Water-Saving Irrigation Strategies in Potato Fields: Effects on Physiological Characteristics and Water Use in Arid Region
Abstract
1. Introduction
2. Materials and Methods
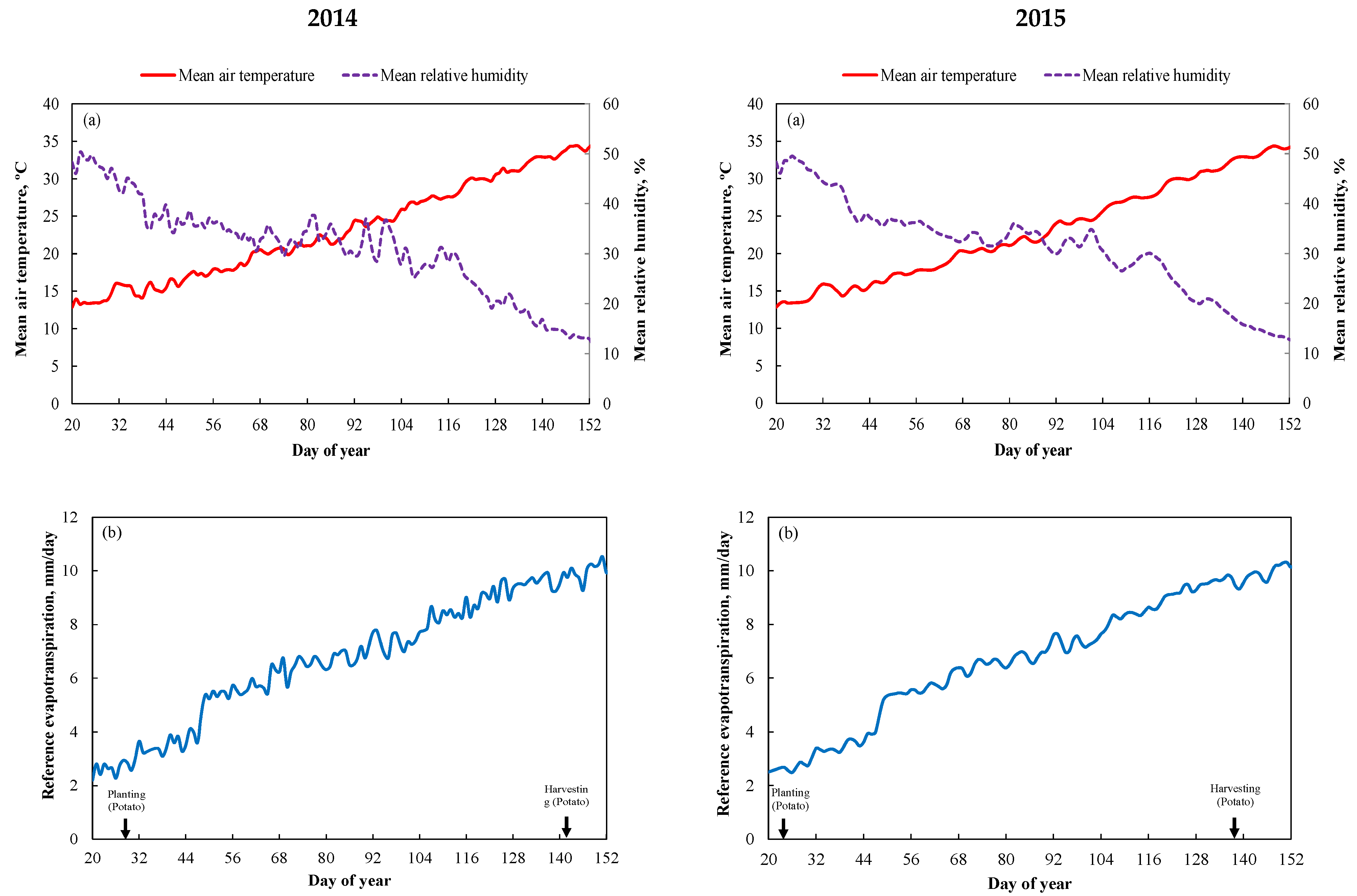
2.1. Experimental Setup and Design
2.2. Physiological Measurements
2.3. Estimation of Yield and Water Productivity
2.4. Statistical Analysis
3. Results
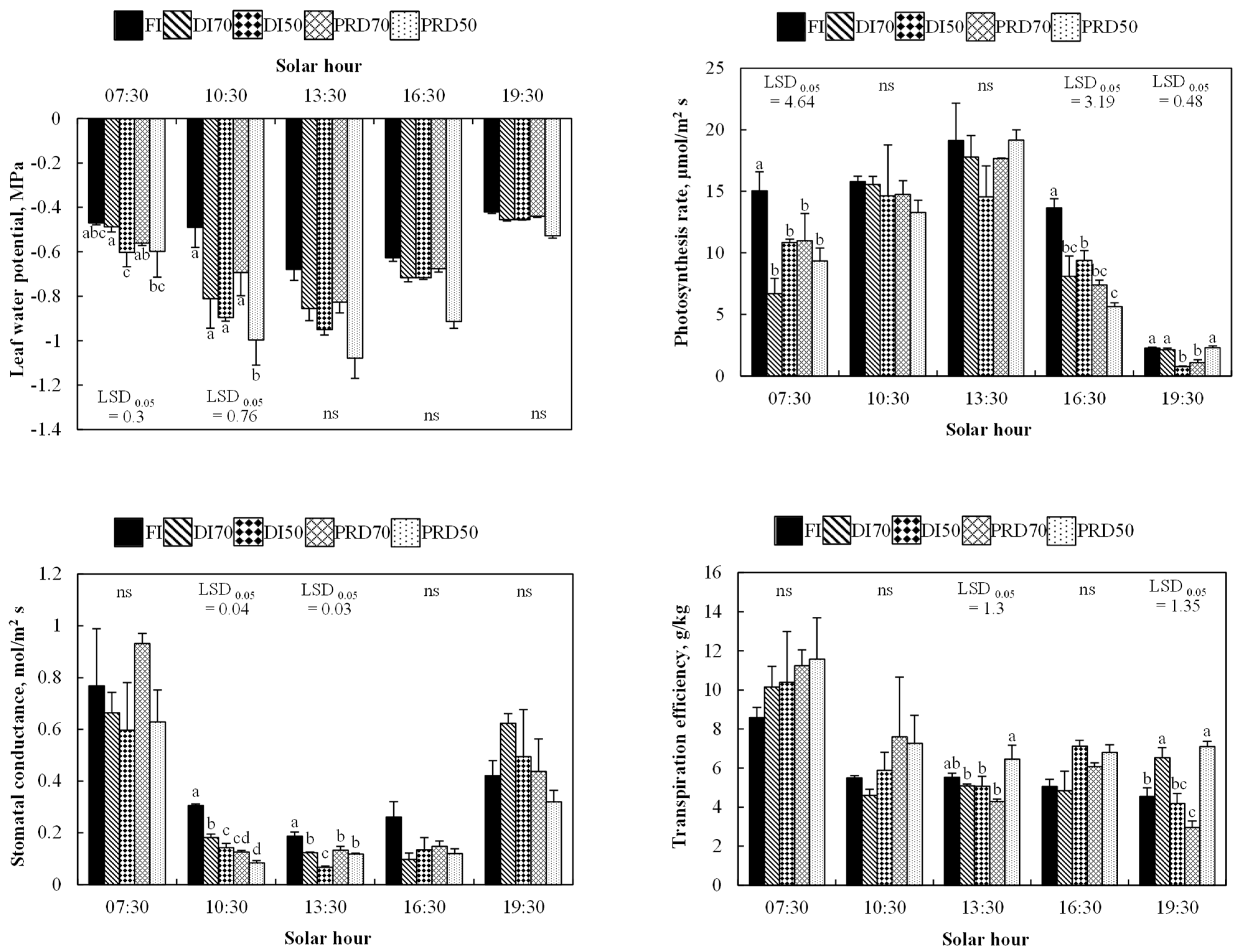
3.1. Physiological Responses
3.1.1. Relative Chlorophyll
3.1.2. Gas-Exchanges
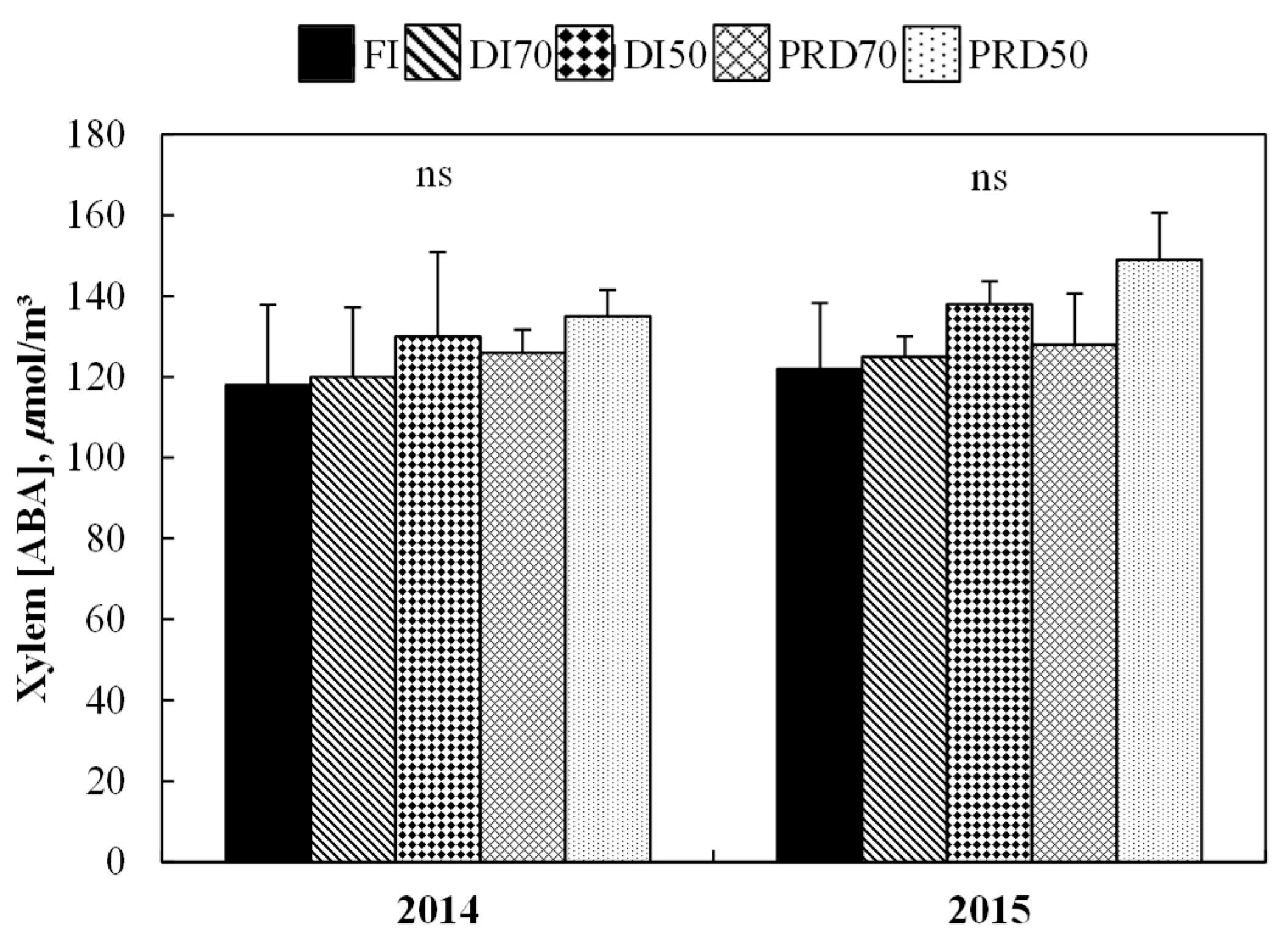
3.1.3. Xylem (ABA)
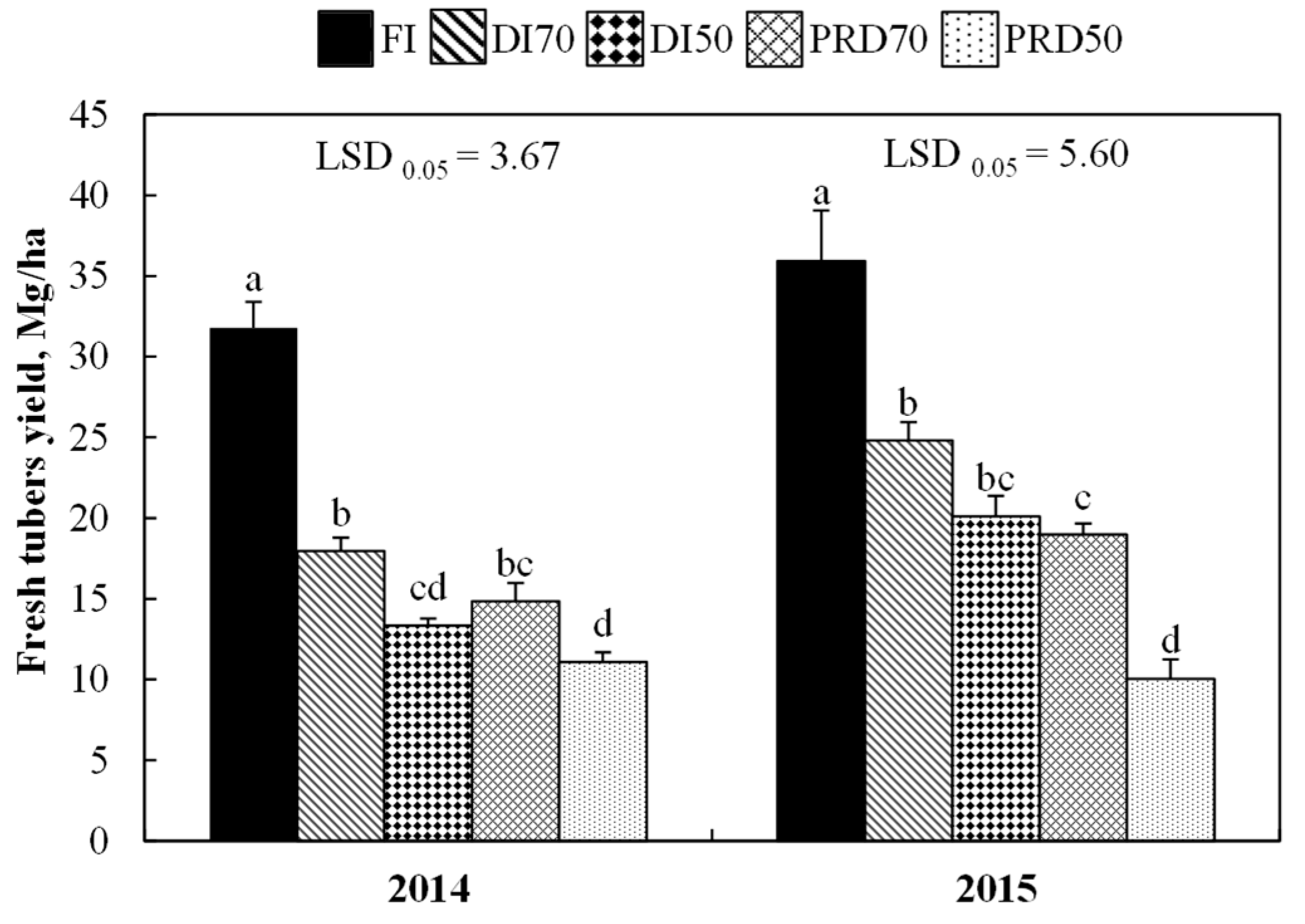
3.2. Tuber Yields
3.3. Water Productivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fabeiro, C.; Martín de Santa Olalla, F.; de Juan, J.A. Yield and size of deficit irrigated potatoes. Agric. Water Manag. 2001, 48, 255–266. [Google Scholar] [CrossRef]
- Ati, A.S.; Iyada, A.D.; Najim, S.M. Water use efficiency of potato (Solanum tuberosum L.) under different irrigation methods and potassium fertilizer rates. Ann. Agric. Sci. 2012, 57, 99–103. [Google Scholar] [CrossRef]
- English, M.J.; Raja, S.N. Perspectives on deficit irrigation. Agric. Water Manag. 1996, 32, 1–14. [Google Scholar] [CrossRef]
- Andersen, M.N.; Asch, F.; Wu, Y.; Jensen, C.R.; Naested, H.; Mogensen, V.O.; Koch, K.E. Soluble invertase expression is an early target of drought stress during the critical abortion-sensitive phase of young ovary development in maize. Plant Physiol. 2002, 130, 591–604. [Google Scholar] [CrossRef]
- Davies, W.J.; Wilkinson, S.; Loveys, B.R. Stomatal control by chemical signaling and the exploitation of this mechanism to increase water use efficiency in agriculture. New Phytol. 2002, 153, 449–460. [Google Scholar] [CrossRef]
- Morison, J.I.L.; Baker, N.R.; Mullineaux, P.M.; Davies, W.J. Improving water use in crop production. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008, 363, 639–658. [Google Scholar] [CrossRef] [PubMed]
