Effect of Traditional Cultivation Management on CO2 Flux in the Dry Tropical Cropland of South India
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Study Sites
2.2. Experimental Design
- Traditional cultivation management plot (sorghum seeds were broadcast and thinned); hereafter referred as the traditional cultivation management ‘(T) plot’;
- Fixed density plot (sorghum seeds sowed at fixed density (distance between plants was 30 cm) and not thinned); hereafter referred as the fixed density ‘(FD) plot’;
- No thinning plot (sorghum seeds were broadcast but not thinned); hereafter referred as the no thinning ‘(NT) plot’;
- No cultivation plot; hereafter referred as the bare ‘(B) plot’.
2.3. Measurement of Environmental Factors
2.4. Measurement of Heterotrophic Soil CO2 Efflux Rate
2.5. Measurement of Sorghum Root-C as C Input
2.6. Data Analyses
3. Results
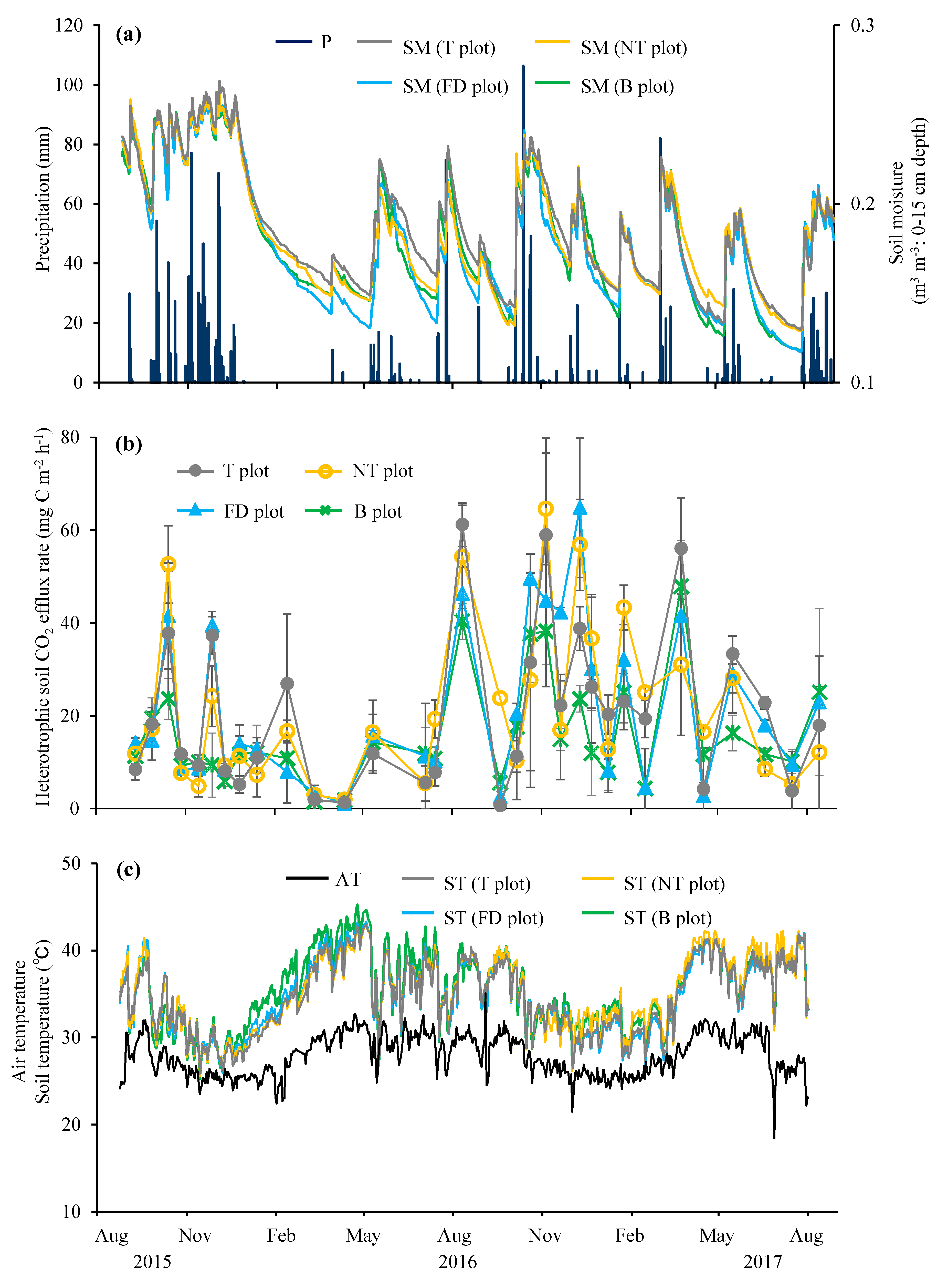
3.1. Environmental Factors
3.2. Seasonal Fluctuation of Heterotrophic Soil CO2 Efflux Rate
3.3. Estimation of Annual CO2 Flux
4. Discussion
4.1. Effect of Cultivation Management on the CO2 Flux
4.2. Effect of Traditional Cultivation Management on The Balance of C Output and Input
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Loveland, P.; Webb, J. Is there a critical level of organic matter in the agricultural soils of temperate regions: A review. Soil Till. Res. 2003, 70, 1–18. [Google Scholar] [CrossRef]
- Raich, J.W.; Schlesinger, W.H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 1992, 44, 81–99. [Google Scholar] [CrossRef] [Green Version]
- Powlson, D.S.; Stirling, C.M.; Thierfelder, C.; White, R.P.; Jat, M.L. Does conservation agriculture deliver climate change mitigation through soil carbon sequestration in tropical agro-ecosystems? Agric. Ecosyst. Environ. 2016, 220, 164–174. [Google Scholar] [CrossRef]
- Wani, S.P.; Pathak, P.; Jangawad, L.S.; Eswaran, H.; Singh, P. Improved management of Vertisols in the semiarid tropics for increased productivity and soil carbon sequestration. Soil Use Manag. 2003, 19, 217–222. [Google Scholar] [CrossRef]
- Ghosh, A.; Bhattacharyya, R.; Dwivedi, B.S.; Meena, M.C.; Agarwal, B.K.; Mahapatra, P.; Shahi, D.K.; Salwani, R.; Agnihorti, R. Temperature sensitivity of soil organic carbon decomposition as affected by long-term fertilization under a soybean based cropping system in a sub-tropical Alfisol. Agric. Ecosyst. Environ. 2016, 233, 202–213. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Venkateswarlu, B.; Lal, R.; Singh, A.K.; Kundu, S. Sustainable Management of Soils of Dryland Ecosystems of India for Enhancing Agronomic Productivity and Sequestering Carbon. Adv. Agron. 2013, 121, 253–329. [Google Scholar]
