Effect of Senescence Phenotypes and Nitrate Availability on Wheat Leaf Metabolome during Grain Filling
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Growth Conditions
2.2. Determination of Physiological Parameters
2.3. Determination of Chl Contents
2.4. Metabolite Extraction
2.5. Determination of Ion Contents
2.6. Determination of Amino Acid Contents
2.7. Profiling of Primary Metabolism
2.8. Statistical Analysis
3. Results
3.1. Difference in Chlorophyll Content in the Leaves of Wheat under Different Nitrogen Conditions
3.2. The Effect of Nitrogen Supply on Grain and Straw Yield, and %N in Grain and %N in the Straw
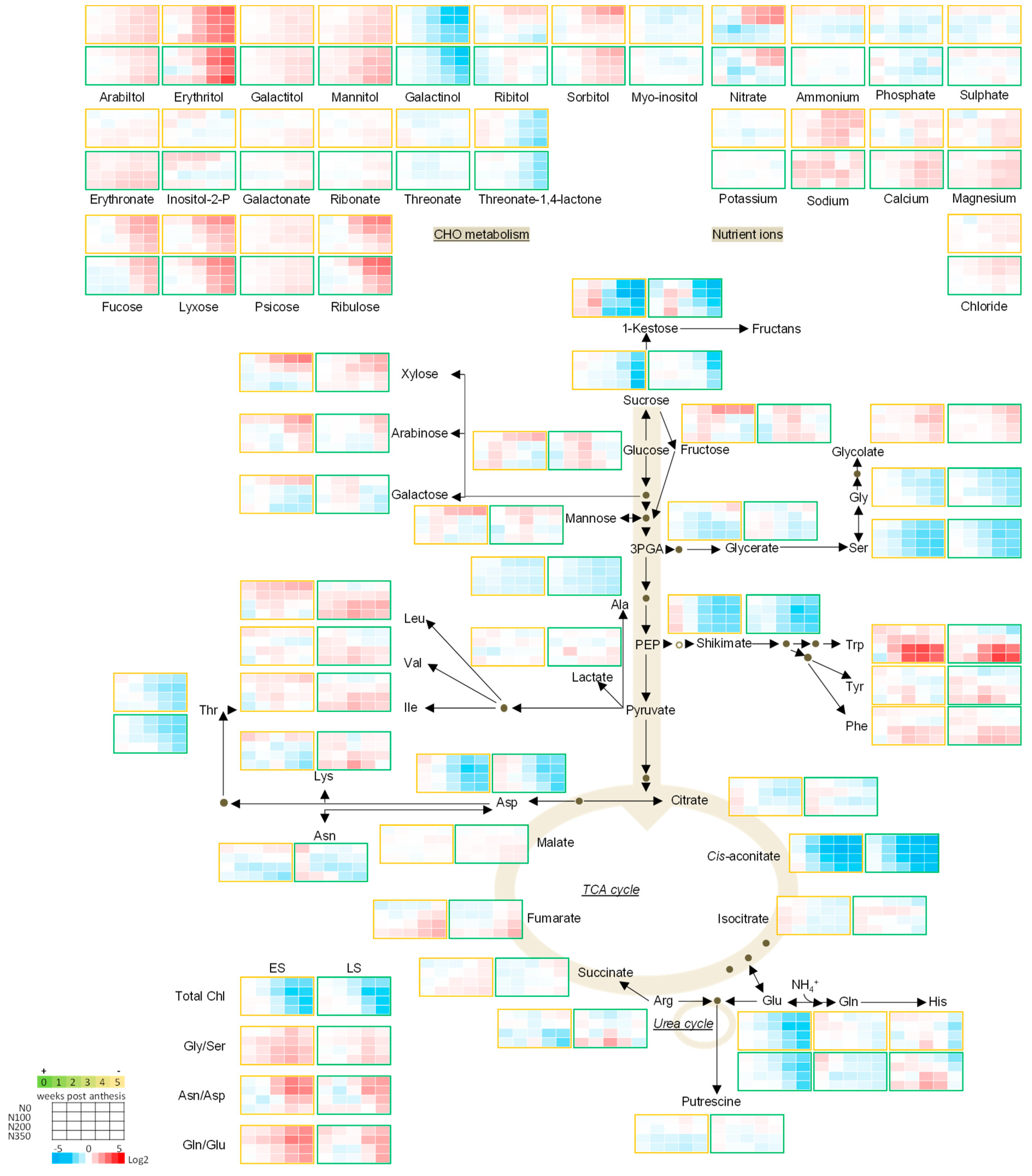
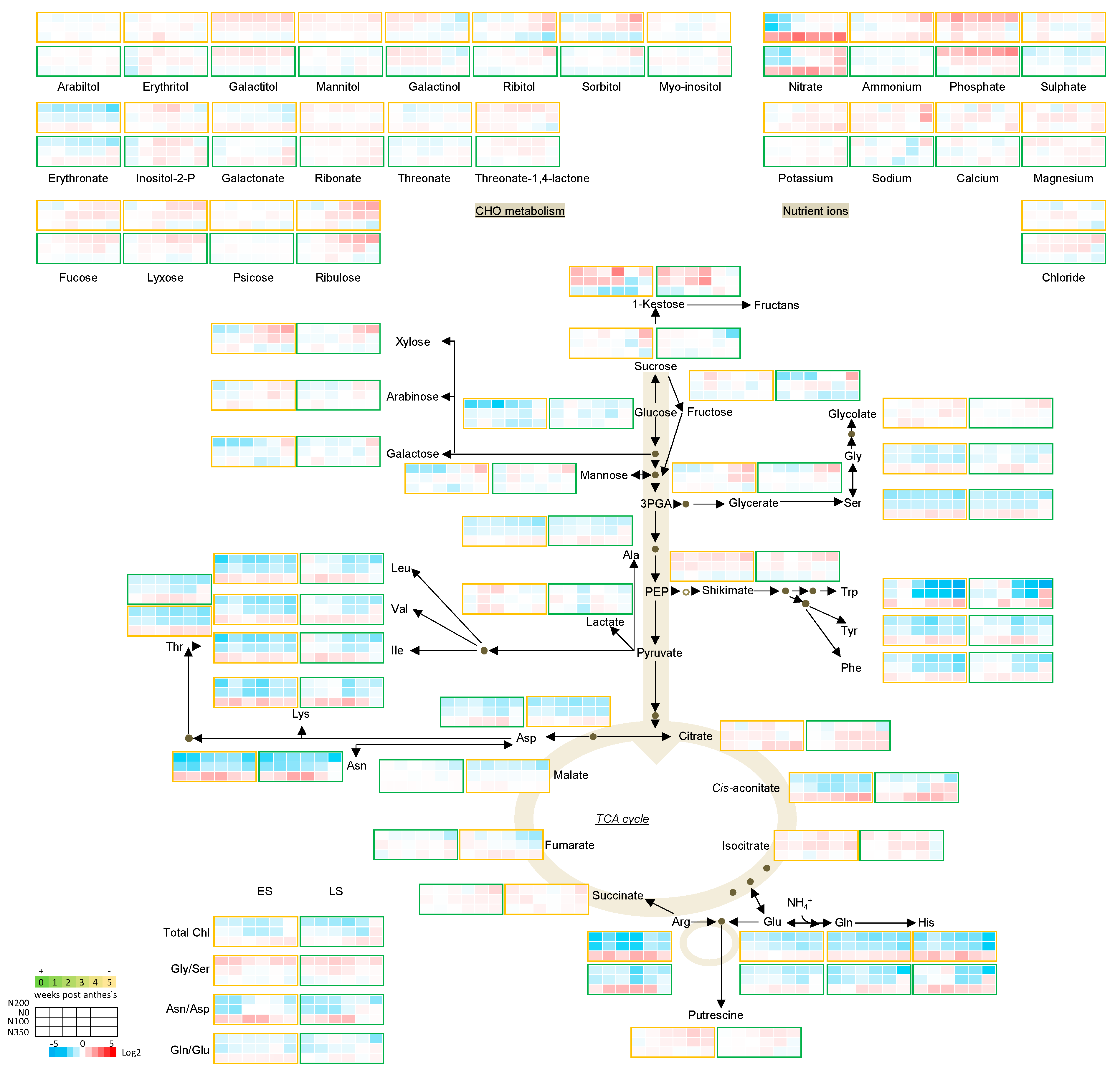
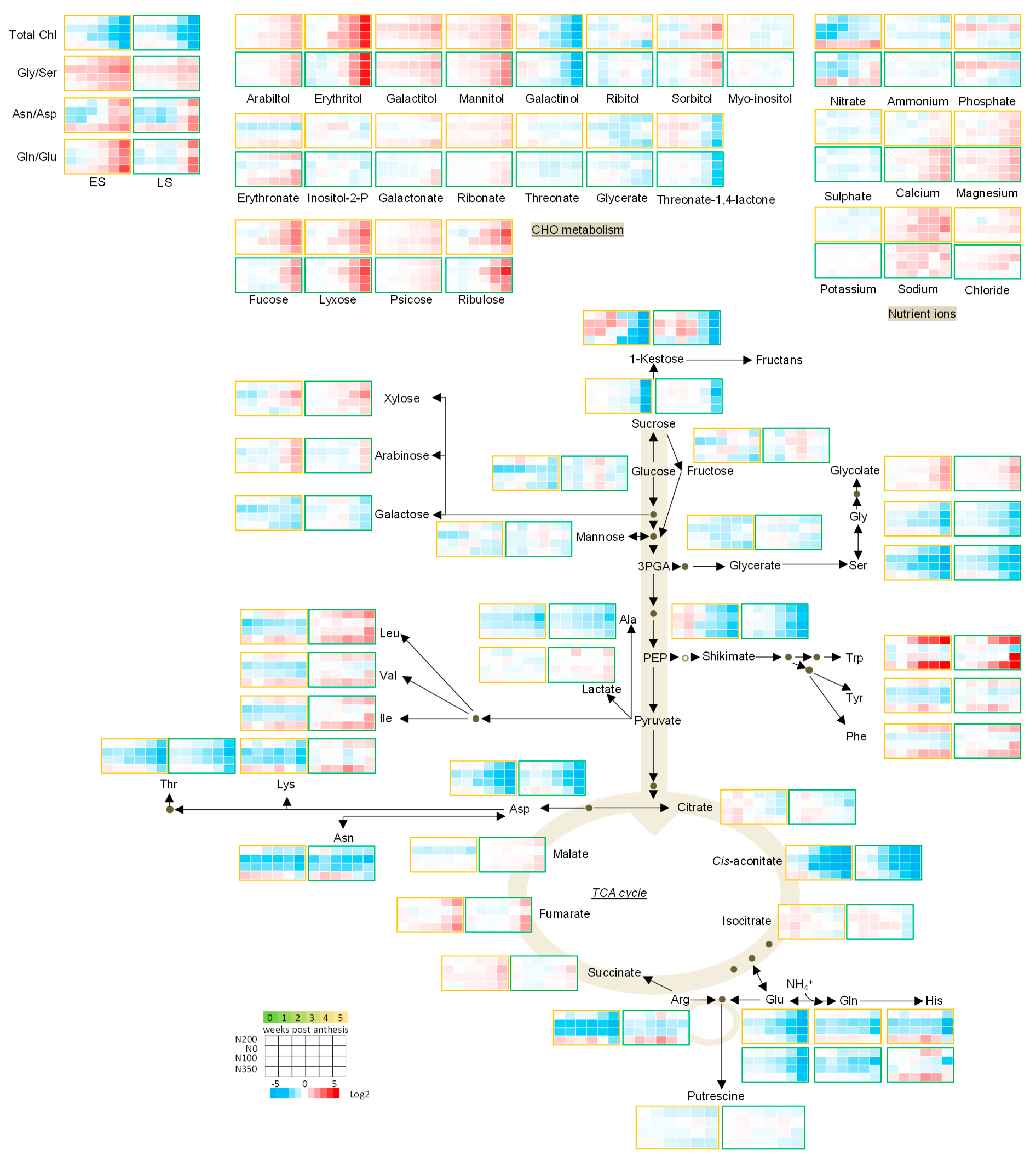
3.3. The Metabolic Profile of Leaves during Post Anthesis Senescence
3.4. Senescence Related Changes in Metabolite Levels in Wheat during Grain Filling
3.4.1. Nutrient Ions
3.4.2. Amino Acids
3.4.3. Sugars
3.4.4. The TCA Cycle Metabolites
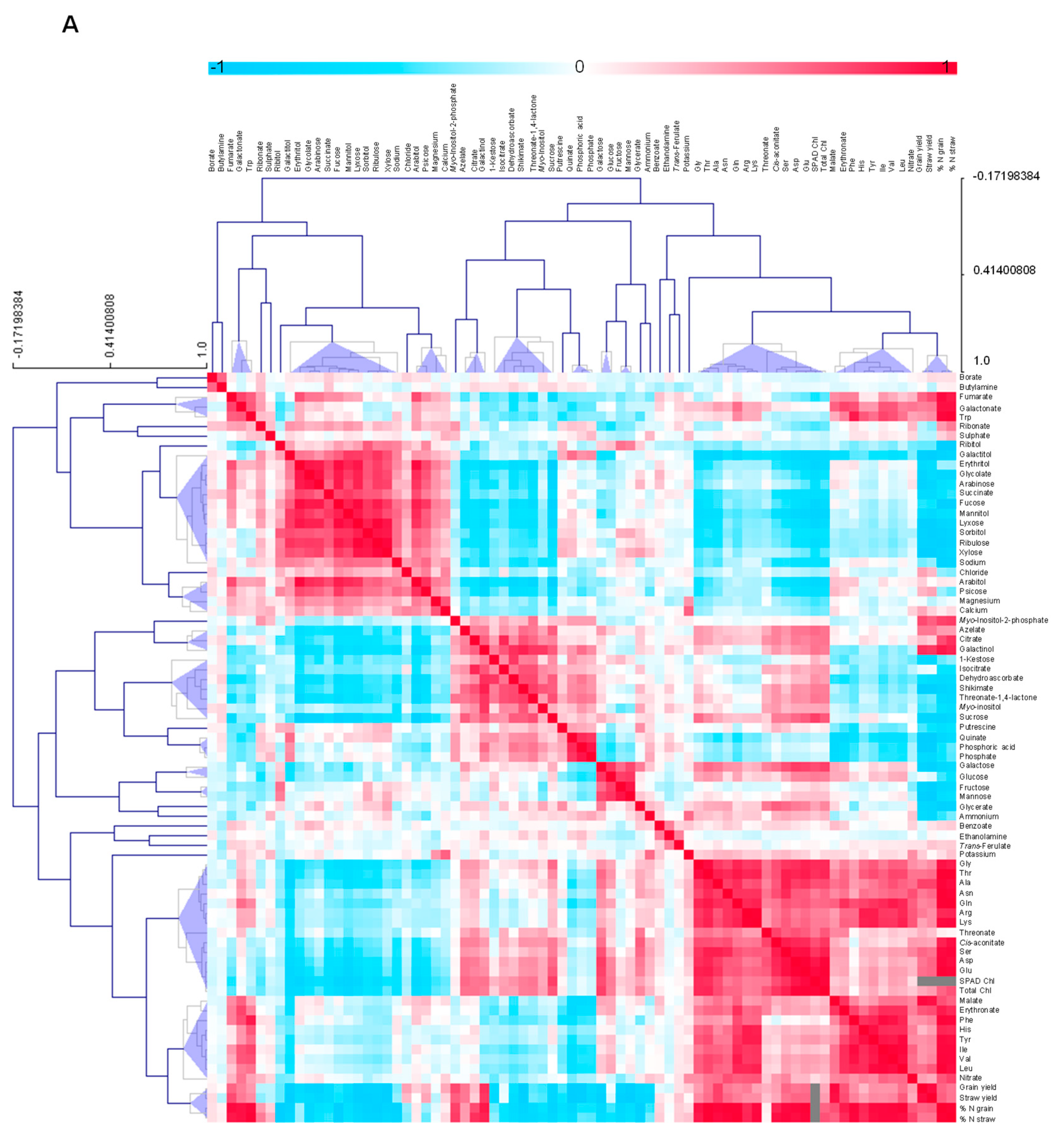
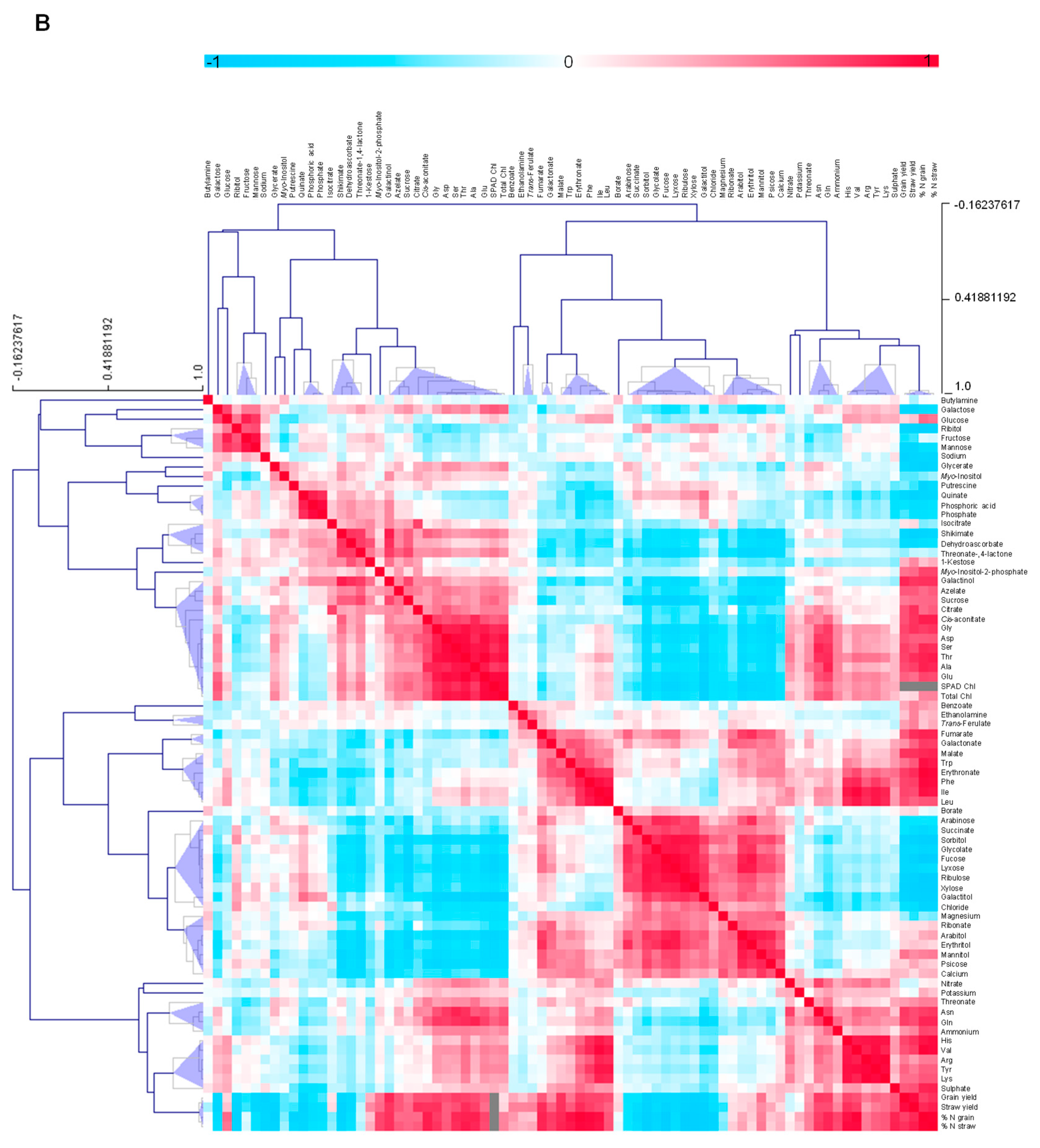
3.4.5. Metabolite-Metabolite Correlation Analysis with Reference to Yield Characteristics
4. Discussion
4.1. Metabolite Alterations Following Anthesis
4.2. Metabolic Alterations in Response to Differential N-Supply
4.3. N-Supply and the Responsiveness of the Genotype Determined the Degree of Senescence
4.3.1. Sugars and Amino Acids Distinguish the Timing of Senescence
4.3.2. The Influence of Senescence and N-Supply on Crop Yield and Plant Biomass Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hawkesford, M.J. Reducing the reliance on nitrogen fertilizer for wheat production. J. Cereal Sci. 2014, 59, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Makino, A. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiol. 2011, 155, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Gaju, O.; DeSilva, J.; Carvalho, P.; Hawkesford, M.J.; Griffiths, S.; Greenland, A.; Foulkes, M.J. Leaf photosynthesis and associations with grain yield, biomass and nitrogen-use efficiency in landraces, synthetic-derived lines and cultivars in wheat. Field Crop. Res. 2016, 193, 1–15. [Google Scholar] [CrossRef]
- Richards, R.A. Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 2000, 51, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Mayes, S.; Sparkes, D.L. Carpel size, grain filling, and morphology determine individual grain weight in wheat. J. Exp. Bot. 2015, 66, 6715–6730. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Sibley, A.; Ivan Ortiz-Monasterio, J. Extreme heat effects on wheat senescence in india. Nat. Clim. Chang. 2012, 2, 186–189. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A nac gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Maillard, A.; Diquélou, S.; Billard, V.; Laîné, P.; Garnica, M.; Prudent, M.; Garcia-Mina, J.-M.; Yvin, J.-C.; Ourry, A. Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 2015, 6, 317. [Google Scholar] [CrossRef]
