The Growth and Development of ‘Mini Chal’ Tomato Plug Seedlings Grown under Various Wavelengths Using Light Emitting Diodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
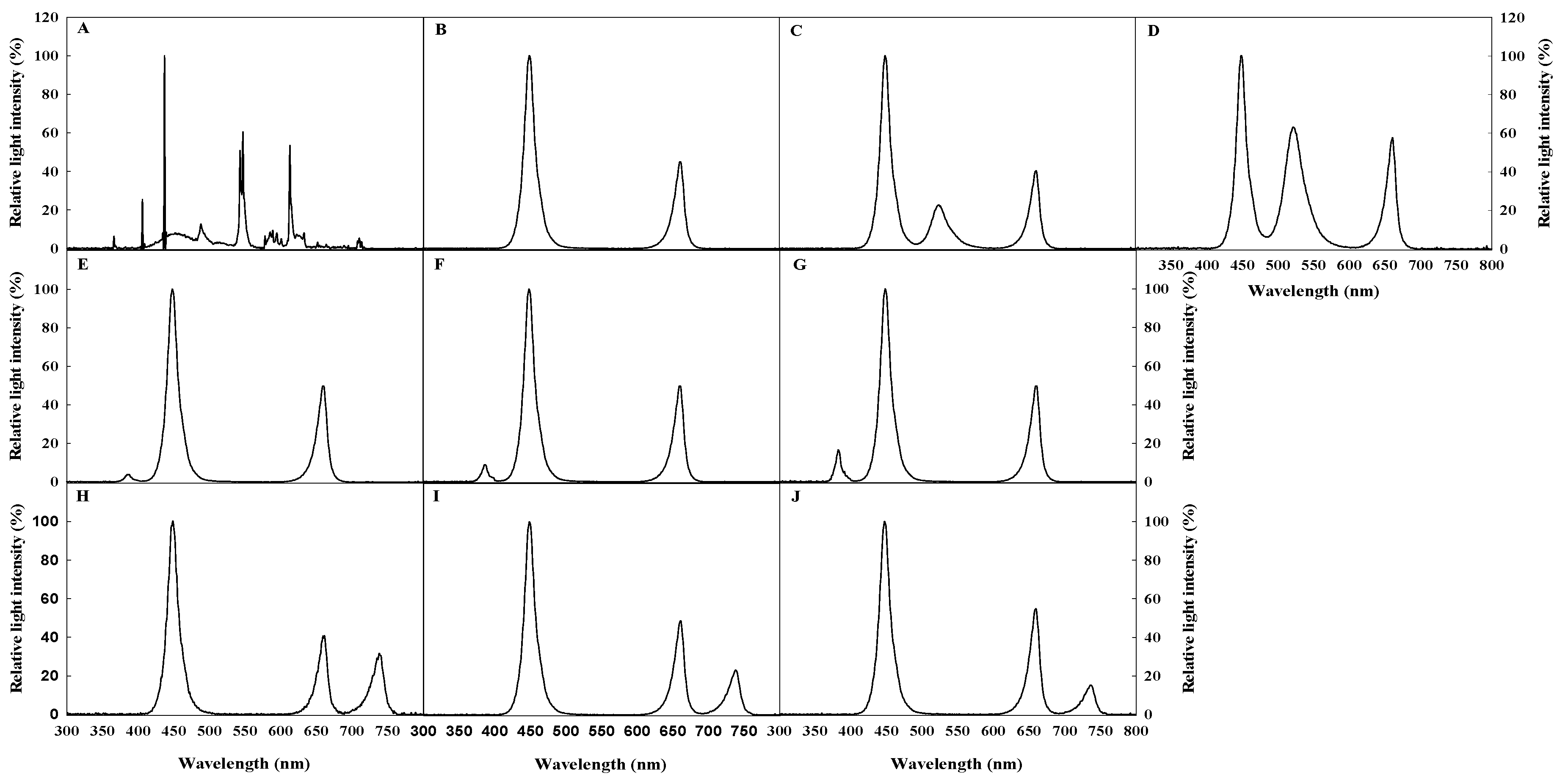
2.2. Light Quality Treatments
2.3. Measurements of Plant Growth Characteristics
2.4. Statistical Analysis
3. Results and Discussion
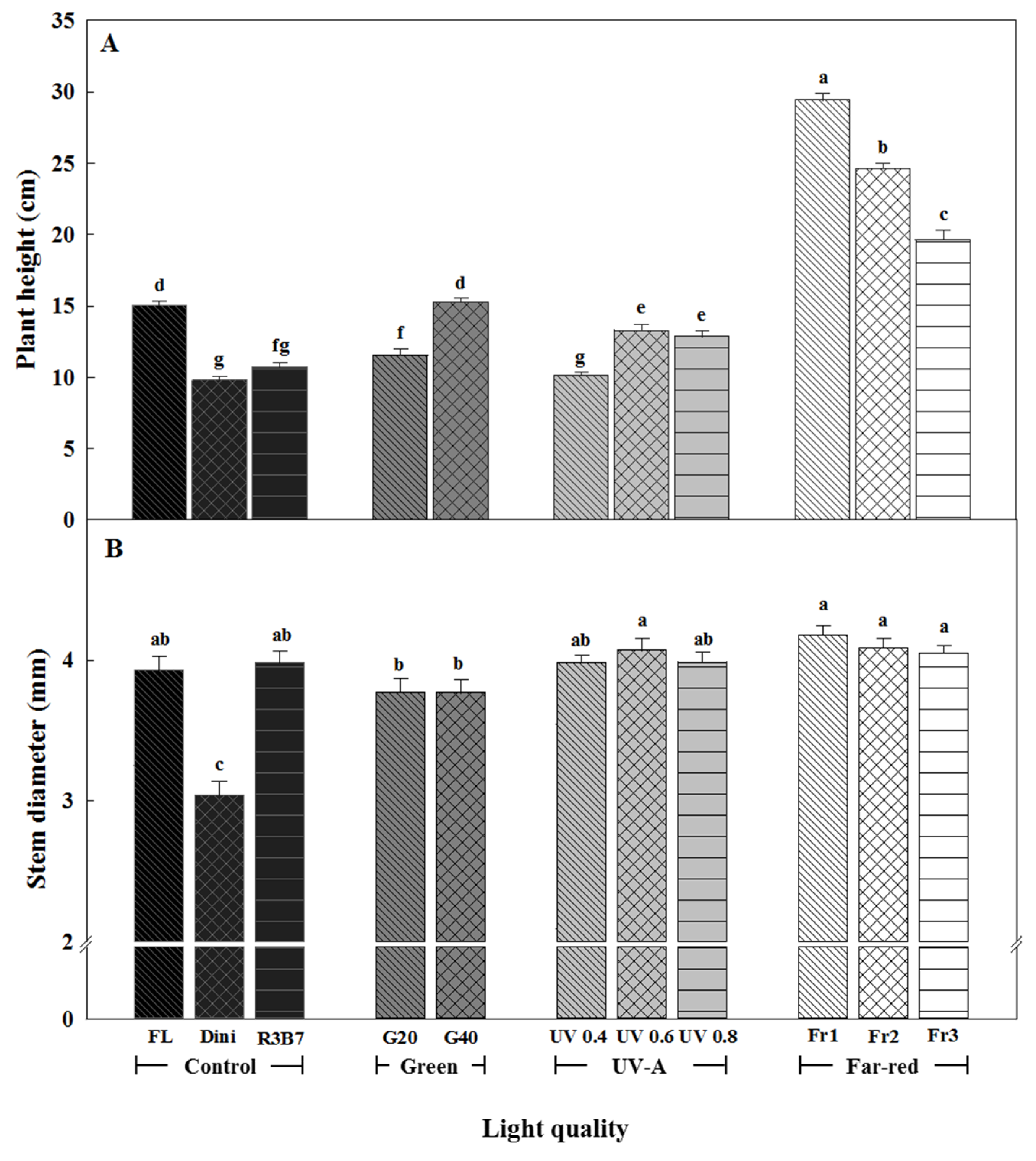
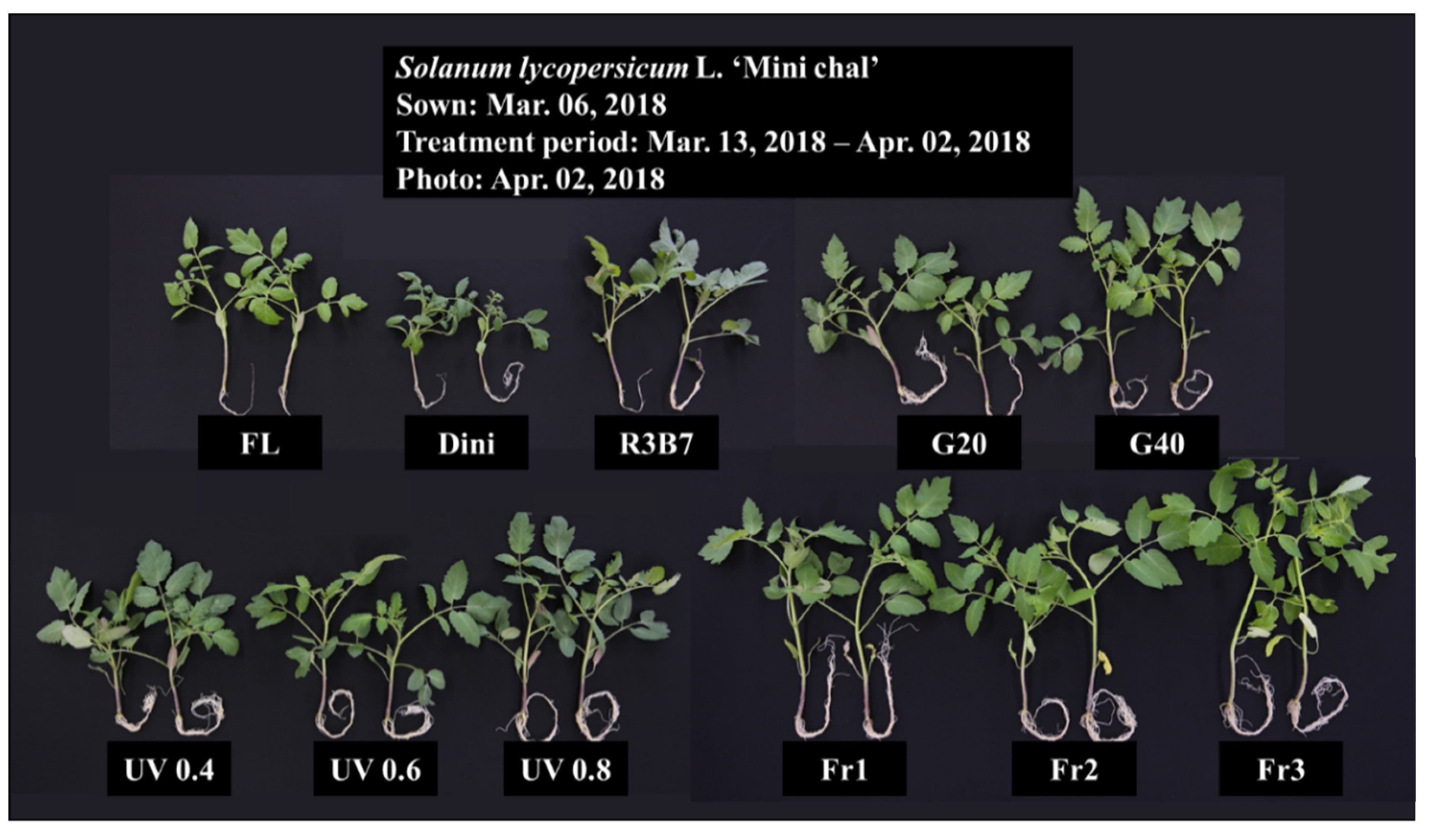
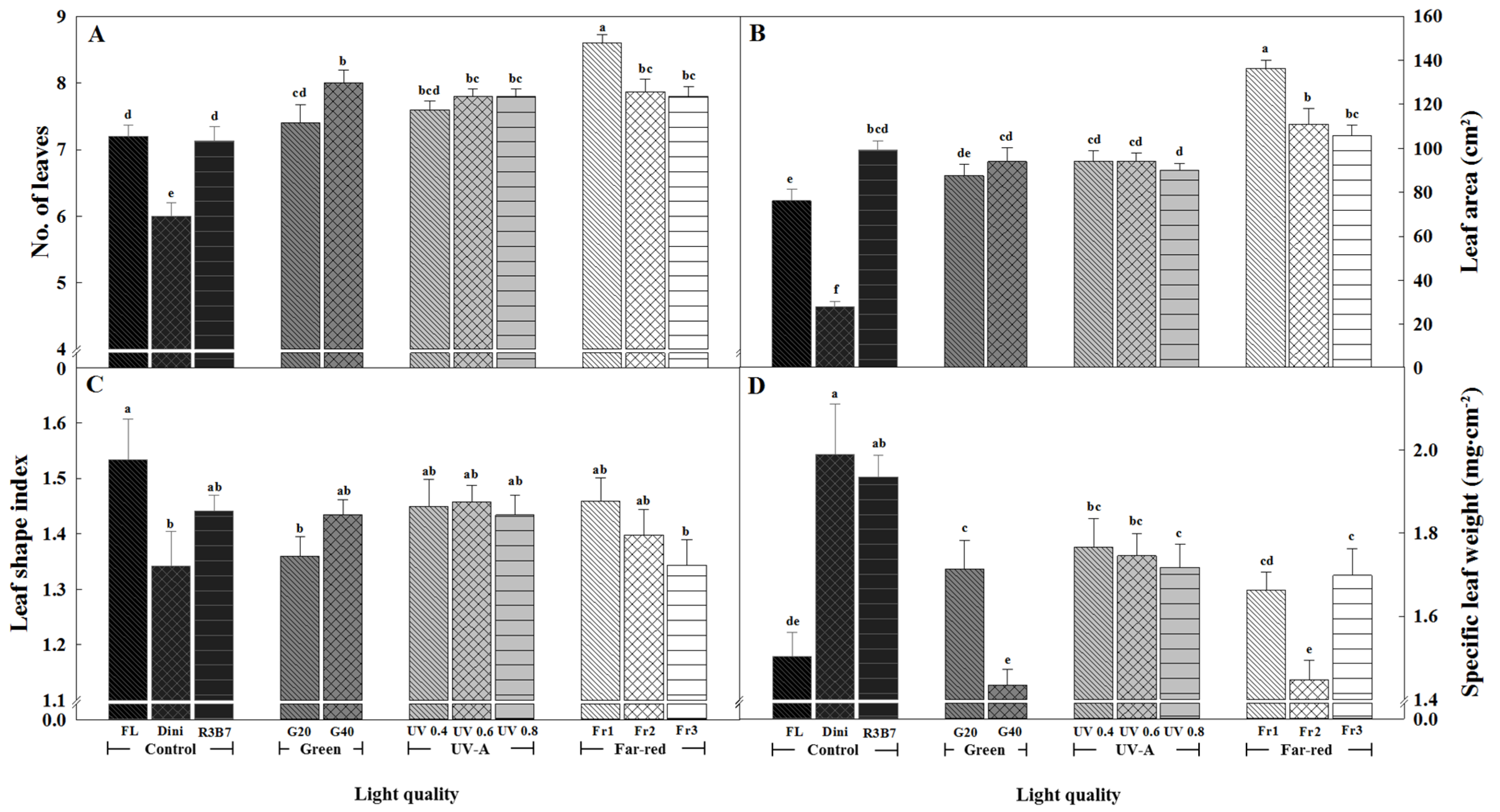
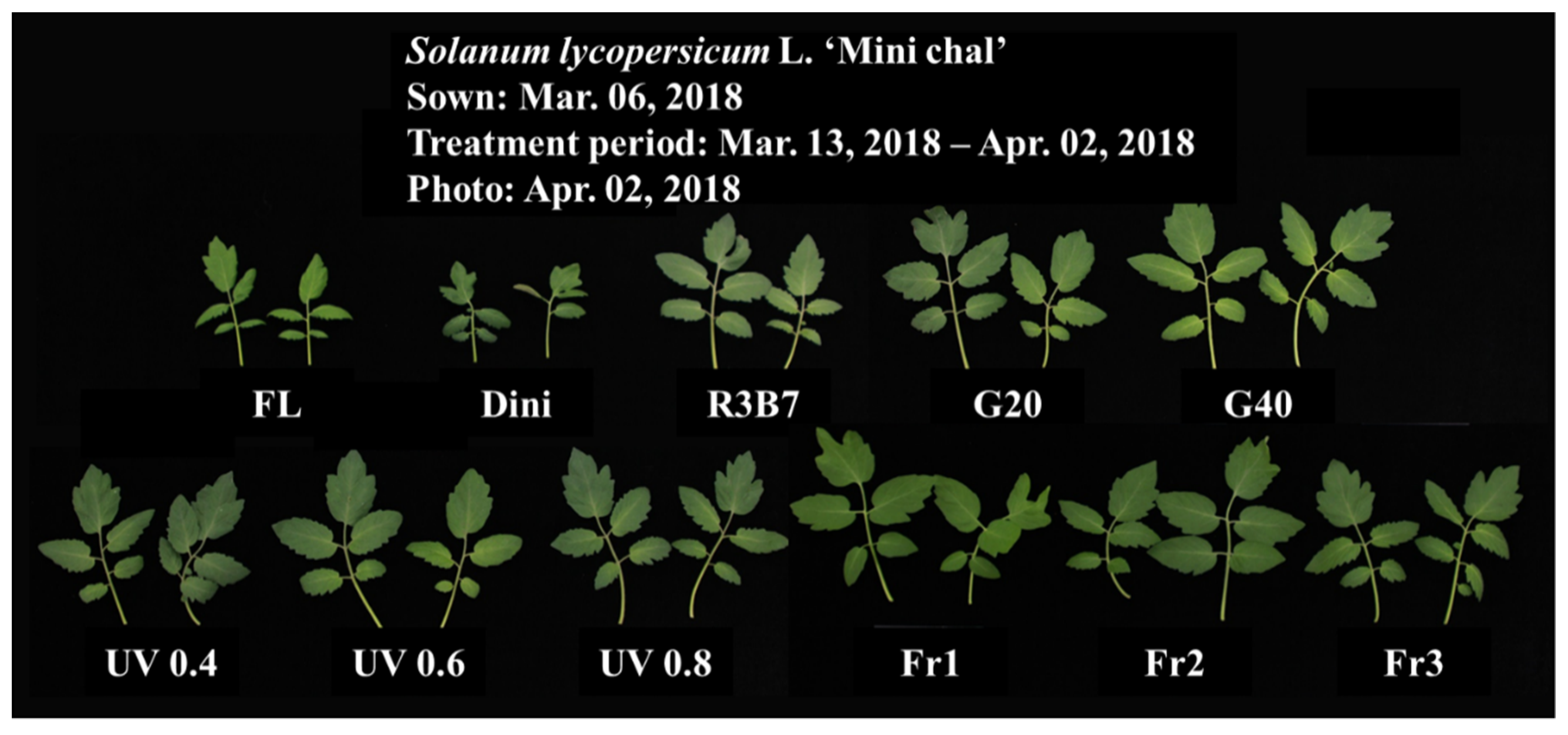
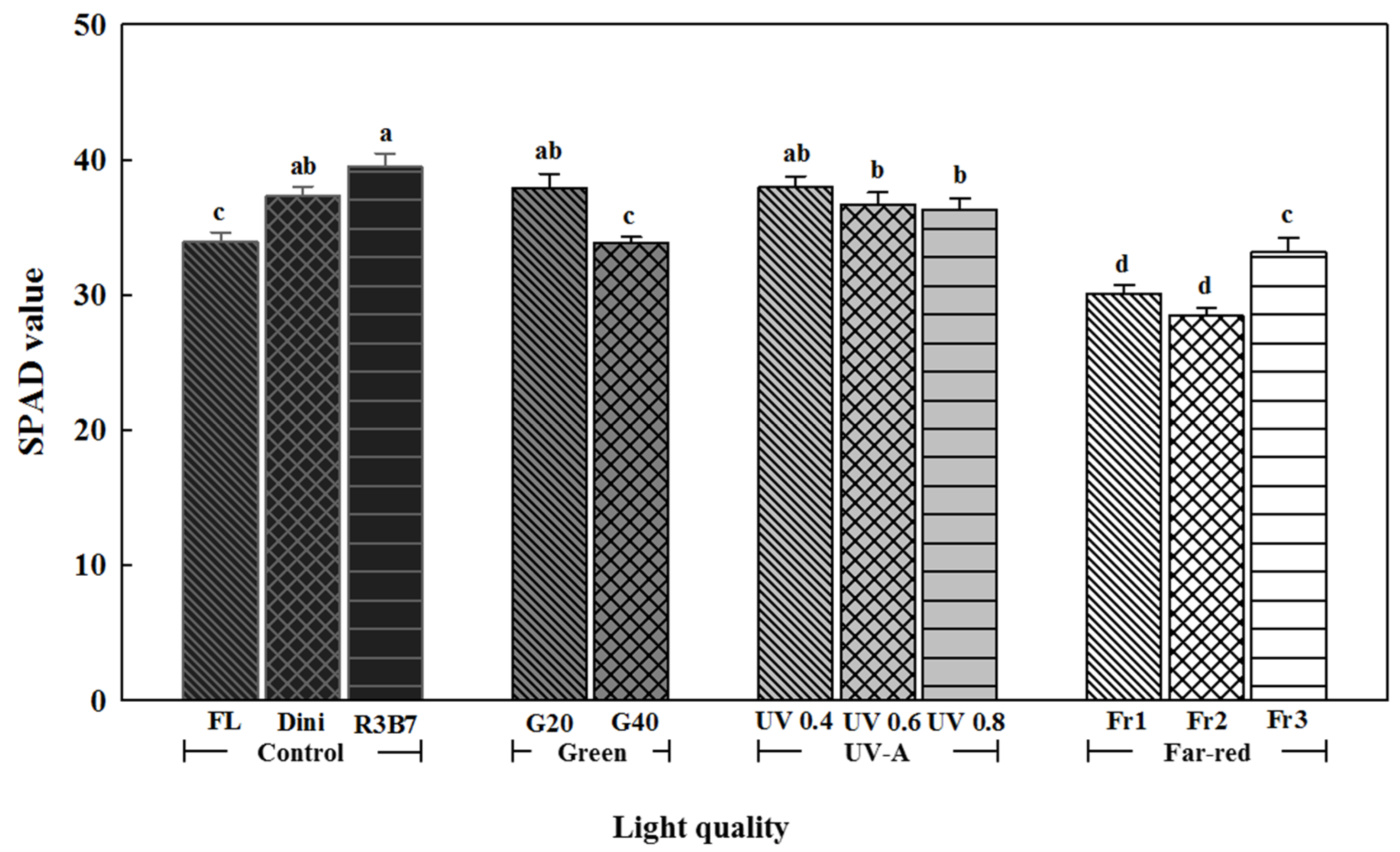
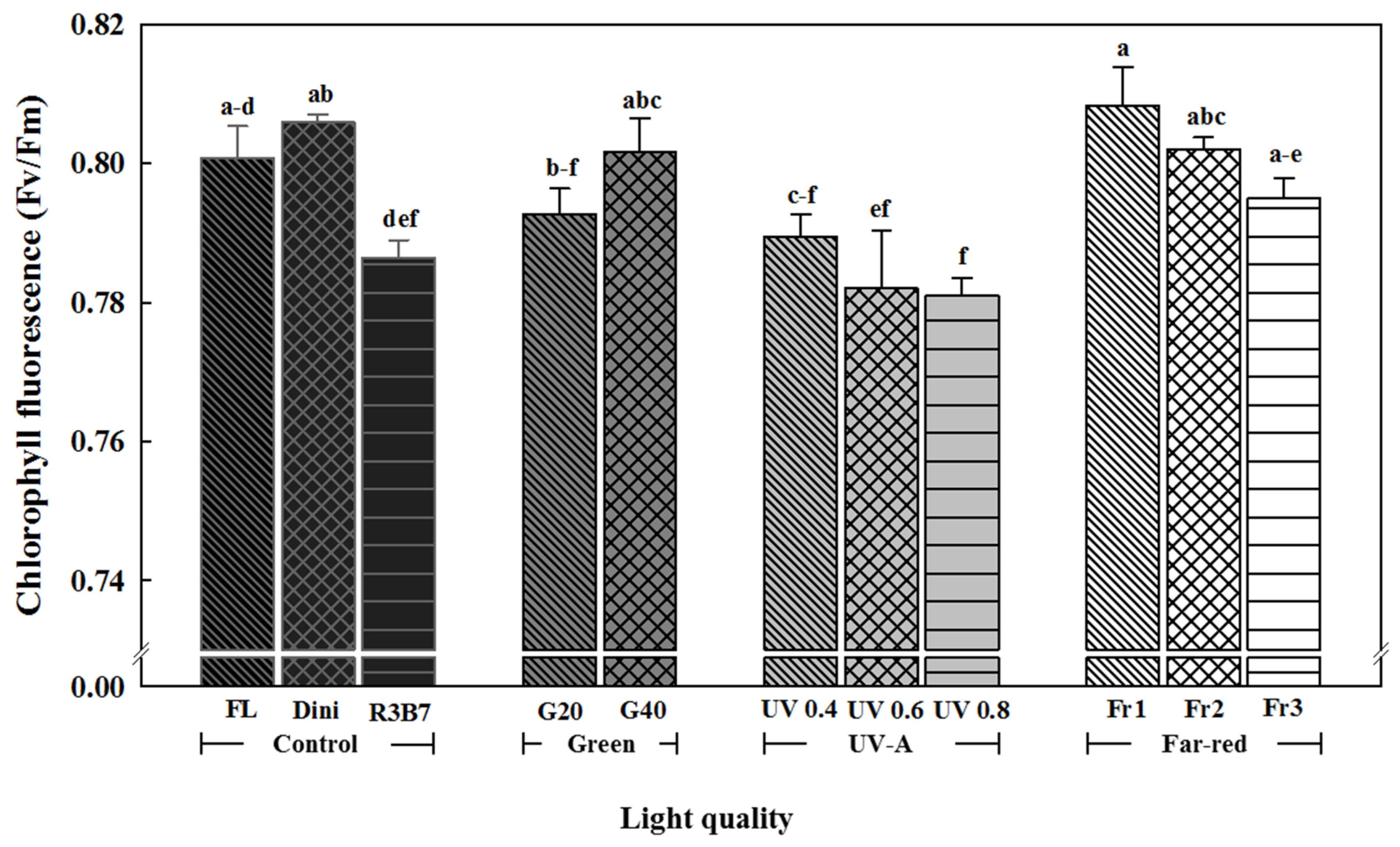
3.1. Growth Characteristics
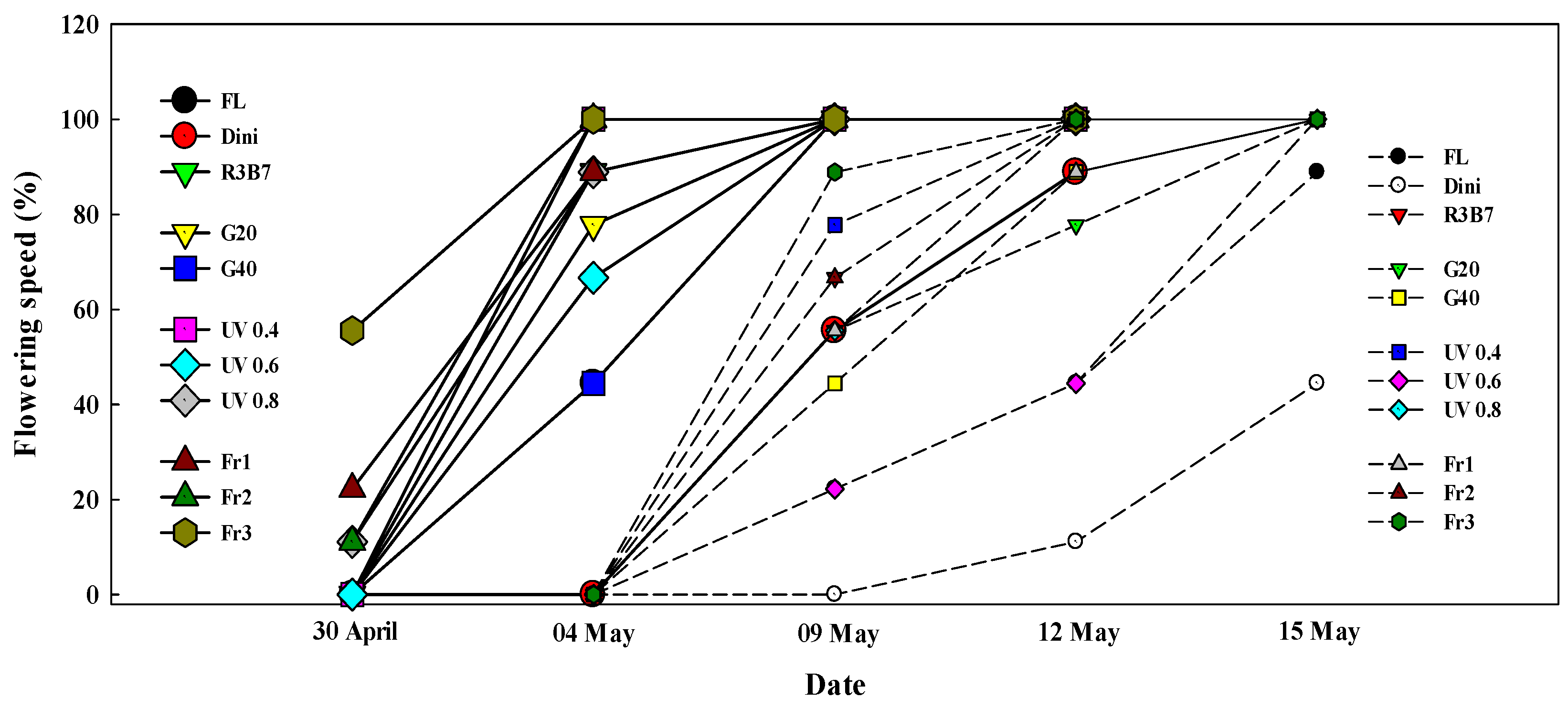
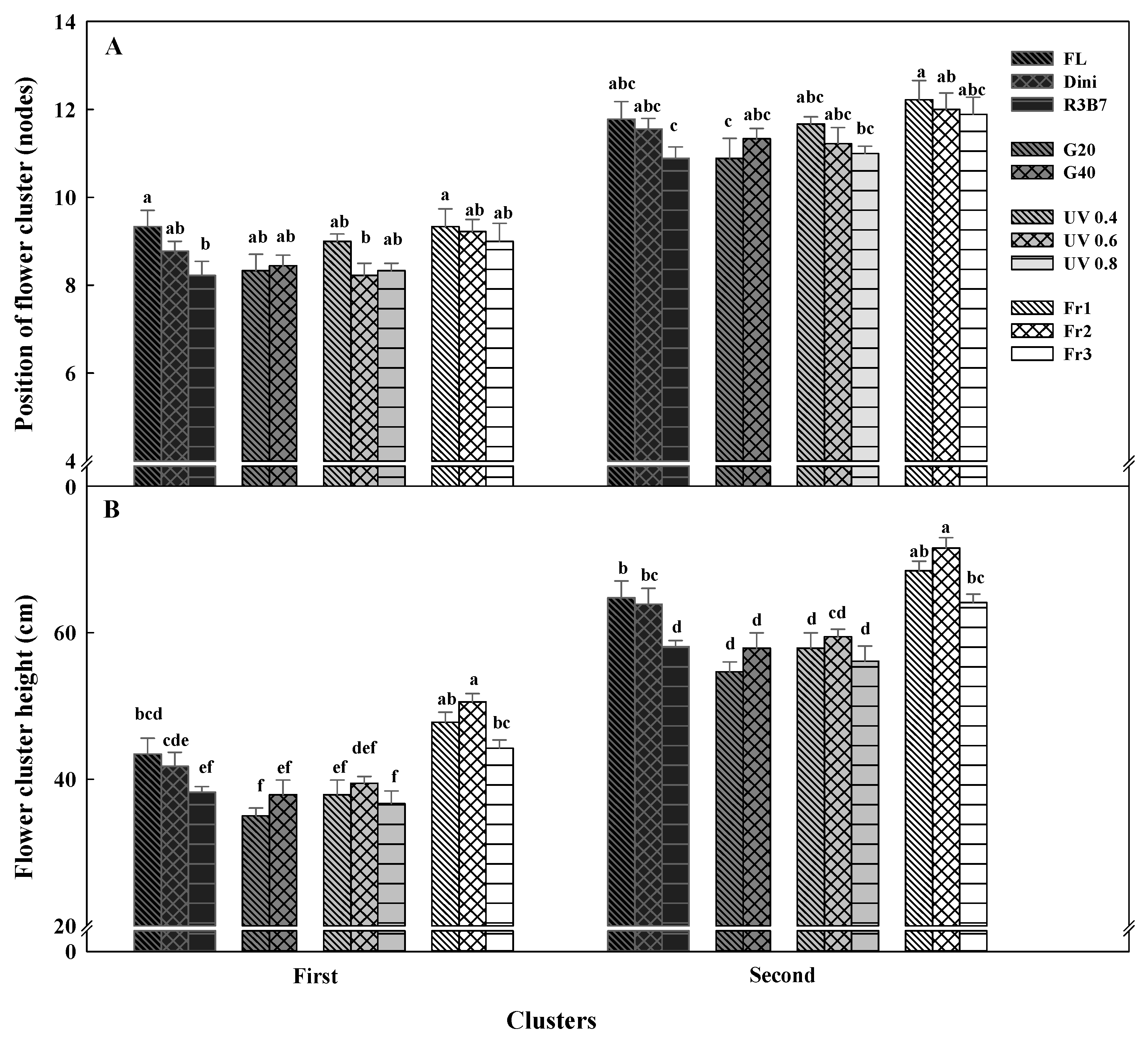
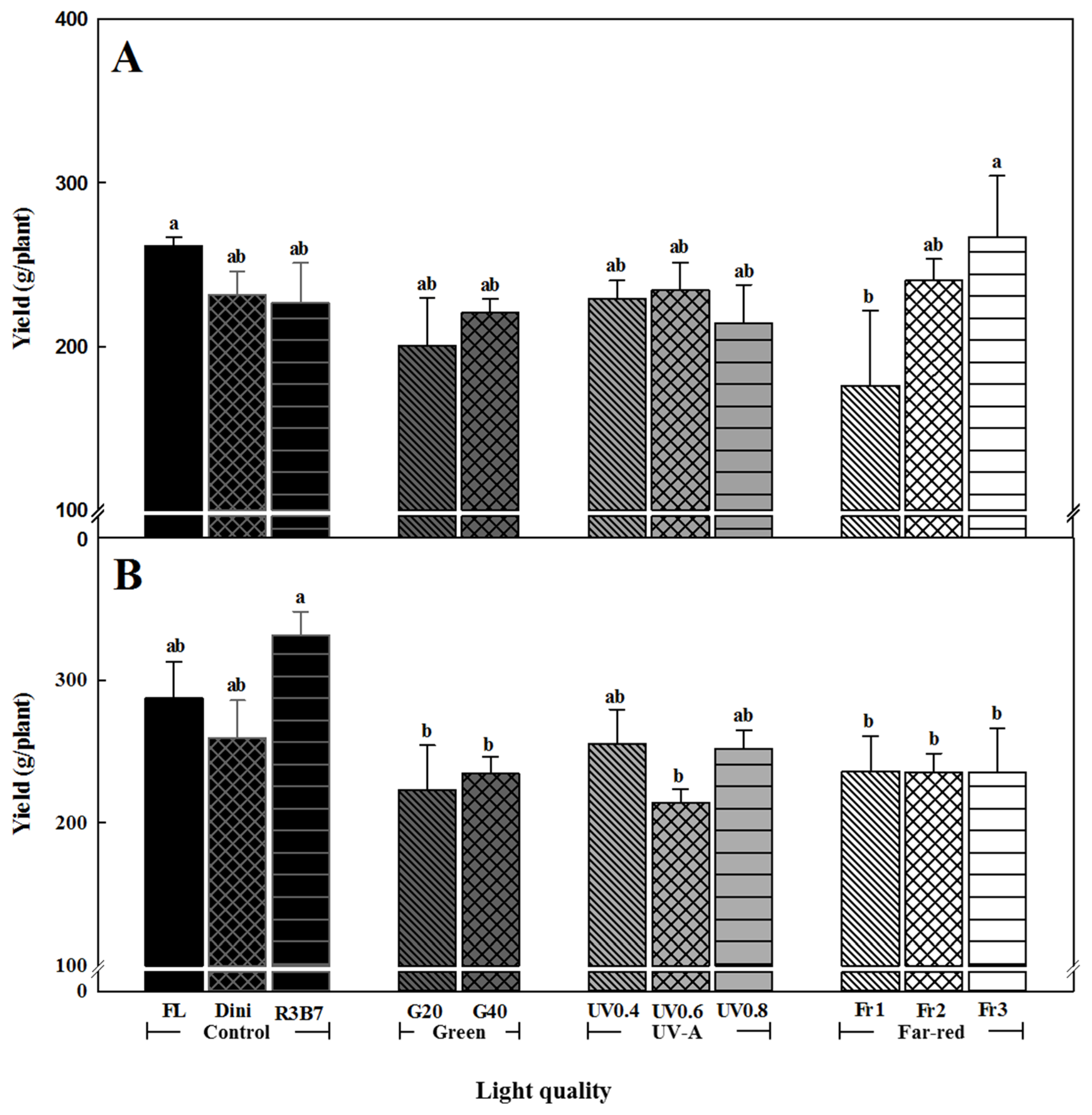
3.2. Development of Tomato Plants after Transplanting
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jenkins, G.I. The UV-B photoreceptor UVR8: From structure to physiology. Plant Cell 2014, 26, 21–37. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies and Vegetation Processes; Wiley: Chichester, UK, 1979. [Google Scholar]
