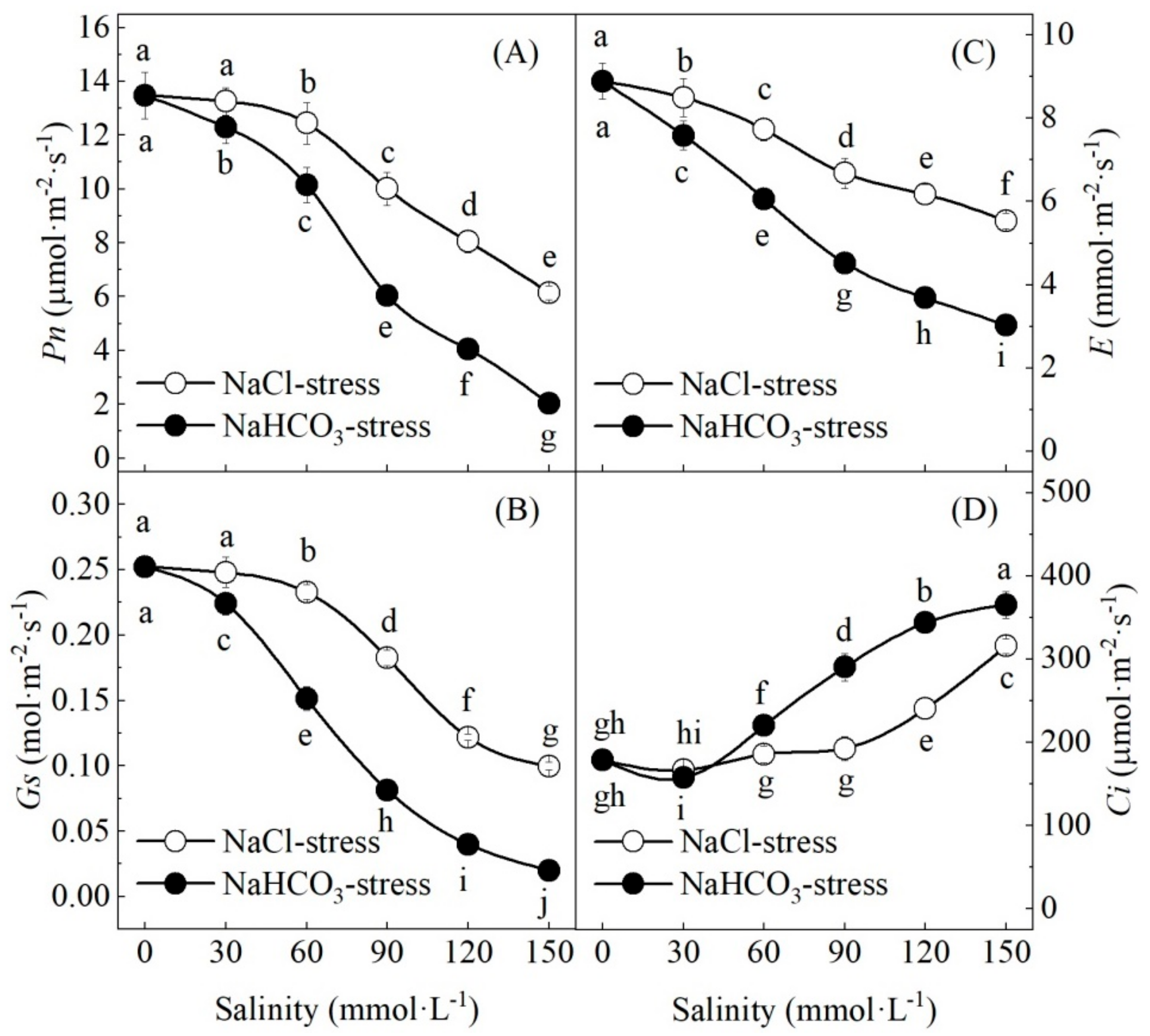
3.3. Effects of Neutral Salt and Alkaline Salt Stresses on Gas Exchange and Pigments
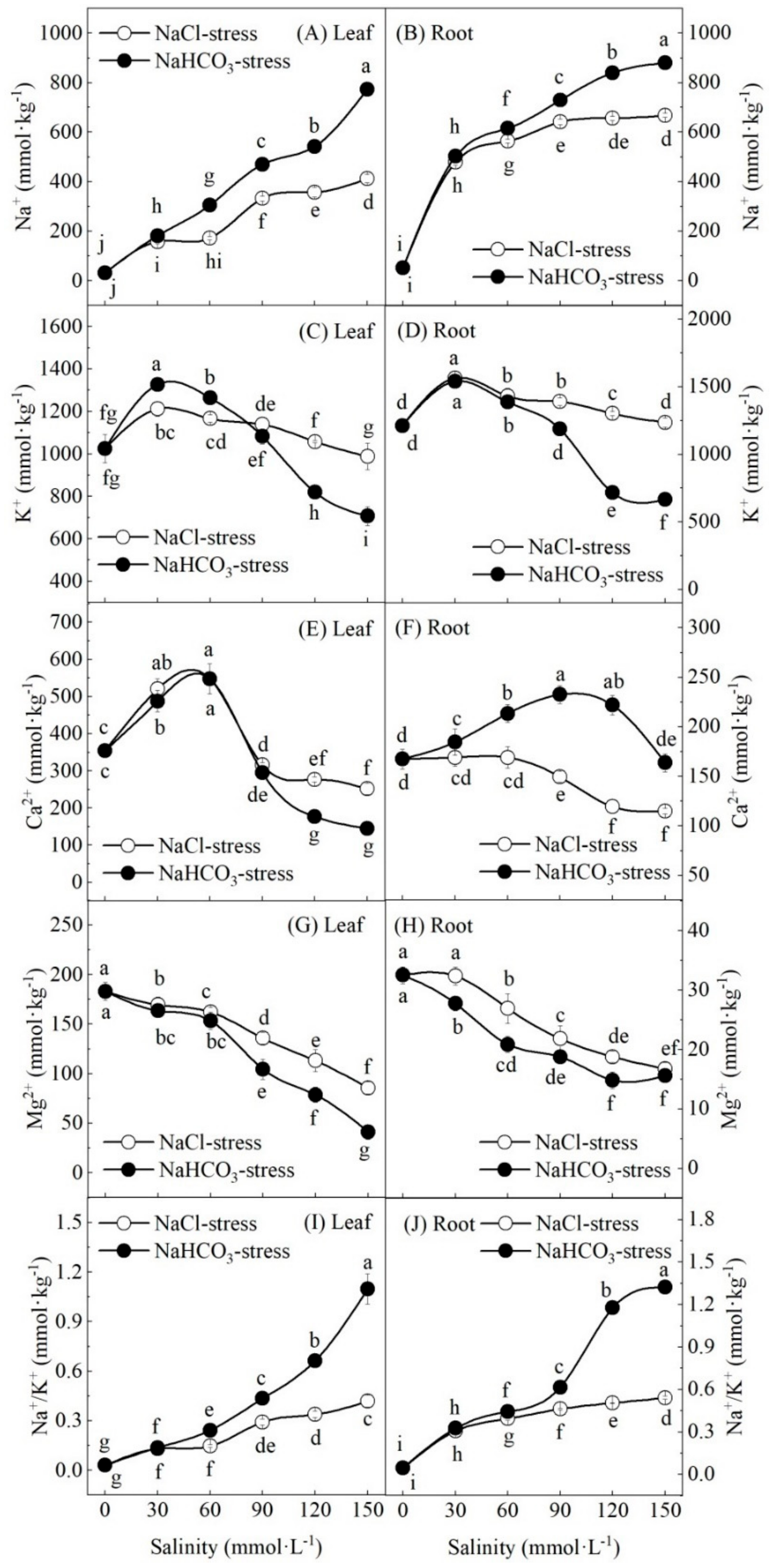
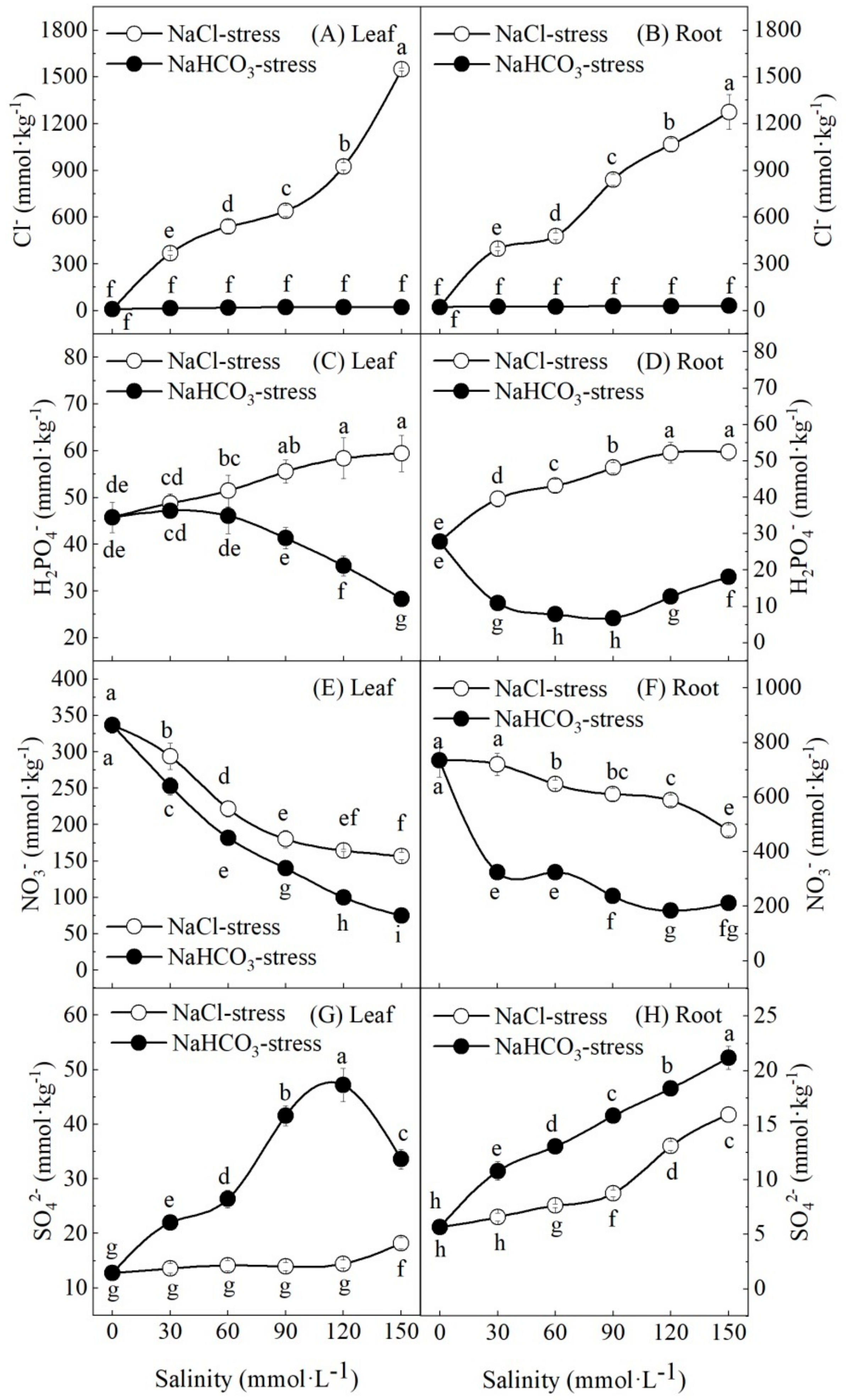
3.4. Effect of Salt and Alkali Stresses on Inorganic Ion Content
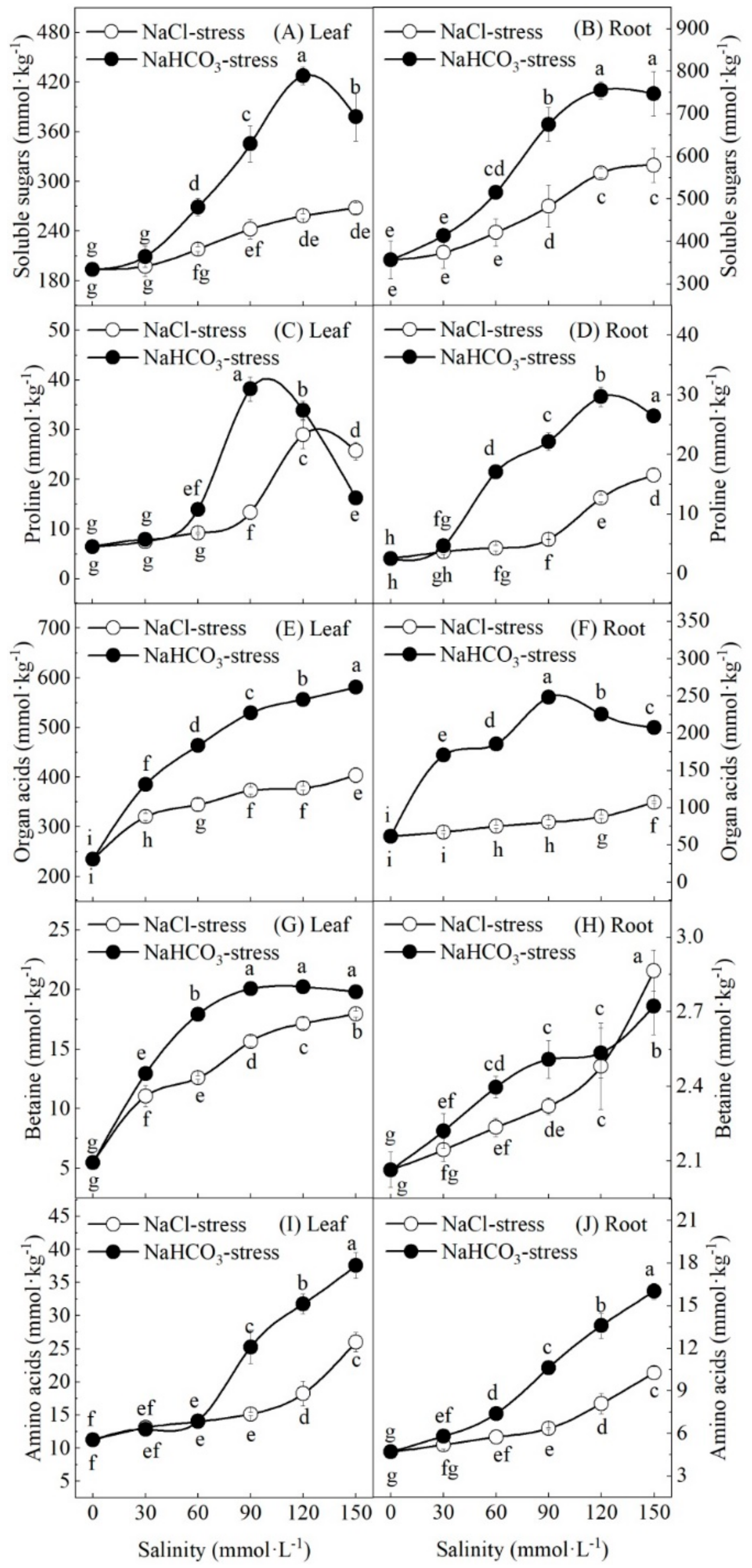
3.5. Effect of Salt and Alkali Stresses on Organic Solute Content
3.6. Effects of Neutral Salt and Alkaline Salt Stresses on Contribution Rates of Each Osmoregulators
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X.X.; Takano, T.; Liu, S.K. Identification of a mitochondrial ATP synthase small subunit gene (RMtATP6) expressed in response to salts and osmotic stresses in rice (Oryza sativa L.). J. Exp. Bot. 2006, 57, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, A.A.; Noller, J.S. Application of remote-sensing data and decision-tree analysis to mapping salt-affected soils over large areas. Remote Sens. 2009, 2, 151–165. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Zhu, Y.M.; Bai, X.; Lv, D.K.; Guo, D.J.; Ji, W.; Cai, H. Global transcriptome profiling of wild soybean (Glycine soja) roots under NaHCO3 treatment. BMC Plant Biol. 2010, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Nishiuchi, S.; Fujihara, K.; Liu, S.K.; Takano, T. Analysis of expressed sequence tags from a NaHCO3-treated alkali-tolerant plant, Chloris virgata. Plant Physiol. Biochem. 2010, 48, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; He, H.S.; Zu, Y.G.; Guan, Y.; Liu, Z.G.; Zhang, Z.H.; Xu, H.N.; Yu, X.Y. Addition of HPMA affects seed germination, plant growth and properties of heavy saline-alkali soil in northeastern China: Comparison with other agents and determination of the mechanism. Plant Soil 2011, 339, 177–191. [Google Scholar] [CrossRef]
- Yang, C.W.; Jianaer, A.; Li, C.Y.; Shi, D.C.; Wang, D.L. Comparison of the effects of salt-stress and alkali-stress on photosynthesis and energy storage of an alkali-resistant halophyte Chloris virgate. Photosynthetica 2008, 46, 273–278. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; de Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidant enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Veselov, D.S.; Sharipova, G.V.; Akhiyarova, G.R.; Kudoyarova, G.R. Fast growth responses of barley and durum wheat plants to NaCl- and PEG-treatment: Resolving the relative contributions of water deficiency and ion toxicity. Plant Growth Regul. 2009, 58, 125–129. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.Y.; Zhang, X.Z.; Luo, H.J.; Fu, J.M. Toxic effect of NaCl on ion metabolism, antioxidative enzymes and gene expression of perennial ryegrass. Ecotoxicol. Environ. Saf. 2011, 74, 2050–2056. [Google Scholar] [CrossRef]
- Yang, C.W.; Shi, D.C.; Wang, D.L. Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge.). Plant Growth Regul. 2008, 56, 179–190. [Google Scholar] [CrossRef]
- Yang, C.W.; Chong, J.N.; Li, C.Y.; Kim, C.M.; Shi, D.C.; Wang, D.L. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 2007, 294, 263–276. [Google Scholar] [CrossRef]
- Abd EI-Samad, H.M.; Shaddad, M.A.K. Comparative effect of sodium carbonate, sodium sulphate, and sodium chloride on the growth and related metabolic activities of pea plants. J. Plant Nutr. 1996, 19, 717–728. [Google Scholar] [CrossRef]
- Brand, J.D.; Tang, C.; Rathjen, A.J. Screening rough-seeded lupins (Lupinus pilosus Murr. and Lupinus atlanticus Glads.) for tolerance to calcareous soils. Plant Soil 2002, 245, 261–275. [Google Scholar] [CrossRef]
- Hartung, W.; Leport, L.; Ratcliffe, R.G.; Sauter, A.; Duda, R.; Turner, N.C. Abscisic acid concentration, root pH and anatomy do not explain growth differences of chickpea (Cicer arietinum L.) and lupin (Lupinus angustifolius L.) on acid and alkaline soils. Plant Soil 2002, 240, 191–199. [Google Scholar] [CrossRef]
- Shi, D.C.; Sheng, Y.M. Effect of various slat-alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. Environ. Exp. Bot. 2005, 54, 8–21. [Google Scholar] [CrossRef]
- Gong, B.; Wen, D.; Van den Langenberg, K.; Wei, M.; Yang, F.J.; Shi, Q.H.; Wang, X.F. Comparative effects of NaCl and NaHCO3 stress on photosynthetic parameters, nutrient metabolism, and the antioxidant system in tomato leaves. Sci. Hortic. 2013, 157, 1–12. [Google Scholar] [CrossRef]
- Guo, R.; Yang, Z.Z.; Li, F.; Yan, C.R.; Zhong, X.L.; Liu, Q.; Xia, X.; Li, H.R.; Zhao, L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef]