- Srinivasarao, C.; Vittal, K.P.R.; Venkateswarlu, B.; Wani, S.P.; Sahrawat, K.L.; Subramanian, M.; Kundu, S. Carbon Stocks in Different Soil Types under Diverse Rainfed Production Systems in Tropical India. Commun. Soil Sci. Plan. 2009, 40, 2338–2356. [Google Scholar] [CrossRef]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Venkateswarlu, B.; Lal, R.; Singh, A.K.; Kundu, S.; Vittal, K.P.R.; Patel, J.J.; Patel, M.M. Long-term manuring and fertilizer effects on depletion of soil organic carbon stocks under pearl millet-cluster bean-castor rotation in Western India. Land Degrad. Dev. 2014, 25, 173–183. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Ghosh, B.N.; Mishra, P.K.; Mandal, B.; Srinivasarao, C.; Sarkar, D.; Das, K.; Anil, K.S.; Lalitha, M.; Hati, K.M.; et al. Soil degradation in India: Challenges and potential solutions. Sustainability 2015, 7, 3528–3570. [Google Scholar] [CrossRef]
- Das, S.; Ganguly, D.; Ray, R.; Jana, T.K.; De, T.K. Microbial activity determining soil CO2 emission in the Sundarban mangrove forest, India. Trop. Ecol. 2017, 58, 525–537. [Google Scholar]
- Devi, N.B.; Yadava, P.S. Seasonal dynamics in soil microbial biomass C, N and P in a mixes-oak forest ecosystem of Manipur, North-east India. Appl. Soil Ecol. 2006, 31, 220–227. [Google Scholar] [CrossRef]
- Mohanty, R.B.; Panda, T. Soil respiration and microbial population in a tropical deciduous forest soil of Orissa, India. Flora 2011, 206, 1040–1044. [Google Scholar] [CrossRef]
- Gupta, S.R.; Singh, J.S. Soil respiration in a tropical grassland. Soil Biol. Biochem. 1981, 13, 261–268. [Google Scholar] [CrossRef]
- Thokchom, A.; Yadava, P.S. Carbon dynamics in an Imperata grassland in Northeast India. Trop. Grassl. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Grace, J.; Jose, J.S.; Meir, P.; Miranda, H.S.; Montes, R.A. Productivity and carbon fluxes of tropical savannas. J. Biogeogr. 2006, 33, 387–400. [Google Scholar] [CrossRef]
- Sahu, C.; Basti, S.; Sahu, S.K. Carbon dioxide evolution and enzymatic activities of soil under different land use practices located near Bhawanipatna town in Odisha, India. Fresen. Environ. Bull. 2016, 25, 5432–5439. [Google Scholar]
- Sandeep, S.; Manjaiah, K.M.; Mayadevi, M.R.; Singh, A.K. Monitoring temperature sensitivity of soil organic carbon decomposition under maize–wheat cropping systems in semi-arid India. Environ. Monit. Assess. 2016, 188, 451. [Google Scholar] [CrossRef]
- Fatichi, S.; Ivanov, V.Y.; Caporali, E. Investigating Interannual Variability of Precipitation at the Global scale: Is There a Connection with Seasonality? J. Clim. 2012, 25, 5512–5523. [Google Scholar] [CrossRef]
- Hu, C.; Zheng, C.; Sadras, V.O.; Ding, M.; Yang, X.; Zhang, S. Effect of straw mulch and seeding rate on the harvest index, yield and water use efficiency of winter wheat. Sci. Rep. 2018, 8, 8167. [Google Scholar] [CrossRef] [Green Version]
- Kuhling, I.; Redozubov, D.; Broll, G.; Trautz, D. Impact of tillage, seeding rate and seeding depth on soil moisture and dryland spring wheat yield in Western Siberia. Soil Till. Res. 2017, 170, 43–52. [Google Scholar] [CrossRef]