- Loveys, B.R. Diurnal changes in water relations and abscisic acid in field-grown Vitisvinifera cultivars. III. The influence of xylem-derived abscisic acid on leaf gas exchange. New Phytol. 1984, 98, 563–573. [Google Scholar] [CrossRef]
- Zhang, J.; Davis, W.J. Change in the concentration of ABA in xylem sap as a function of changing soil water status can account for changes in leaf conductance and growth. Plant Cell Environ. 1990, 13, 277–285. [Google Scholar] [CrossRef]
- Davies, W.J.; Zhang, J. Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Sauter, A.; Davies, W.J.; Hartung, W. The long-distance abscisic acid signal in the droughted plant: The fate of the hormone on its way from root-to-shoot. J. Exp. Bot. 2001, 52, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.L.; Jensen, C.R.; Andersen, M.N. A review of drought adaptation in crop plants: Changes in vegetative and reproductive physiology induced by ABA-based chemical signals. Aust. J. Agric. Res. 2005, 56, 1245–1252. [Google Scholar] [CrossRef]
- Dodd, I.C. Soil moisture heterogeneity during deficit irrigation alters root-to-shoot signaling of abscisic acid. Funct. Plant Biol. 2007, 34, 439–448. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Goodger, J.Q.D. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008, 13, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Stoll, M.; Loveys, B.R.; Dry, P. Hormonal changes induced by partial root-zone drying of irrigated grapevine. J. Exp. Bot. 2000, 51, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.L.; Shahnazari, A.; Andersen, M.N.; Jacobsen, S.E.; Jensen, C.R. Physiological responses of potato (Solanumtuberosum L.) to partial root-zone drying: ABA signaling, leaf gas exchange, and water use efficiency. J. Exp. Bot. 2006, 57, 3727–3735. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.L.; Shahnazari, A.; Andersen, M.N.; Jacobsen, S.E.; Jensen, C.R. Effects of deficit irrigation (DI) and partial root drying (PRD) on gas exchange, biomass partitioning, and water use efficiency in potato. Sci. Hortic. 2006, 109, 113–117. [Google Scholar] [CrossRef]