- Balazadeh, S.; Schildhauer, J.; Araújo, W.L.; Munné-Bosch, S.; Fernie, A.R.; Proost, S.; Humbeck, K.; Mueller-Roeber, B. Reversal of senescence by n resupply to n-starved arabidopsis thaliana: Transcriptomic and metabolomic consequences. J. Exp. Bot. 2014, 65, 3975–3992. [Google Scholar] [CrossRef]
- Wuest, S.E.; Philipp, M.A.; Guthörl, D.; Schmid, B.; Grossniklaus, U. Seed production affects maternal growth and senescence in arabidopsis. Plant Physiol. 2016, 171, 392–404. [Google Scholar] [CrossRef]
- Borrill, P.; Fahy, B.; Smith, A.M.; Uauy, C. Wheat grain filling is limited by grain filling capacity rather than the duration of flag leaf photosynthesis: A case study using nam rnai plants. PLoS ONE 2015, 10, e0134947. [Google Scholar] [CrossRef] [PubMed]
- Schippers, J.H.M.; Schmidt, R.; Wagstaff, C.; Jing, H.-C. Living to die and dying to live: The survival strategy behind leaf senescence. Plant Physiol. 2015, 169, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Bieker, S.; Zentgraf, U. Plant Senescence and Nitrogen Mobilization and Signaling; IntechOpen: London, UK, 2013. [Google Scholar]
- Distelfeld, A.; Avni, R.; Fischer, A.M. Senescence, nutrient remobilization, and yield in wheat and barley. J. Exp. Bot. 2014, 65, 3783–3798. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, K.S.V.; Kavi Kishor, P.B.; Bahuguna, R.N.; von Wirén, N.; Sreenivasulu, N. Staying alive or going to die during terminal senescence-an enigma surrounding yield stability. Front. Plant Sci. 2015, 6, 1070. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Kuai, B.-K.; Jia, J.-Z.; Jing, H.-C. Regulation of leaf senescence and crop genetic improvementf. J. Integr. Plant Biol. 2012, 54, 936–952. [Google Scholar] [CrossRef]
- Gregersen, P.L.; Holm, P.B.; Krupinska, K. Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol. 2008, 10, 37–49. [Google Scholar] [CrossRef]
- Araújo, W.L.; Ishizaki, K.; Nunes-Nesi, A.; Tohge, T.; Larson, T.R.; Krahnert, I.; Balbo, I.; Witt, S.; Dörmann, P.; Graham, I.A.; et al. Analysis of a range of catabolic mutants provides evidence that phytanoyl-coenzyme a does not act as a substrate of the electron-transfer flavoprotein/electron-transfer flavoprotein:Ubiquinone oxidoreductase complex in arabidopsis during dark-induced senescence. Plant Physiol. 2011, 157, 55–69. [Google Scholar]
- Yoshimoto, K.; Avila-Ospina, L.; Moison, M.; Masclaux-Daubresse, C. Autophagy, plant senescence, and nutrient recycling. J. Exp. Bot. 2014, 65, 3799–3811. [Google Scholar]