- Gong, J.; Wang, Y.; Liu, M.; Huang, Y.; Yan, X.; Zhang, Z.; Zhang, W. Effects of land use on soil respiration in the temperate steppe of Inner Mongolia, China. Soil Till. Res. 2014, 144, 20–31. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Z.; Wang, C.H.; Jiang, F.; Xia, J. Effects of mowing and nitrogen addition on soil respiration in three patches in an oldfield grassland in Inner Mongolia. J. Plant Ecol. 2012, 5, 219–228. [Google Scholar] [CrossRef]
- Soil Survey Staff. Key to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Department of Evaluation and Applied Research. Tamil Nadu—An Economical Appraisal 2011–2012 to 2013–2014. Available online: http://www.tn.gov.in/dear/ (accessed on 19 March 2019).
- Shinjo, H.; Kato, A.; Fujii, K.; Mori, K.; Funakawa, S.; Kosaki, T. Carbon dioxide emission derived from soil organic matter decomposition and root respiration in Japanese forests under different ecological conditions. Soil Sci. Plant Nutr. 2006, 52, 233–242. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Chang. Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef]
- Sugihara, S.; Funakawa, S.; Kilasara, M.; Kosaki, T. Effect of land management on CO2 flux and soil C stock in two Tanzanian croplands with contrasting soil texture. Soil Biol. Biochem. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Funakawa, S.; Nakamura, I.; Akshalov, K.; Kosaki, T. Soil organic matter dynamics under grain farming in Northern Kazakhstan. Soil Sci. Plant Nutr. 2004, 50, 1211–1218. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Deshpande, A.N.; Venkateswarlu, B.; Lal, R.; Singh, A.K.; Kundu, S.; Vittal, K.P.R.; Mishra, P.K.; Prasad, J.V.N.S.; Mandal, U.K.; et al. Grain yield and carbon sequestration potential of post monsoon sorghum cultivation in Vertisols in the semi arid tropics of central India. Geoderma 2012, 175–176, 90–97. [Google Scholar] [CrossRef]
- Shrinivasarao, C.; Venkateswarlu, B.; Lal, R.; Singh, A.K.; Kundu, S.; Vittal, K.P.R.; Balaguravaiah, G.; Malayanur, V.S.B.; Chary, G.R.; Prasadbabu, M.B.B.; et al. Soil carbon sequestration and agronomic productivity of an Alfisol for a groundnut-based system in a semiarid environment in southern India. Eur. J. Agron. 2012, 43, 40–48. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Bian, C.; Changjian, M.; Lang, K.; Han, H.; Li, Q. Response of Soil CO2 Emission and Summer Maize Yield to Plant Density and Straw Mulching in the North China Plain. Sci. World J. 2014, 2014, 180219. [Google Scholar]
- Sugihara, S.; Funakawa, S.; Ikazaki, K.; Shinjo, H.; Kosaki, T. Rewetting of Dry Soil did not Stimulate the Carbon and Nitrogen Mineralization in Croplands with Plant Residue Removed in the Sahel, West Africa. Trop. Agric. Dev. 2014, 58, 8–17. [Google Scholar]