- Gong, B.; Wen, D.; Bloszies, S.; Li, X.; Wei, M.; Yang, F.J.; Shi, Q.H.; Wang, X.F. Comparative effects of NaCl and NaHCO3 stresses on respiratory metabolism, antioxidant system, nutritional status, and organic acid metabolism in tomato roots. Acta Physiol. Plant 2014, 36, 2167–2181. [Google Scholar] [CrossRef]
- Katerji, N.; van Hoorn, J.W.; Hamdy, A.; Mastrorilli, M. Salt tolerance of crops according to three classification methods and examination of some hypothesis about salt tolerance. Agric. Water Manag. 2001, 47, 1–8. [Google Scholar] [CrossRef]
- Bayuelo-Jiménes, J.S.; Craig, R.; Lynch, J.P. Salinity tolerance of Phaseolus species during germination and early seedling growth. Crop Sci. 2002, 42, 1584–1594. [Google Scholar] [CrossRef]
- Neel, J.P.S.; Alloush, G.A.; Belesky, D.P.; Clapham, W.M. Influence of rhizosphere ionic strength on mineral composition, dry matter yield and nutritive value of forage chicory. J. Agron. Crop Sci. 2002, 188, 398–407. [Google Scholar] [CrossRef]
- Bayuelo-Jiménes, J.S.; Debouck, D.G.; Lynch, J.P. Salinity tolerance of Phaseolus species during early vegetative growth. Crop Sci. 2002, 42, 2184–2192. [Google Scholar] [CrossRef]
- Wang, L.; Seki, K.; Miyazaki, T.; Ishihama, Y. The causes of soil alkalinization in the Songnen Plain of Northeast China. Paddy Water Environ. 2009, 7, 259–270. [Google Scholar] [CrossRef]
- Seemann, J.R.; Critchley, C. Effects of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L. Planta 1985, 164, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Bayuelo-Jiménez, J.S.; Debouck, D.G.; Lynch, J.P. Growth, gas exchange, water relations, and ion composition of Phaseolus species grown under saline conditions. Field Crops Res. 2003, 80, 207–222. [Google Scholar] [CrossRef]
- Palma, F.; Lluch, C.; Iribarne, C.; García-Garrido, J.M.; Tejera García, N.A. Combined effect of salicylic acid and salinity on some antioxidant activities, oxidative stress and metabolite accumulation in Phaseolus vulgaris. Plant Growth Regul. 2009, 58, 307–316. [Google Scholar] [CrossRef]