- Dodd, I.C. Rhizposphere manipulations to maximize ‘crop per drop’ during deficit irrigation. J. Exp. Bot. 2009, 60, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, F.L.; Andersen, M.N.; Jensen, C.R. Improved plant nitrogen nutrition contributes to higher water use efficiency in tomatoes under alternate partial root-zone irrigation. Funct. Plant Biol. 2010, 37, 175–182. [Google Scholar] [CrossRef]
- Ali, M.; Jensen, C.R.; Mogensen, V.O. Early signals in field grown wheat in response to hallow soil drying. Aus. J. Plant Physiol. 1998, 25, 871–882. [Google Scholar]
- Holbrook, N.M.; Shashidhar, V.R.; James, R.A.; Munns, R. Stomatal control in tomato with ABA-deficient roots: Response of grafted plants to soil drying. J. Exp. Bot. 2002, 53, 1503–1514. [Google Scholar]
- Bahrun, A.; Jensen, C.R.; Asch, F.; Mogensen, V.O. Drought-induced changes in xylem pH, ionic composition, and ABA concentration act as early signals in field-grown maize (Zea mays L.). J. Exp. Bot. 2002, 53, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Z.; Zhang, J.H. Controlled alternate partial root-zone irrigation: Its physiological consequences and impact on water use efficiency. J. Exp. Bot. 2004, 55, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.H.; Andersen, M.N.; Plauborg, F.; Poulsen, R.T.; Jensen, C.R.; Sepaskhah, A.R.; Hansen, S. Effects of irrigation strategies and soils on field grown potatoes: Gas exchange and xylem [ABA]. Agric. Water Manag. 2010, 97, 1486–1494. [Google Scholar] [CrossRef]
- Jensen, C.R.; Battilani, A.; Plauborg, F.; Psarras, G.; Chartzoulakis, K.; Janowiak, F.; Stikic, R.; Jovanovic, Z.; Li, G.; Qi, X.; et al. Deficit irrigation based on drought tolerance and root signaling in potatoes and tomatoes. Agric. Water Manag. 2010, 98, 403–413. [Google Scholar] [CrossRef]