- Mapanda, F.; Mupini, J.; Wuta, M.; Nyamangara, J.; Rees, R.M. A cross-ecosystem assessment of the effects of land cover and land use on soil emission of selected greenhouse gases and related soil properties in Zimbabwe. Eur. J. Soil Sci. 2010, 61, 721–733. [Google Scholar] [CrossRef]
- La Scala, N., Jr.; Marques, J., Jr.; Pereira, G.T.; Cora, J.E. Carbon dioxide emission related to chemical properties of a tropical B soil. Soil Biol. Biochem. 2000, 32, 1469–1473. [Google Scholar] [CrossRef]
- Ritchie, J.T. Model for Predicting Evaporation from a Row Crop with Incomplete Cover. Water Resour. Res. 1972, 8, 1204–1213. [Google Scholar] [CrossRef]
- Pal, D.K.; Wani, S.P.; Sahrawat, K.L. Vertisols of tropical Indian environments: Pedology and edaphology. Geoderma 2012, 189–190, 28–49. [Google Scholar] [CrossRef]
- Fu, W.; Huang, M.; Shao, M.A.; Horton, R. Soil carbon dioxide (CO2) efflux of two shrubs in response to plant density in the northern Loess Plateau of China. Afr. J. Biotechnol. 2010, 9, 6916–6926. [Google Scholar]
- Adachi, M.; Bekku, Y.S.; Rashidah, W.; Okuda, T.; Koizumi, H. Differences in soil respiration between different tropical ecosystems. Appl. Soil Ecol. 2006, 34, 258–265. [Google Scholar] [CrossRef]
- Linn, D.M.; Doran, J.W. Effect of Water-Filled Pore Space on Carbon Dioxide and Nitrous Oxide Production in Tilled and Nontilled Soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- Liang, G.; Houssou, A.; Wu, H.; Cai, D.; Wu, X.; Gao, L.; Li, J.; Wang, B.; Li, S. Seasonal Patterns of Soil Respiration and Related Soil Biochemical Properties under Nitrogen Addition in Winter Wheat Field. PLoS ONE 2015, 10, e0144115. [Google Scholar] [CrossRef]
- Sugihara, S.; Funakawa, S.; Kadono, A.; Tanaka, Y.; Sawada, K.; Fujii, K.; Kosaki, T. In situ short-term dynamics of CO2 flux and microbial biomass after simulated rainfall in dry croplands in four tropical and continental ecosystems. Soil Sci. Plant Nutr. 2015, 61, 392–403. [Google Scholar] [CrossRef]
- Domanski, G.; Kuzyakov, Y.; Siniakina, S.V.; Stahr, K. Carbon flows in the rhizosphere of ryegrass (Lolium perenne). J. Plant Nutr. Soil Sci. 2001, 164, 381–387. [Google Scholar]
- Manna, M.C.; Subba Rao, A.; Mandal, A. Impact of agricultural land management practices on soil carbon sequestration. Indian J. Soil Conserv. 2015, 43, 204–212. [Google Scholar]
- Pathak, H.; Byjesh, K.; Chakrabarti, B.; Aggarwal, P.K. Potential and cost of carbon sequestration in Indian agriculture: Estimates from long-term field experiments. Field Crop. Res. 2011, 120, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S.; Singh, M.; Saha, J.K.; Biswas, A.; Tripathi, A.K.; Acharya, C.L. Relationship between C addition and storage in a Vertisol under soybean-wheat cropping system in sub-tropical central India. J. Plant Nutr. Soil Sci. 2001, 164, 483–486. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration in India. Clim. Chang. 2004, 65, 277–296. [Google Scholar] [CrossRef]