- Shiga, T.; Shoji, K.; Shimada, H.; Hashida, S.N.; Goto, F.; Yoshihara, T. Effect of light quality on rosmarinic acid content and antioxidant activity of sweet basil, Ocimum basilicum L. Plant Biotechnol. 2009, 26, 255–259. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Kim, H.R.; You, Y.H. Effects of red, blue, white, and far-red LED source on growth responses of Wasabia japonica seedlings in plant factory. Korean J. Hortic. Sci. Technol. 2013, 31, 415–422. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hort. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Verdaguer, D.; Jansen, M.A.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.S.; Murthy, H.N.; Heo, J.W.; Hahn, E.J.; Paek, K.Y. The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiol. Plant 2008, 30, 339–343. [Google Scholar] [CrossRef]
- Klein, R.M. Effects of green light on biological systems. Biol. Rev. 1992, 67, 199–284. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Nishio, J.N.; Vogelmann, T.C. Green light drives CO2 fixation deep within leaves. Plant Cell Physiol. 1998, 39, 1020–1026. [Google Scholar] [CrossRef]
- Folta, K.M. Green light stimulates early stem elongation, antagonizing light-mediated growth inhibition. Plant Physiol. 2004, 135, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Weerheim, K.; Schipper, R.; Dieleman, J.A. Partial replacement of red and blue by green light increases biomass and yield in tomato. Sci. Hortic. 2019, 249, 271–279. [Google Scholar] [CrossRef]