- Araújo, W.L.; Ishizaki, K.; Nunes-Nesi, A.; Larson, T.R.; Tohge, T.; Krahnert, I.; Witt, S.; Obata, T.; Schauer, N.; Graham, I.A.; et al. Identification of the 2-hydroxyglutarate and isovaleryl-coa dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of arabidopsis mitochondria. Plant Cell 2010, 22, 1549–1563. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J.; Hölzer, R.; Feller, U. Influence of nitrogen deficiency on senescence and the amounts of rna and proteins in wheat leaves. Physiol. Plantarum 1998, 102, 192–200. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J.; Klein, R.R.; Klein, P.; Hölzer, R.; Feller, U. Coordination of protein and mrna abundances of stromal enzymes and mrna abundances of the clp protease subunits during senescence of phaseolus vulgaris (l.) leaves. Planta 1996, 200, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Soltabayeva, A.; Srivastava, S.; Kurmanbayeva, A.; Bekturova, A.; Fluhr, R.; Sagi, M. Early senescence in older leaves of low nitrate-grown ATXDH1 uncovers a role for purine catabolism in n supply. Plant Physiol. 2018, 178, 1027–1044. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.K.; Medina-Escobar, N.; Zulawski, M.; Sparkes, I.A.; Cao, F.-Q.; Witte, C.-P. The ureide-degrading reactions of purine ring catabolism employ three amidohydrolases and one aminohydrolase in arabidopsis, soybean, and rice. Plant Physiol. 2013, 163, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Han, B. Natural variations and genome-wide association studies in crop plants. Ann. Rev. Plant Biol. 2014, 65, 531–551. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wingen, L.; Orford, S.; Fenwick, P.; Wang, J.; Griffiths, S. Using the uk reference population avalon × cadenza as a platform to compare breeding strategies in elite western european bread wheat. Mol. Breed. 2015, 35, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Derkx, A.P.; Liu, D.C.; Buchner, P.; Hawkesford, M.J. Overexpression of a nac transcription factor delays leaf senescence and increases grain nitrogen concentration in wheat. Plant Biol. 2015, 17, 904–913. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Erban, A.; Schauer, N.; Fernie, A.; Kopka, J. Nonsupervised construction and application of mass spectral and retention time index libraries from time-of-flight gas chromatography-mass spectrometry metabolite profiles. In Metabolomics; Weckwerth, W., Ed.; Humana Press: New York, NY, USA, 2007; Volume 358, pp. 19–38. [Google Scholar]
- Watanabe, M.; Balazadeh, S.; Tohge, T.; Erban, A.; Giavalisco, P.; Kopka, J.; Mueller-Roeber, B.; Fernie, A.R.; Hoefgen, R. Comprehensive dissection of spatiotemporal metabolic shifts in primary, secondary, and lipid metabolism during developmental senescence in arabidopsis. Plant Physiol. 2013, 162, 1290–1310. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protocols 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Luedemann, A.; Strassburg, K.; Erban, A.; Kopka, J. Tagfinder for the quantitative analysis of gas chromatography—mass spectrometry (gc-ms)-based metabolite profiling experiments. Bioinformatics 2008, 24, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Kopka, J.; Schauer, N.; Krueger, S.; Birkemeyer, C.; Usadel, B.; Bergmüller, E.; Dörmann, P.; Weckwerth, W.; Gibon, Y.; Stitt, M.; et al. Gmd@csb.Db: The golm metabolome database. Bioinformatics 2005, 21, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Stacklies, W.; Redestig, H.; Scholz, M.; Walther, D.; Selbig, J. Pcamethods—a bioconductor package providing pca methods for incomplete data. Bioinformatics 2007, 23, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Parry, M.; Noctor, G. Markers and signals associated with nitrogen assimilation in higher plants. J. Exp. Bot. 2003, 54, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.W. Carbon and nitrogen assimilation in relation to yield: Mechanisms are the key to understanding production systems. J. Exp. Bot. 2002, 53, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Kojima, M.; Gepstein, A.; Sakakibara, H.; Mittler, R.; Gepstein, S.; Blumwald, E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc. Nat. Acad. Sci. USA 2007, 104, 19631–19636. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and heat-stress effects on seed filling in food crops: Impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Guo, X.; Pan, X.; Li, Z. Chlorophyll composition, chlorophyll fluorescence, and grain yield change in esl mutant rice. Int. J. Mol. Sci. 2018, 19, 2945. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, J.; Ren, T.; Hussain, S.; Guo, C.; Wang, S.; Cong, R.; Li, X. Effects of nitrogen and tiller type on grain yield and physiological responses in rice. AoB PLANTS 2017, 9, plx012. [Google Scholar] [CrossRef] [PubMed]
- Tischner, R. Nitrate uptake and reduction in higher and lower plants. Plant Cell Envir. 2000, 23, 1005–1024. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Li, X.; Zhang, F.; Liu, C.; Du, Y.; Gao, X.; Zhang, Z.; Zhang, X.; Hou, Z.; et al. Intergrative metabolomic and transcriptomic analyses unveil nutrient remobilization events in leaf senescence of tobacco. Sci. Rep. 2017, 7, 12126. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.M.; Habash, D.Z. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009, 182, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Kamachi, K.; Yamaya, T.; Mae, T.; Ojima, K. A role for glutamine synthetase in the remobilization of leaf nitrogen during natural senescence in rice leaves. Plant Physiol. 1991, 96, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Mendoza, M.; Velasco-Arroyo, B.; Santamaria, M.E.; González-Melendi, P.; Martinez, M.; Diaz, I. Plant senescence and proteolysis: Two processes with one destiny. Genet. Mol. Biol. 2016, 39, 329–338. [Google Scholar] [CrossRef]
- Araújo, W.L.; Tohge, T.; Ishizaki, K.; Leaver, C.J.; Fernie, A.R. Protein degradation—An alternative respiratory substrate for stressed plants. Trends Plant Sci. 2011, 16, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.-P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Pires, M.V.; Pereira Júnior, A.A.; Medeiros, D.B.; Daloso, D.M.; Pham, P.A.; Barros, K.A.; Engqvist, M.K.M.; Florian, A.; Krahnert, I.; Maurino, V.G.; et al. The influence of alternative pathways of respiration that utilize branched-chain amino acids following water shortage in arabidopsis. Plant Cell Environ. 2016, 39, 1304–1319. [Google Scholar] [CrossRef] [PubMed]