- Shahabian, M.; Samar, S.M.; Talaie, A.; Emdad, M.R. Response of orange trees to deficit irrigation strategies in the north of Iran. Arch. Agron. Soil Sci. 2012, 58, 267–276. [Google Scholar] [CrossRef]
- Xie, K.; Wang, X.X.; Zhang, R.; Gong, X.; Zhang, S.; Mares, V.; Gavilán, C.; Posadas, A.; Quiroz, R. Partial root-zone drying irrigation and water utilization efficiency by the potato crop in semi-arid regions in China. Sci. Hortic. 2012, 134, 20–25. [Google Scholar] [CrossRef]
- Shahnazari, A.; Liu, F.; Andersen, M.N.; Jacobsen, S.E.; Jensen, C.R. Effects of partial root-zone drying on yield, tuber size and water use efficiency in potato under filed conditions. Field Crops Res. 2007, 100, 117–124. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Andersen, M.N.; Plauborg, F.; Poulsen, R.T.; Jensen, C.R.; Sepaskhah, A.R.; Hansen, S. Effects of irrigation strategies and soils on field grown potatoes: Yield and water productivity. Agric. Water Manag. 2010, 97, 1923–1930. [Google Scholar] [CrossRef]
- Yactayo, W.; Ramírez, D.A.; Gutiérrez, R.; Mares, V.; Posadas, A.; Quiroz, R. Effect of partial root-zone drying irrigation timing on potato tuber yield and water use efficiency. Agric. Water Manag. 2013, 123, 65–70. [Google Scholar] [CrossRef]
- Fernández, J.E.; Perez-Martin, A.; Torres-Ruiz, J.M.; Cuevas, M.V.; Rodriguez-Dominguez, C.M.; Elsayed-Farag, S.; Morales-Sillero, A.; García, J.M.; Hernandez-Santana, V.; Diaz-Espejo, A. A regulated deficit irrigation strategy for hedgerow olive orchards with high plant density. Plant Soil 2013, 372, 279–295. [Google Scholar] [CrossRef]
- Karandish, F. Improved soil–plant water dynamics and economic water use efficiency in a maize field under locally water stress. Arch. Agron. Soil Sci. 2016, 69, 1311–1323. [Google Scholar] [CrossRef]