- Robson, T.M.; Klem, K.; Urban, O.; Jansen, M.A. Re-interpreting plant morphological responses to UV-B radiation. Plant Cell Environ. 2015, 38, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Bornman, J.F.; Reuber, S.; Cen, Y.-P.; Weissenböck, G. Ultraviolet radiation as a stress factor and the role of protective pigments. In Plants and UV-B: Responses to Environmental Change; Lumsden, P.J., Ed.; Cambridge University Press: Cambridge, UK, 1997; pp. 157–168. [Google Scholar]
- Burchard, P.; Bilger, W.; Weissenböck, G. Contribution of hydroxycinnamates and flavonoids to epidermal shielding of UV-A and UV-B radiation in developing rye primary leaves as assessed by ultraviolet-induced chlorophyll fluorescence measurements. Plant Cell Environ. 2000, 23, 1373–1380. [Google Scholar] [CrossRef]
- Bilger, W.; Johnsen, T.; Schreiber, U. UV-excited chlorophyll fluorescence as a tool for the assessment of UV-protection by the epidermis of plants. J. Exp. Bot. 2001, 52, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Kolb, C.; Käser, M.; Kopecký, J.; Zotz, G.; Riederer, M.; Pfündel, E. Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiol. 2001, 127, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, J.-P.; Jungblut, T.P.; Heller, W.; Köfferlein, M.; Hutzler, P.; Heinzmann, U.; Schmelzer, E.; Ernst, D.; Langebartels, C.; Sandermann, H., Jr. Tissue localization of UV-B-screening pigments and of chalcone synthase mRNA in needles of scots pine seedlings. New Phytol. 1996, 132, 247–258. [Google Scholar] [CrossRef]
- Schmitz-Hoerner, R.; Weissenböck, G. Contribution of phenolic compounds to the UV-B screening capacity of developing barley primary leaves in relation to DNA damage and repair under elevated UV-B levels. Phytochemistry 2003, 64, 243–255. [Google Scholar] [CrossRef]
- McKenzie, R.; Smale, D.; Kotkamp, M. Relationship between UVB and erythemally weighted radiation. Photochem. Photobiol. Sci. 2004, 3, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Nagatani, A. Phytochrome: Structural basis for its functions. Curr. Opin. Plant Biol. 2010, 13, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Quail, P.H. Phytochromes. Curr. Biol. 2010, 20, R503–R504. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Baek, G.Y.; Kwon, S.J.; Yoon, Y.C.; Kim, H.T. Effect of LED light wavelength on lettuce growth, vitamin C and anthocyanin contents. Prot. Hortic. Plant Fact. 2014, 23, 19–25. [Google Scholar] [CrossRef]