- Talaat, N.B. Effective microorganisms enhance the scavenging capacity of the ascorbate-glutathione cycle in common bean (Phaseolus vulgaris L.) plants grown in salty soils. Plant Physiol. Bioch. 2014, 80, 136–143. [Google Scholar] [CrossRef]
- Chen, J.B.; Yang, J.W.; Zhang, Z.Y.; Feng, X.F.; Wang, S.M. Two P5CS genes from common bean exhibiting different tolerance to salt stress in transgenic Arabidopsis. J. Genet. 2013, 92, 461–469. [Google Scholar] [CrossRef]
- Hernández-Lucero, E.; Rodríguez-Hernández, A.A.; Ortega-Amaro, M.A.; Jiménez-Bremont, J.F. Differential expression of genes for tolerance to salt stress in common bean (Phaseolus vulgaris L.). Plant Mol. Biol. Rep. 2014, 32, 318–327. [Google Scholar] [CrossRef]
- Hiz, M.C.; Canher, B.; Niron, H.; Turet, M. Transcriptome analysis of salt tolerant common bean (Phaseolusvulgaris L.) under saline conditions. PLoS ONE 2014, 9, e92598. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Lei, Y.T.; Xu, Y.X.; Hettenhausen, C.; Lu, C.K.; Shen, G.J.; Zhang, C.P.; Li, J.; Song, J. Comparative analysis of alfalfa (Medicago sativa L.) leaf transcriptomes reveals genotype-specific salt tolerance mechanisms. BMC Plant Biol. 2018, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Kochert, G. Carbohydrate Determination by Phenol-Sulfuric Acid Method. In Handbook of Phycological Methods: Physiological and Biochemical Methods; Hellebust, J.A., Craigie, J.S., Eds.; Cambridge University Press: London, UK, 1978; pp. 95–97. [Google Scholar]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of the free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Yao, L.H.; Liu, X.; Jiang, Y.M.; Caffin, N. Compositional analysis of teas from Australian supermarkets. Food Chem. 2006, 94, 115–122. [Google Scholar] [CrossRef]
- Liu, J.; Guo, W.Q.; Shi, D.C. Seed germination, seedling survival, and physiological response of sunflowers under saline and alkaline conditions. Photosynthetica 2010, 48, 278–286. [Google Scholar] [CrossRef]
- Shi, D.C.; Wang, D.L. Effects of various salt-alkali mixed stresses on Aneurolepidium chinense (Trin.) Kitag. Plant Soil 2005, 271, 15–26. [Google Scholar] [CrossRef]