- Padilla-Díaz, C.M.; Rodriguez-Dominguez, C.M.; Hernandez-Santana, V.; Perez-Martin, A.; Fernández, J.E. Scheduling regulated deficit irrigation in a hedgerow olive orchard from leaf turgor pressure related measurements. Agric. Water Manag. 2016, 164, 28–37. [Google Scholar] [CrossRef]
- Hernandez-Santana, V.; Fernández, J.E.; Cuevas, M.V.; Perez-Martin, A.; Diaz-Espejo, A. Photosynthetic limitations by water deficit: Effect on fruit and olive oil yield, leaf area and trunk diameter and its potential use to control vegetative growth of super-high density olive orchards. Agric. Water Manag. 2017, 184, 9–18. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Doorenbos, J.; Pruitt, W.O. Guidelines for Predicting Crop Water Requirements; Irrigation and Drainage Paper No. 24; FAO: Rome, Italy, 1977; p. 179. [Google Scholar]
- Klute, A. Water retention: Laboratory methods. In Methods of Soil Analysis. Part 1: Physical and Mineralogical Methods; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986. [Google Scholar]
- Dirksen, C. Soil Physics Measurements; Catena Verlag: Reiskirchen, Germany, 1999. [Google Scholar]
- Blacke, G.R. Bulk density. In Methods of Soil Analysis. Part 1: Physical and Mineralogical Methods; Black, C.A., Evans, D.D., Ensminger, L.E., White, J.L., Clark, F.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 374–390. [Google Scholar]
- Asch, F. Determination of Abscisic Acid by Indirect Enzyme Linked Immunosorbent Assay (ELISA); Technical Report; Laboratory for Agrohydrology and Bioclimatology, Department of Agricultural Sciences, The Royal Veterinary and Agricultural University: Taastrup, Denmark, 2000. [Google Scholar]
- Kirda, C.; Topcu, S.; Kaman, H.; Ulger, A.C.; Yazici, A.; Cetin, M.; Derici, M.R. Grain yield response and N-fertilizer recovery of maize under deficit irrigation. Field Crops Res. 2005, 93, 132–141. [Google Scholar] [CrossRef]
- CoStat Version 6.311 Copyright_ 1998–2005 CoHort Software798 Lighthouse Ave; PMB 320; CoHort Software: Monterey, CA, USA, 2005.