| Year | Month | Crop Cultivation | Land Management |
|---|---|---|---|
| 2015 | End of August | Farmyard manure application (1.1 Mg C ha−1) | |
| Mid October | Seeding | Chemical fertilizer application (40 kg N; 20 kg P; 0 kg K ha−1) | |
| Mid November | Thinning | ||
| 2016 | End of January | Harvesting | |
| Early October | Seeding | Chemical fertilizer application (40 kg N; 20 kg P; 0 kg K ha−1) | |
| Early November | Thinning | ||
| 2017 | End of January | Harvesting |
| September 2015–August 2016 | September 2016–August 2017 | |||
|---|---|---|---|---|
| Soil Moisture | CO2 Flux | Soil Moisture | CO2 Flux | |
| F Value | F Value | F Value | F Value | |
| Broadcasting | 197.7 ** | 0.2 | 3.8 | 0.0 |
| Sampling time | 14.1 ** | 17.5 ** | 13.5 ** | 3.0 ** |
| Broadcasting × Sampling time | 0.3 | 0.7 | 0.4 | 0.5 |
| Thinning | 95.4 ** | 0.1 | 3.6 | 0.0 |
| Sampling time | 14.6 ** | 22.0 ** | 10.8 ** | 4.3 ** |
| Thinning × Sampling time | 0.1 | 1.1 | 0.3 | 1.2 |
| Cultivation | 82.0 ** | 5.4 * | 93.5 ** | 3.7 |
| Sampling time | 32.4 ** | 20.3 ** | 18.0 ** | 4.2 ** |
| Cultivation × Sampling time | 0.2 | 3.6 ** | 0.6 | 0.5 |
| Plot | September 2015–August 2016 | September 2016–August 2017 | For Two Years | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CO2 Flux | Factor | R | p Value | CO2 Flux | Factor | R | p Value | CO2 Flux | |
| T | 0.8 | SM | 0.53 | 0.04 | 1.7 | SM | 0.63 | 0.01 | 2.6 |
| FD | 0.9 | SM | 0.53 | 0.04 | 1.8 | SM | 0.70 | 0.00 | 2.7 |
| NT | — | SM | 0.42 | 0.11 | 1.7 | SM | 0.54 | 0.03 | — |
| B | 0.8 | ST | 0.56 | 0.03 | 1.4 | SM | 0.66 | 0.01 | 2.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seki, M.; Sugihara, S.; Miyazaki, H.; Araki, R.; Jegadeesan, M.; Ishiyama, S.; Tanaka, U.; Tanaka, H. Effect of Traditional Cultivation Management on CO2 Flux in the Dry Tropical Cropland of South India. Agronomy 2019, 9, 347. https://doi.org/10.3390/agronomy9070347
Seki M, Sugihara S, Miyazaki H, Araki R, Jegadeesan M, Ishiyama S, Tanaka U, Tanaka H. Effect of Traditional Cultivation Management on CO2 Flux in the Dry Tropical Cropland of South India. Agronomy. 2019; 9(7):347. https://doi.org/10.3390/agronomy9070347
Chicago/Turabian StyleSeki, Mayuko, Soh Sugihara, Hidetoshi Miyazaki, Ryoichi Araki, Muniandi Jegadeesan, Shun Ishiyama, Ueru Tanaka, and Haruo Tanaka. 2019. "Effect of Traditional Cultivation Management on CO2 Flux in the Dry Tropical Cropland of South India" Agronomy 9, no. 7: 347. https://doi.org/10.3390/agronomy9070347
APA StyleSeki, M., Sugihara, S., Miyazaki, H., Araki, R., Jegadeesan, M., Ishiyama, S., Tanaka, U., & Tanaka, H. (2019). Effect of Traditional Cultivation Management on CO2 Flux in the Dry Tropical Cropland of South India. Agronomy, 9(7), 347. https://doi.org/10.3390/agronomy9070347