- Thompson, D.I.; Edwards, T.J.; Van Staden, J. A novel dual-phase culture medium promotes germination and seedling establishment from immature embryos in South African Disa (Orchidaceae) Species. Plant Growth Regul. 2007, 53, 163–171. [Google Scholar] [CrossRef]
- Li, C.Y.; Fang, B.; Yang, C.W.; Shi, D.C.; Wang, D.L. Effects of various salt–alkaline mixed stresses on the state of mineral elements in nutrient solutions and the growth of alkali resistant halophyte Chlorisvirgata. J. Plant Nutr. 2009, 32, 1137–1147. [Google Scholar] [CrossRef]
- Yang, C.W.; Zhang, M.L.; Liu, J.; Shi, D.C.; Wang, D.L. Effects of buffer capacity on growth, photosynthesis, and solute accumulation of a glycophyte (wheat) and a halophyte (Chloris virgata). Photosynthetica 2009, 47, 55–60. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, X.; Tao, H.B.; Wang, P. Growth performance and physiological response in the halophyte Lycium barbarum grown at salt-affected soil. Ann. Appl. Biol. 2006, 149, 263–269. [Google Scholar] [CrossRef]
- Bethke, P.C.; Drew, M.C. Stomatal and non-stomatal components to inhibition of photosynthesis in leaves of Capsicum annuum during progressive exposure to NaCl salinity. Plant Physiol. 1992, 99, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Koyro, H.W. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- Sultana, N.; Ikeda, T.; Itoh, R. Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environ. Exp. Bot. 1999, 42, 211–220. [Google Scholar] [CrossRef]
- Ma, J.F.; Zheng, S.J.; Matsumoto, H.; Hiradate, S. Detoxifying aluminum with buckwheat. Nature 1997, 390, 569–570. [Google Scholar] [CrossRef]
- James, R.A.; Munns, R.; Von Cammerer, S.; Trejo, C.; Miller, C.; Condon, T.A.G. Photosynthetic capacity is related to the cellular and subcelluar partitioning of Na+, K+ and Cl− in salt-affected barley and durum wheat. Plant Cell Environ. 2006, 29, 2185–2197. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Sagi, M.; Dovrat, A.; Kipnis, T.; Lips, H. Ionic balance, biomass production, and organic nitrogen as affected by salinity and nitrogen source in annual ryegrass. J. Plant Nutr. 1997, 20, 1291–1316. [Google Scholar] [CrossRef]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Santa-Cruz, A.; Martinez-Rodriguez, M.M.; Perez-Alfocea, F.; Romero-Aranda, R.; Bolarin, M.C. The rootstock effect on the tomato salinity response depends on the shoot genotype. Plant Sci. 2002, 162, 825–831. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Kerepesi, I.; Galiba, G. Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci. 2000, 40, 482–487. [Google Scholar] [CrossRef]