- Liu, F.L.; Song, R.; Zhang, X.; Shahnazari, A.; Andersen, M.N.; Plauborg, F.; Jacobsen, S.E.; Jensen, C.R. Measurement and modeling of ABA signaling in potato (Solanumtuberosum L.) during partial root-zone drying. Environ. Exp. Bot. 2008, 63, 385–391. [Google Scholar] [CrossRef]
- Bowen, W.T. Water Productivity and Potato Cultivation. In Water Productivity in Agriculture: Limits and Opportunities for Improvement; Kijne, J.W., Barker, R., Molden, D., Eds.; CABI Publishing: Oxfordshire, England, 2003; p. 332. [Google Scholar]
- Vos, J.; Haverkort, A.J. Water availability and potato crop performance. In Potato Biology and Biotechnology: Advances and Perspectives; Vreugdenhil, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 333–351. [Google Scholar]
- Vos, J.; Groenwold, J. Genetic differences in water-use efficiency, stomatal conductance and carbon isotope fractionation in potato. Potato Res. 1989, 32, 113–121. [Google Scholar] [CrossRef]
- Jones, H.G. Plants and Microclimate. In A Quantitative Approach to Environmental Plant Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Shimsi, D.; Shalhavet, J.; Meir, T. Irrigation regime effects on some physiological responses of potato. Agron. J. 1983, 75, 262–267. [Google Scholar] [CrossRef]
- Levy, D. Varietal differences in the response of potatoes to repeated short periods of water stress in hot climates. 1. Turgor maintenance and stomatal behaviors. Potato Res. 1983, 26, 303–313. [Google Scholar] [CrossRef]
- Stark, J.C. Stomatal behavior of potatoes under nonlimiting soil water conditions. Am. Potato J. 1987, 64, 301–309. [Google Scholar] [CrossRef]
- Vos, J.; Groenwold, J. Characteristics of photosynthesis and conductance of potato canopies and the effects of cultivar and transient drought. Field Crops Res. 1989, 20, 237–250. [Google Scholar] [CrossRef]
- Darwish, T.M.; Atallah, T.W.; Hajhasan, S.; Haidar, A. Nitrogen and water use efficiency of fertigated processing potato. Agric. Water Manag. 2006, 85, 95–104. [Google Scholar] [CrossRef]
- Shae, J.B.; Steele, D.D.; Gregor, B.L. Irrigation scheduling methods for potatoes in the northern Great Plains. Trans ASABE 1999, 42, 351–360. [Google Scholar] [CrossRef]
- Kaman, H.; Kirda, C.; Sesveren, S. Genotypic differences of maize in grain yield response to deficit irrigation. Agric. Water Manag. 2011, 98, 801–807. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Agharezaee, M.; Kamgar-Haghighib, A.; Sepaskhah, A.R. Effects of dynamic and static deficit and partial root zone drying irrigation strategies on yield, tuber sizes distribution, and water productivity of two field grown potato cultivars. Agric. Water Manag. 2014, 134, 126–136. [Google Scholar] [CrossRef]
- De la Hera, M.L.; Romero, P.; Gomez-Plaza, E.; Martinez, A. Is partial root-zone drying an effective irrigation technique to improve water use efficiency and fruit quality in field–grown wine grapes under semiarid conditions? Agric. Water Manag. 2007, 87, 261–274. [Google Scholar] [CrossRef]
- Ferreira, T.C.; Goncalves, D.A. Crop-yield/water-use production function of potatoes (Solanum tubersoum L.) grown under differential nitrogen and irrigation treatments in a hot, dry climate. Agric. Water Manag. 2007, 90, 45–55. [Google Scholar] [CrossRef]
- Kriedmann, P.E.; Goodwin, I. Regulated Deficit Irrigation and Partial Root-Zone Drying; Irrigation Insights No. 4; Land and Water Australia: Canberra, Australia, 2003; p. 102. [Google Scholar]
- Sepaskhah, A.R.; Parand, A.R. Effects of alternate furrow irrigation with supplemental every-furrow irrigation at different growth stages on the yield of maize (Zea mays L.). Plant Prod. Sci. 2006, 9, 415–421. [Google Scholar] [CrossRef]