- Peterhansel, C.; Horst, I.; Niessen, M.; Blume, C.; Kebeish, R.; Kürkcüoglu, S.; Kreuzaler, F. Photorespiration. Arabidopsis Book 2010, 8, e0130. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, H.; Noda, T.; Shirano, Y.; Kato, T.; Hayashi, H.; Shibata, D.; Tabata, S.; Ohsumi, Y. Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an arabidopsis autophagy gene. Plant Physiol. 2002, 129, 1181–1193. [Google Scholar] [CrossRef]
- Wingler, A.; Masclaux-Daubresse, C.; Fischer, A.M. Sugars, senescence, and ageing in plants and heterotrophic organisms. J. Exp. Bot. 2009, 60, 1063–1066. [Google Scholar] [CrossRef]
- Quirino, B.F.; Noh, Y.-S.; Himelblau, E.; Amasino, R.M. Molecular aspects of leaf senescence. Trends Plant Sci. 2000, 5, 278–282. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Ann. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Maire, V.; Martre, P.; Kattge, J.; Gastal, F.; Esser, G.; Fontaine, S.; Soussana, J.-F. The coordination of leaf photosynthesis links c and n fluxes in c3 plant species. PLoS ONE 2012, 7, e38345. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Bauwe, H.; Badger, M. Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem ii by suppression of repair but not acceleration of damage processes in arabidopsis. Plant Physiol. 2007, 144, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Guiboileau, A.; Sormani, R.; Meyer, C.; Masclaux-Daubresse, C. Senescence and death of plant organs: Nutrient recycling and developmental regulation. CR Biol. 2010, 333, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.R.; Kim, H.J.; Nam, H.G.; Lim, P.O. Plant leaf senescence and death – regulation by multiple layers of control and implications for aging in general. J. Cell Sci. 2013, 126, 4823–4833. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Hubberten, H.M.; Saito, K.; Hoefgen, R. General regulatory patterns of plant mineral nutrient depletion as revealed by serat quadruple mutants disturbed in cysteine synthesis. Mol. Plant 2010, 3, 438–466. [Google Scholar] [CrossRef]
- Whitcomb, S.J.; Heyneke, E.; Aarabi, F.; Watanabe, M.; Hoefgen, R. Mineral nutrient depletion affects plant development and crop yield. In Nutrient Use Efficiency in Plants: Concepts and Approaches; Hawkesford, M.J., Kopriva, S., De Kok, L.J., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 205–228. [Google Scholar]
- Gregersen, P.; Culetic, A.; Boschian, L.; Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 2013, 82, 603–622. [Google Scholar] [CrossRef]
- Martre, P.; Dambreville, A. A model of leaf coordination to scale-up leaf expansion from the organ to the canopy. Plant Physiol. 2018, 176, 704–716. [Google Scholar] [CrossRef]
- Galili, G.; Avin-Wittenberg, T.; Angelovici, R.; Fernie, A.R. The role of photosynthesis and amino acid metabolism in the energy status during seed development. Front. Plant Sci. 2014, 5, 447. [Google Scholar] [CrossRef] [PubMed]