- Stoop, J.M.H.; Pharr, D.M. Growth substrate and nutrient salt environment alter mannitol-hexose partitioning in celery petioles. J. Am. Soc. Hortic. Sci. 1994, 119, 237–242. [Google Scholar] [CrossRef]
- Van den Ende, W.; Valluru, R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef]
- Timpa, J.D.; Burke, J.J.; Quisenberry, J.E.; Wendt, C.W. Effects of water stress on the organic acid and carbohydrate compositions of cotton plants. Plant Physiol. 1986, 82, 724–728. [Google Scholar] [CrossRef]
- Fougère, F.; Le Rudulier, D.; Streeter, J.G. Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids and cytosol of alfalfa (Medicago sativa L.). Plant Physiol. 1991, 96, 1228–1236. [Google Scholar] [CrossRef]
- Chen, W.; Feng, C.; Guo, W.; Shi, D.; Yang, C. Comparative effects of osmotic-, salt- and alkali stress on growth, photosynthesis, and osmotic adjustment of cotton plants. Photosynthetica 2011, 49, 417–425. [Google Scholar] [CrossRef]
- Backhausen, J.E.; Kitzmahn, C.; Scheibe, R. Competition between electron acceptors in photosynthesis: Regulation of the malate valve during CO2 fixation and nitrate reduction. Photosynth. Res. 1994, 42, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Scheible, W.R.; González-Fontes, A.; Lauerer, M.; Muller-Rober, B.; Caboche, M.; Stitt, M. Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell 1997, 9, 783–798. [Google Scholar] [CrossRef] [PubMed]
| Salinity (mmol·L−1) | NaCl Stress | Salinity (mmol·L−1) | NaHCO3 Stress | ||||
|---|---|---|---|---|---|---|---|
| Chla (g·kg−1) | Chlb (g·kg−1) | Car (g·kg−1) | Chla (g·kg−1) | Chlb (g·kg−1) | Car (g·kg−1) | ||
| 0 | 2.58 ± 0.16a | 0.73 ± 0.06a | 0.60 ± 0.05ab | 0 | 2.58 ± 0.16ab | 0.73 ± 0.06a | 0.60 ± 0.05a |
| 30 | 2.72 ± 0.13a | 0.69 ± 0.02ab | 0.66 ± 0.03a | 30 | 2.66 ± 0.13a | 0.66 ± 0.02b | 0.58 ± 0.02ab |
| 60 | 2.65 ± 0.06a | 0.66 ± 0.03ab | 0.62 ± 0.03a | 60 | 2.34 ± 0.10b | 0.61 ± 0.03b | 0.52 ± 0.02bc |