| Depth (cm) | Particle Size (%) | Texture | FC (%) | WP (%) | Ks (mm/h) | ρb, (g/cm3) | ||
|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | ||||||
| 0–20 | 71.8 | 16.3 | 11.9 | sandy loam | 14.2 | 6 | 37.8 | 1.6 |
| 20–40 | 66.7 | 18 | 15.3 | sandy loam | 17.1 | 8.1 | 24.6 | 1.6 |
| 40–60 | 69.1 | 18.3 | 12.6 | sandy loam | 18.5 | 9.9 | 19.6 | 1.6 |
| Year | Treatments | 48 DAP | 55 DAP | 62 DAP | 69 DAP | 76 DAP | 83 DAP |
|---|---|---|---|---|---|---|---|
| 2014 | FI | 29.63 (±3.44) | 32.25 (±3.78) | 18.76 (±0.04) b | 17.37 (±0.87) | 19.47 (±3.12) | 9.17 (±1.22) c |
| DI70 | 33.15 (±2.28) | 36.12 (±2.51) | 18.52 (±2.69) b | 19.50 (±3.90) | 16.17 (±2.07) | 10.00 (±1.27) bc | |
| DI50 | 32.85 (±3.20) | 35.79 (±3.52) | 28.35 (±1.92) a | 19.90 (±3.42) | 23.73 (±1.47) | 12.10 (±1.60) abc | |
| PRD70 | 34.93 (±3.30) | 38.08 (±3.63) | 26.66 (±3.49) a | 15.80 (±1.37) | 22.43 (±2.69) | 13.17 (±1.94) ab | |
| PRD50 | 29.45 (±2.28) | 30.30 (±3.05) | 16.70 (±1.22) b | 15.30 (±1.48) | 14.77 (±0.94) | 14.95 (±1.24) a | |
| p-Value | 0.72 | 0.59 | 0.01 | 0.70 | 0.06 | 0.05 | |
| LSD 0.05 | - | - | 6.93 | - | - | 3.96 | |
| 2015 | FI | 24.40 (±2.17) | 27.40 (±1.38) | 19.37 (±0.84) | 14.77 (±1.14) b | 15.66 (±0.54) b | 9.32 (±0.63) b |
| DI70 | 25.93 (±3.12) | 27.82 (±1.55) | 19.00 (±1.20) | 18.80 (±1.08) ab | 17.44 (±0.85) ab | 8.32 (±0.36) b | |
| DI50 | 26.70 (±2.32) | 29.12 (±0.82) | 24.57 (±0.32) | 22.18 (±1.64) a | 22.98 (±2.00) ab | 12.09 (±0.37) a | |
| PRD70 | 27.60 (±3.27) | 32.67 (±1.99 | 24.95 (±1.05) | 21.98 (±1.15) a | 22.99 (±2.49) a | 12.91 (±0.52) a | |
| PRD50 | 24.43 (±2.00) | 29.43 (±1.51) | 19.37 (±2.71) | 17.8 (±1.79) ab | 18.97 (±0.94) ab | 9.56 (±1.18) b | |
| p-Value | 0.88 | 0.08 | 0.06 | 0.03 | 0.05 | <0.01 | |
| LSD 0.05 | - | - | - | 4.57 | 5.57 | 1.86 |
| Year | Treatments | 48 DAP | 55 DAP | 62 DAP | 69 DAP | 76 DAP | 83 DAP |
|---|---|---|---|---|---|---|---|
| 2014 | FI | 26.2 (±1.57) ab | 28.38 (±1.72) ab | 21.60 (±2.86) a | 16.00 (±2.13) a | 14.92 (±0.73) a | 25.29 (±0.86) a |
| DI70 | 19.00 (±1.22) bc | 20.45 (±1.35) bc | 16.15 (±2.75) b | 5.13 (±1.68) b | 9.98 (±1.52) b | 13.90 (±1.98) bc | |
| DI50 | 16.43 (±1.10) c | 17.63 (±1.21) c | 9.59 (±1.81) c | 8.25 (±0.99) b | 10.81 (±0.72) b | 16.97 (±0.27) b | |
| PRD70 | 28.77 (±0.87 a | 31.21 (±0.96) a | 21.83 (±1.44) a | 9.93 (±1.93) b | 15.93 (±1.51) a | 15.05 (±3.54) bc | |
| PRD50 | 27.77 (±4.84) a | 30.09 (±5.33) a | 23.08 (±0.90) a | 7.59 (±0.92) b | 14.71 (±0.99) a | 10.40 (±0.81) c | |
| p-Value | 0.02 | 0.02 | <0.01 | 0.01 | 0.01 | 0.01 | |
| LSD 0.05 | 7.74 | 8.53 | 5.09 | 5.37 | 3.53 | 6.49 | |
| 2015 | FI | 23.83 (±1.00) a | 23.81 (±0.52) a | 22.24 (±1.01) | 18.54 (±0.20) a | 16.99 (±0.64) a | 15.56 (±0.63) |
| DI70 | 22.38 (±0.30) a | 22.01 (±1.12) b | 20.58 (±1.69) | 11.27 (±0.09) b | 13.38 (±0.17) b | 14.73 (±1.79) | |
| DI50 | 18.63 (±0.13) b | 18.33 (±0.50) c | 19.65 (±1.05) | 8.55 (±1.04) c | 13.56 (±0.29) b | 9.34 (±1.05) | |
| PRD70 | 21.37 (±1.28) ab | 20.60 (±1.10) b | 20.39 (±0.16) | 12.26 (±1.32) b | 14.01 (±0.23) b | 14.54 (±2.52) | |
| PRD50 | 23.45 (±1.44) a | 18.63 (±0.13) c | 21.26 (±0.76) | 11.29 (±0.24) b | 7.22 (±0.25) c | 11.69 (±1.47) | |
| p-Value | 0.03 | <0.01 | 0.56 | <0.01 | <0.01 | 0.06 | |
| LSD 0.05 | 3.02 | 1.59 | - | 2.21 | 1.29 | - |
| Year | Treatments | 48 DAP | 55 DAP | 62 DAP | 69 DAP | 76 DAP | 83 DAP |
|---|---|---|---|---|---|---|---|
| 2014 | FI | 0.64 (±0.05) | 0.7 (±0.05) | 0.89 (±0.09) a | 0.79 (±0.18) a | 0.37 (±0.03) | 0.67 (±0.02) a |
| DI70 | 0.81 (±0.03) | 0.89 (±0.04) | 0.38 (±0.03) b | 0.28 (±0.07) b | 0.39 (±0.14) | 0.55 (±0.04) ab | |