- Mouradov, A.; Spangenberg, G. Flavonoids: A metabolic network mediating plants adaptation to their real estate. Front. Plant Sci. 2014, 5, 620. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Timm, S.; Florian, A.; Wittmiß, M.; Jahnke, K.; Hagemann, M.; Fernie, A.R.; Bauwe, H. Serine acts as a metabolic signal for the transcriptional control of photorespiration-related genes in arabidopsis. Plant Physiol. 2013, 162, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, M.; Bräutigam, A.; Timm, S.; Florian, A.; Tohge, T.; Fernie, A.R.; Bauwe, H.; Weber, A.P.M. Photorespiration is crucial for dynamic response of photosynthetic metabolism and stomatal movement to altered Co2 availability. Mol. Plant 2017, 10, 47–61. [Google Scholar] [CrossRef]
- Abadie, C.; Boex-Fontvieille, E.R.A.; Carroll, A.J.; Tcherkez, G. In vivo stoichiometry of photorespiratory metabolism. Nature Plants 2016, 2, 15220. [Google Scholar] [CrossRef]
- Kan, C.-C.; Chung, T.-Y.; Juo, Y.-A.; Hsieh, M.-H. Glutamine rapidly induces the expression of key transcription factor genes involved in nitrogen and stress responses in rice roots. BMC Genomics 2015, 16, 1–15. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Reisdorf-Cren, M.; Orsel, M. Leaf nitrogen remobilisation for plant development and grain filling. Plant Biol. 2008, 10, 23–36. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Heidlebaugh, N.M.; Parrott, D.L.; Fischer, I.A.; McInnerney, K.; Fischer, A.M. Comparative transcriptome profiling of near-isogenic barley (hordeum vulgare) lines differing in the allelic state of a major grain protein content locus identifies genes with possible roles in leaf senescence and nitrogen reallocation. New Phytol. 2008, 177, 333–349. [Google Scholar] [CrossRef]
- Avin-Wittenberg, T.; Bajdzienko, K.; Wittenberg, G.; Alseekh, S.; Tohge, T.; Bock, R.; Giavalisco, P.; Fernie, A.R. Global analysis of the role of autophagy in cellular metabolism and energy homeostasis in arabidopsis seedlings under carbon starvation. Plant Cell 2015, 27, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Howarth, J.R.; Parmar, S.; Jones, J.; Shepherd, C.E.; Corol, D.-I.; Galster, A.M.; Hawkins, N.D.; Miller, S.J.; Baker, J.M.; Verrier, P.J.; et al. Co-ordinated expression of amino acid metabolism in response to n and s deficiency during wheat grain filling. J. Exp. Bot. 2008, 59, 3675–3689. [Google Scholar] [CrossRef] [PubMed]
- Uauy, C.; Brevis, J.C.; Dubcovsky, J. The high grain protein content gene gpc-b1 accelerates senescence and has pleiotropic effects on protein content in wheat. J. Exp. Bot. 2006, 57, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Ritsema, T.; Smeekens, S. Fructans: Beneficial for plants and humans. Curr. Opin. Plant Biol. 2003, 6, 223–230. [Google Scholar] [CrossRef]