| 90 | 2.48 ± 0.10a | 0.63 ± 0.04bc | 0.55 ± 0.04b | 90 | 2.01 ± 0.13c | 0.48 ± 0.05c | 0.47 ± 0.04c |
| 120 | 2.21 ± 0.16b | 0.57 ± 0.02cd | 0.43 ± 0.03c | 120 | 1.89 ± 0.09cd | 0.40 ± 0.04d | 0.39 ± 0.02d |
| 150 | 2.20 ± 0.15b | 0.55 ± 0.04d | 0.40 ± 0.02c | 150 | 1.74 ± 0.13d | 0.39 ± 0.03d | 0.34 ± 0.02d |
| Treatment | Na+ (%) | K+ (%) | Ca2+ (%) | Mg2+ (%) | Cl− (%) | H2PO4− (%) | NO3− (%) | SO42− (%) | Soluble Sugars (%) | Proline (%) | Organ Acids (%) | Betaine (%) | Amino Acids (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl Stress | Control | 43.65 | 1.32 | 14.47 | 7.48 | 0.35 | 1.87 | 14.34 | 0.54 | 8.25 | 0.28 | 10.01 | 0.23 | 0.48 |
| 30 | 37.43 | 4.88 | 15.61 | 5.08 | 11.41 | 1.46 | 9.08 | 0.42 | 6.11 | 0.23 | 9.90 | 0.34 | 0.41 | |
| 60 | 34.47 | 5.06 | 15.78 | 4.67 | 15.92 | 1.48 | 6.56 | 0.42 | 6.45 | 0.27 | 10.18 | 0.37 | 0.41 | |
| 90 | 30.52 | 8.91 | 9.09 | 3.91 | 17.16 | 1.60 | 4.82 | 0.37 | 6.50 | 0.36 | 10.00 | 0.42 | 0.41 | |
| 120 | 25.92 | 8.76 | 7.55 | 3.08 | 22.65 | 1.59 | 4.03 | 0.35 | 6.33 | 0.71 | 9.25 | 0.42 | 0.45 | |
| 150 | 20.08 | 8.38 | 5.91 | 2.01 | 31.46 | 1.40 | 3.19 | 0.37 | 5.44 | 0.52 | 8.21 | 0.36 | 0.53 | |
| NaHCO3 Stress | 30 | 43.65 | 1.32 | 15.61 | 5.23 | 0.35 | 1.87 | 14.34 | 0.54 | 8.25 | 0.28 | 10.01 | 0.23 | 0.48 |
| 60 | 43.32 | 5.92 | 16.50 | 4.62 | 0.46 | 1.51 | 8.25 | 0.72 | 6.83 | 0.26 | 12.58 | 0.42 | 0.42 | |
| 90 | 38.25 | 9.23 | 9.36 | 3.31 | 0.56 | 1.39 | 5.50 | 0.80 | 8.15 | 0.42 | 14.03 | 0.54 | 0.43 | |
| 120 | 30.10 | 13.08 | 6.09 | 2.72 | 0.66 | 1.31 | 3.89 | 1.16 | 9.61 | 1.06 | 14.72 | 0.56 | 0.70 | |
| 150 | 23.68 | 15.67 | 5.05 | 1.44 | 0.69 | 1.22 | 2.89 | 1.36 | 12.36 | 0.98 | 16.08 | 0.58 | 0.92 | |
| Treatment | Na+ (%) | K+ (%) | Ca2+ (%) | Mg2+ (%) | Cl− (%) | H2PO4− (%) | NO3− (%) | SO42− (%) | Soluble Sugars (%) | Proline (%) | Organ Acids (%) | Betaine (%) | Amino Acids (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl Stress | Control | 45.83 | 1.99 | 6.23 | 1.21 | 0.87 | 1.05 | 27.74 | 0.21 | 13.48 | 0.10 | 2.33 | 0.08 | 0.18 |
| 30 | 40.05 | 12.26 | 4.37 | 0.84 | 10.17 | 1.01 | 18.44 | 0.17 | 9.56 | 0.09 | 1.72 | 0.05 | 0.13 | |