- Im, J.U.; Yoon, Y.C.; Seo, K.W.; Kim, K.H.; Moon, A.K.; Kim, H.T. Effect of LED light wavelength on chrysanthemum growth. Prot. Hortic. Plant Fact. 2013, 22, 49–54. [Google Scholar] [CrossRef]
- Lee, J.E.; Shin, Y.S.; Do, H.W.; Cheung, J.D.; Kang, Y.H. Effect of seedling quality and growth after transplanting of Korean melon nursed under LED light sources and intensity. Prot. Hortic. Plant Fact. 2016, 4, 294–301. [Google Scholar] [CrossRef]
- Sonneveld, C.; Straver, N. Nutrient Solutions for Vegetables and Flower Grow in Water on Substrates; Research Station for Floriculture and Glasshouse Vegetables: Aalsmeer/Naaldwijk, The Netherlands, 1994; Volume 8, p. 45. [Google Scholar]
- Banerjee, R.; Schleicher, E.; Meier, S.; Viana, R.M.; Pokorny, R.; Ahmad, M.; Bittl, R.; Batschauer, A. The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone. J. Biol. Chem. 2007, 282, 14916–14922. [Google Scholar] [CrossRef]
- Bouly, J.P.; Schleicher, E.; Dionisio-Sese, M.; Vandenbussche, F.; Van Der Straeten, D.; Bakrim, N.; Meier, S.; Batschauer, A.; Galland, P.; Bittl, R. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J. Biol. Chem. 2007, 282, 9383–9391. [Google Scholar] [CrossRef]