- Li, H.; Cai, J.; Jiang, D.; Liu, F.; Dai, T.; Cao, W. Carbohydrates accumulation and remobilization in wheat plants as influenced by combined waterlogging and shading stress during grain filling. J. Agron. Crop Sci. 2013, 199, 38–48. [Google Scholar] [CrossRef]
- Fahnenstich, H.; Saigo, M.; Niessen, M.; Zanor, M.I.; Andreo, C.S.; Fernie, A.R.; Drincovich, M.F.; Flügge, U.-I.; Maurino, V.G. Alteration of organic acid metabolism in arabidopsis overexpressing the maize c4 nadp-malic enzyme causes accelerated senescence during extended darkness. Plant Physiol. 2007, 145, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Gibon, Y.; Usadel, B.; Blaesing, O.; Kamlage, B.; Hoehne, M.; Trethewey, R.; Stitt, M. Integration of metabolite with transcript and enzyme activity profiling during diurnal cycles in arabidopsis rosettes. Genome Biol. 2006, 7, R76. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.; Purdy, S.; Christ, A.; Morot-Gaudry, J.-F.; Wingler, A.; Masclaux-Daubresse, C. Characterization of markers to determine the extent and variability of leaf senescence in arabidopsis. A metabolic profiling approach. Plant Physiol. 2005, 138, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Xu, Y.C.; Li, W.L.; Yang, L.; Yue, X.; Zhang, X.S.; Zhao, X.Y. Transcriptional analyses of natural leaf senescence in maize. PLoS ONE 2014, 9, e115617. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. The biosynthetic pathways for shikimate and aromatic amino acids in arabidopsis thaliana. Arabidopsis Book 2010, e0132. [Google Scholar] [CrossRef]
- Wittstock, U.; Halkier, B.A. Glucosinolate research in the arabidopsis era. Trends Plant Sci. 2002, 7, 263–270. [Google Scholar] [CrossRef]
- Perrotta, C.; Platani, C.; Lawlor, D.W.; Ronga, G.; Spano, G.; Napier, J.A.; Di Fonzo, N.; Shewry, P.R. Physiological characterization of ‘stay green’ mutants in durum wheat. J. Exp. Bot. 2003, 54, 1415–1420. [Google Scholar]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; van den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef] [PubMed]

| AxC181 | AxC112 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chl (SPAD unit) | Chl (mg/gDW) | Grain Yield (t/ha) | Straw Yield (t/ha) | %N in Grain | %N in Straw | Chl (SPAD unit) | Chl (mg/gDW) | Grain Yield (t/ha) | Straw Yield (t/ha) | %N in Grain | %N in Straw | |
| N0 0WPA | 26.40 (±1.71) | 2.62 (±0.39) | 25.83 (±0.4) | 1.99 (±0.19) | ||||||||
| N0 1WPA | 27.66 (±2.00) | 2.71(±0.19) | 26.9 (±1.42) | 1.77 (±0.43) | ||||||||
| N0 2WPA | 22.33 (±1.67) | 2.00 (±0.53) | 18.57 (2.87) | 1.47 (±0.25) | ||||||||
| N0 3WPA | 13.7 (±3.08) | 0.79 (±0.15) | 11.6 (±3.47) | 0.75 (±0.12) | ||||||||
| N0 4WPA | 2.70 (±4.68) | 0.27 (±0.02) | 4.1 (±7.1) | 0.3 (±0.16) | ||||||||
| N0 5WPA | 0 (±0) | 0.27 (±0.09) | 3.14 (±0.18) | 1.14 (±0.37) | 1.3 (±0.05) | 0.23 (±0.01) | 0 (±0) | 0.29 (±0.02) | 2.22 (±1.01) | 0.39 (±0.27) | 1.37 (±0.04) | 0.25(±0.03) |
| N100 0WPA | 46.37 (±1.94) | 4.70 (±0.08) | 52.6 (±1.01) | 5.1 (±0.17) | ||||||||