| DI50 | 0.47 (±0.07) | 0.51 (±0.08) | 0.17 (±0.04) c | 0.36 (±0.04) b | 0.31 (±0.03) | 0.43 (±0.10) bc | |
| PRD70 | 0.66 (±0.14) | 0.73 (±0.15) | 0.56 (±0.03) b | 0.31 (±0.07) b | 0.27 (±0.06) | 0.32 (±0.01) c | |
| PRD50 | 0.51 (±0.09) | 0.56 (±0.10) | 0.47 (±0.05) b | 0.28 (±0.01) b | 0.23 (±0.02) | 0.37 (±0.05) c | |
| p-Value | 0.14 | 0.14 | <0.01 | 0.01 | 0.64 | <0.01 | |
| LSD 0.05 | - | - | 0.18 | 0.28 | - | 0.13 | |
| 2015 | FI | 0.59 (±0.06) a | 0.64 (±0.14) a | 0.89 (±0.23) | 0.61 (± 0.08) a | 0.60 (±0.02) a | 0.62 (±0.04) |
| DI70 | 0.53 (±0.03) a | 0.52 (±0.04) abc | 0.52 (±0.15) | 0.44 (± 0.10) ab | 0.45 (±0.01) b | 0.39 (±0.11) | |
| DI50 | 0.3 (±0.03) b | 0.28 (±0.04) c | 0.47 (±0.10) | 0.41 (± 0.03) ab | 0.36 (±0.00) c | 0.32 (±0.04) | |
| PRD70 | 0.55 (±0.08) a | 0.53 (±0.02) ab | 0.52 (±0.04) | 0.29 (± 0.06) b | 0.29 (±0.01) d | 0.43 (±0.13) | |
| PRD50 | 0.45 (±0.10) ab | 0.30 (±0.04) bc | 0.47 (±0.10) | 0.26 (± 0.03) b | 0.25 (±0.01) e | 0.36 (±0.08) | |
| p-Value | 0.03 | 0.04 | 0.35 | 0.03 | <0.01 | 0.32 | |
| LSD 0.05 | 0.17 | 0.25 | - | 0.21 | 0.04 | - |
| Year | Treatments | 48 DAP | 55 DAP | 62 DAP | 69 DAP | 76 DAP | 83 DAP |
|---|---|---|---|---|---|---|---|
| 2014 | FI | 3.40 (±0.24) | 3.29 (±0.26) | 4.82 (±0.41) a | 4.10 (±0.60) a | 2.38 (±0.15) | 2.51 (±0.49) |
| DI70 | 3.83 (±0.15) | 3.76 (±0.16) | 3.16 (±0.70) b | 2.01 (±0.41) b | 2.44 (±0.74) | 3.18 (±0.20) | |
| DI50 | 2.60 (±0.33) | 2.41 (±0.36) | 1.21 (±0.25) c | 1.75 (±0.52) b | 2.03 (±0.18) | 2.66 (±0.56) | |
| PRD70 | 3.09 (±0.43) | 2.95 (±0.48) | 3.36 (±0.17) b | 1.90 (±0.37) b | 1.80 (±0.35) | 1.97 (±0.08) | |
| PRD50 | 2.85 (±0.28) | 2.69 (±0.30) | 2.53 (±0.34) bc | 1.77 (±0.06) b | 1.24 (±0.07) | 2.00 (±0.33) | |
| p-Value | 0.11 | 0.10 | <0.01 | 0.01 | 0.32 | 0.27 | |
| LSD 0.05 | - | - | 1.40 | 1.21 | - | - | |
| 2015 | FI | 3.18 (±0.26) | 2.85 (±0.17) b | 5.17 (±0.52) a | 2.19 (±0.22) a | 3.27 (±0.08) a | 3.4 (±0.18) a |
| DI70 | 2.18 (±0.20) | 3.14 (±0.18) ab | 4.63 (±0.77) a | 1.76 (±0.38) ab | 2.65 (±0.04) b | 1.92 (±0.24) b | |
| DI50 | 2.63 (±0.21) | 1.81 (±0.27) c | 3.48 (±0.31) b | 1.69 (±0.02) ab | 2.43 (±0.02) b | 1.71 (±0.21) b | |
| PRD70 | 2.54 (±0.43) | 3.37 (±0.16) a | 3.38 (±0.22) b | 1.11 (±0.19) bc | 1.80 (±0.04) c | 2.68 (±0.53) ab | |
| PRD50 | 2.67 (±0.23) | 1.87 (±0.25) c | 3.35 (±0.44) b | 0.91 (±0.02) c | 1.19 (±0.25) d | 2.05 (±0.16) b | |
| p-Value | 0.24 | <0.01 | 0.02 | 0.02 | <0.01 | 0.03 | |
| LSD 0.05 | - | 0.46 | 1.12 | 0.69 | 0.37 | 1.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zin El-Abedin, T.K.; Mattar, M.A.; Al-Ghobari, H.M.; Alazba, A.A. Water-Saving Irrigation Strategies in Potato Fields: Effects on Physiological Characteristics and Water Use in Arid Region. Agronomy 2019, 9, 172. https://doi.org/10.3390/agronomy9040172
Zin El-Abedin TK, Mattar MA, Al-Ghobari HM, Alazba AA. Water-Saving Irrigation Strategies in Potato Fields: Effects on Physiological Characteristics and Water Use in Arid Region. Agronomy. 2019; 9(4):172. https://doi.org/10.3390/agronomy9040172
Chicago/Turabian StyleZin El-Abedin, Tarek K., Mohamed A. Mattar, Hussein M. Al-Ghobari, and Abdulrahman A. Alazba. 2019. "Water-Saving Irrigation Strategies in Potato Fields: Effects on Physiological Characteristics and Water Use in Arid Region" Agronomy 9, no. 4: 172. https://doi.org/10.3390/agronomy9040172
APA StyleZin El-Abedin, T. K., Mattar, M. A., Al-Ghobari, H. M., & Alazba, A. A. (2019). Water-Saving Irrigation Strategies in Potato Fields: Effects on Physiological Characteristics and Water Use in Arid Region. Agronomy, 9(4), 172. https://doi.org/10.3390/agronomy9040172