- Zhang, C.; Chun, I.; Park, Y.; Kim, I. Effect of timings and light intensities of supplemental red light on the growth characteristics of cucumber and tomato plug seedlings. J. Bio-Environ. 2003, 12, 173–179. [Google Scholar]
- Son, K.H.; Park, J.H.; Kim, D.I.; Oh, M.M. Leaf shape index, growth, and phytochemicals in two leaf lettuce cultivars grown under monochromatic light-emitting diodes. Korean J. Hortic. Sci. Technol. 2012, 30, 664–672. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.M.; Hwang, S.J. Growth and phytochemical contents of ice plant as affected by light quality in a closed-type plant production system. Korean J. Hortic. Sci. Technol. 2016, 34, 878–885. [Google Scholar]
- Cosgrove, D.J. Rapid suppression of growth by blue light: Occurrence, time course, and general characteristics. Plant Physiol. 1981, 67, 584–590. [Google Scholar] [CrossRef]
- Gaba, V.; Black, M. Photocontrol of hypocotyl elongation in de-etiolated Cucumis sativus L. rapid responses to blue light. Photochem. Photobiol. 1983, 38, 469–472. [Google Scholar] [CrossRef]
- Meijer, G. Rapid growth inhibition of gherkin hypocotyls by blue light. Acta Bot. Neerl. 1968, 17, 9–14. [Google Scholar] [CrossRef]
- Chen, X.L.; Guo, W.Z.; Xue, X.Z.; Wang, L.C.; Qiao, X.J. Growth and quality responses of ‘Green Oak Leaf’ lettuce as affected by monochromic or mixed radiation provided by fluorescent lamp (FL) and light-emitting diode (LED). Sci. Hortic. 2014, 172, 168–175. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Kataria, S.; Guruprasad, K.N. Intraspecific variations in growth, yield and photosynthesis of sorghum varieties to ambient UV (280–400 nm) radiation. Plant Sci. 2012, 196, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.B.; Kirby, J.; Naxakis, G.; Pearson, S. Substantial UV-B-mediated induction of essential oils in sweet basil (Ocimum basilicum L.). Phytochemistry 1999, 51, 507–510. [Google Scholar] [CrossRef]
- Dai, Q.J.; Peng, S.B.; Chavez, A.Q.; Vergara, B.S. Intraspecific responses of 188 rice cultivars to enhanced UV-B radiation. Environ. Exp. Bot. 1994, 34, 422–433. [Google Scholar] [CrossRef]
- Li, Y.; Zu, Y.Q.; Chen, H.Y.; Chen, J.J.; Yang, J.L.; Hu, Z.D. Intraspecific responses in crop growth and yield of 20 wheat cultivars to enhanced ultraviolet-B radiation under field conditions. Field Crops Res. 2000, 67, 25–33. [Google Scholar]