| 60 | 36.33 | 14.30 | 4.36 | 0.69 | 12.14 | 1.10 | 16.42 | 0.19 | 10.69 | 0.11 | 1.90 | 0.06 | 0.15 | |
| 90 | 31.62 | 14.59 | 3.48 | 0.51 | 19.13 | 1.10 | 13.88 | 0.20 | 10.99 | 0.13 | 1.83 | 0.05 | 0.14 | |
| 120 | 28.01 | 14.10 | 2.66 | 0.42 | 22.92 | 1.12 | 12.65 | 0.28 | 12.07 | 0.27 | 1.90 | 0.05 | 0.17 | |
| 150 | 25.83 | 13.95 | 2.51 | 0.37 | 26.65 | 1.10 | 10.00 | 0.33 | 12.10 | 0.35 | 2.25 | 0.06 | 0.21 | |
| NaHCO3 Stress | 30 | 47.58 | 15.51 | 5.72 | 0.86 | 0.82 | 0.34 | 10.00 | 0.33 | 12.80 | 0.14 | 5.27 | 0.07 | 0.18 |
| 60 | 40.92 | 18.14 | 6.39 | 0.62 | 0.80 | 0.23 | 9.60 | 0.38 | 15.18 | 0.50 | 5.47 | 0.07 | 0.22 | |
| 90 | 34.21 | 21.03 | 6.80 | 0.55 | 0.80 | 0.20 | 6.85 | 0.46 | 19.47 | 0.64 | 7.16 | 0.07 | 0.31 | |
| 120 | 23.11 | 27.14 | 7.24 | 0.48 | 0.90 | 0.41 | 5.93 | 0.59 | 24.38 | 0.96 | 7.27 | 0.08 | 0.44 | |
| 150 | 21.54 | 28.49 | 5.45 | 0.52 | 1.02 | 0.58 | 6.84 | 0.68 | 24.18 | 0.86 | 6.72 | 0.09 | 0.52 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Yu, L.; Hou, Y.; Zhang, Y.; Guo, W.; Xue, Y. Contrasting Effects of NaCl and NaHCO3 Stresses on Seed Germination, Seedling Growth, Photosynthesis, and Osmoregulators of the Common Bean (Phaseolus vulgaris L.). Agronomy 2019, 9, 409. https://doi.org/10.3390/agronomy9080409
Yu S, Yu L, Hou Y, Zhang Y, Guo W, Xue Y. Contrasting Effects of NaCl and NaHCO3 Stresses on Seed Germination, Seedling Growth, Photosynthesis, and Osmoregulators of the Common Bean (Phaseolus vulgaris L.). Agronomy. 2019; 9(8):409. https://doi.org/10.3390/agronomy9080409
Chicago/Turabian StyleYu, Song, Lihe Yu, Yulong Hou, Yifei Zhang, Wei Guo, and Yingwen Xue. 2019. "Contrasting Effects of NaCl and NaHCO3 Stresses on Seed Germination, Seedling Growth, Photosynthesis, and Osmoregulators of the Common Bean (Phaseolus vulgaris L.)" Agronomy 9, no. 8: 409. https://doi.org/10.3390/agronomy9080409
APA StyleYu, S., Yu, L., Hou, Y., Zhang, Y., Guo, W., & Xue, Y. (2019). Contrasting Effects of NaCl and NaHCO3 Stresses on Seed Germination, Seedling Growth, Photosynthesis, and Osmoregulators of the Common Bean (Phaseolus vulgaris L.). Agronomy, 9(8), 409. https://doi.org/10.3390/agronomy9080409