| N100 1WPA | 45.70 (±1.51) | 4.69 (±0.26) | 50.6 (±0.26) | 5.42 (±0.1) | ||||||||
| N100 2WPA | 39.47 (±3.29) | 3.03 (±0.33) | 45.67 (±2.65) | 4.37 (±0.36) | ||||||||
| N100 3WPA | 9.17 (±2.66) | 0.90 (±0.42) | 28.97 (±5.22) | 2.12 (±0.38) | ||||||||
| N100 4WPA | 0 (±0) | 0.24 (±0.06) | 0 (±0) | 0.22 (±0.03) | ||||||||
| N100 5WPA | 0 (±0) | 0.27(± 0.09) | 7.29 (±0.39) | 4.56 (±0.57) | 1.49 (±0.1) | 0.25 (±0.01) | 0 (±0) | 0.26 (±0.04) | 7.24 (±0.13) | 2.7 (±0.59) | 1.55 (±0.09) | 0.31 (0.02) |
| N200 0WPA | 53.70 (±2.34) | 5.78 (±0.39) | 57.88 (±1.91) | 6.33 (±0.43) | ||||||||
| N200 1WPA | 54.67 (±0.7) | 6.41 (±0.31) | 58.73 (±1.36) | 7.5 (±0.46) | ||||||||
| N200 2WPA | 54.00 (±1.35) | 6.40 (±0.19) | 57.63 (±0.45) | 6.68(±0.57) | ||||||||
| N200 3WPA | 41.43(±1.04) | 2.80 (±0.78) | 53.4 (±8.1) | 5.05 (±1.13) | ||||||||
| N200 4WPA | 11.00(±9.66) | 0.61 (±0.24) | 16.53 (±4.65) | 0.98 (±0.43) | ||||||||
| N200 5WPA | 0 (±0) | 0.27 (±0.02) | 7.95 (±1.23) | 4.78 (±0.29) | 2.31 (±0.28) | 0.41 (±0.06) | 0 (±0) | 0.26 (±0.01) | 8.3 (±1.33) | 3.37 (±1.01) | 2.09(±0.13) | 0.42 (±0.03) |
| N350 0WPA | 57.40 (±2.34) | 6.64 (±0.35) | 59.73 (±2.38) | 7.37 (±0.47) | ||||||||
| N350 1WPA | 56.73 (±1.16) | 7.60 (±0.38) | 61.7 (±2.6) | 8.08 (±0.22) | ||||||||
| N350 2WPA | 56.13 (±0.92) | 6.84 (±0.35) | 60.9 (±1.95) | 7.08 (±0.18) | ||||||||
| N350 3WPA | 40.23 (±9.88) | 3.50 (±0.93) | 61.2 (±4.42) | 7.36 (±1.14 | ||||||||
| N350 4WPA | 17.43 (±0.49) | 0.95 (±0.32) | 36.1 (±3.02) | 1.91 (±0.46) | ||||||||
| N350 5WPA | 0 (±0) | 0.39 (±0.04) | 7.84 (±1.15) | 4.76 (±0.8) | 2.86 (0.04) | 0.61(±0.01) | 0 (±0) | 0.36 (±0.01) | 8.94 (±0.84) | 3.99 (±0.25) | 2.47 (±0.06) | 0.51 (±0.01) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heyneke, E.; Watanabe, M.; Erban, A.; Duan, G.; Buchner, P.; Walther, D.; Kopka, J.; Hawkesford, M.J.; Hoefgen, R. Effect of Senescence Phenotypes and Nitrate Availability on Wheat Leaf Metabolome during Grain Filling. Agronomy 2019, 9, 305. https://doi.org/10.3390/agronomy9060305
Heyneke E, Watanabe M, Erban A, Duan G, Buchner P, Walther D, Kopka J, Hawkesford MJ, Hoefgen R. Effect of Senescence Phenotypes and Nitrate Availability on Wheat Leaf Metabolome during Grain Filling. Agronomy. 2019; 9(6):305. https://doi.org/10.3390/agronomy9060305
Chicago/Turabian StyleHeyneke, Elmien, Mutsumi Watanabe, Alexander Erban, Guangyou Duan, Peter Buchner, Dirk Walther, Joachim Kopka, Malcolm John Hawkesford, and Rainer Hoefgen. 2019. "Effect of Senescence Phenotypes and Nitrate Availability on Wheat Leaf Metabolome during Grain Filling" Agronomy 9, no. 6: 305. https://doi.org/10.3390/agronomy9060305
APA StyleHeyneke, E., Watanabe, M., Erban, A., Duan, G., Buchner, P., Walther, D., Kopka, J., Hawkesford, M. J., & Hoefgen, R. (2019). Effect of Senescence Phenotypes and Nitrate Availability on Wheat Leaf Metabolome during Grain Filling. Agronomy, 9(6), 305. https://doi.org/10.3390/agronomy9060305