- Li, Y.; Zu, Y.Q.; Chen, J.J.; Chen, H.Y. Intraspecific responses in crop growth and yield of 20 soybean cultivars to enhanced ultraviolet-B radiation under field conditions. Field Crops Res. 2002, 78, 1–8. [Google Scholar]
- Hidema, J.; Kumagai, T. Sensitivity of rice to ultraviolet-B radiation. Ann. Bot. 2006, 97, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, L.; Zu, Y. Intraspecific variation in sensitivity to ultraviolet-B radiation in endogenous hormones and photosynthetic characteristics of 10 wheat cultivars grown under field conditions. S. Afr. J. Bot. 2010, 76, 493–498. [Google Scholar] [CrossRef]
- Page, E.R.; Tollenaar, M.; Lee, E.A.; Lukens, L.; Swanton, C.J. Shade avoidance: An integral component of crop-weed competition. Weed Res. 2010, 50, 281–288. [Google Scholar] [CrossRef]
- Afifi, M.; Swanton, C. Maize seed and stem roots differ in response to neigh-boring weeds. Weed Res. 2011, 51, 442–450. [Google Scholar] [CrossRef]
- Zhang, L.; Allen, L.H.; Vaughan, M.M.; Hauser, B.A.; Boote, K.J. Solar ultraviolet radiation exclusion increases soybean internode lengths and plant height. Agric. For. Meteorol. 2014, 184, 170–178. [Google Scholar] [CrossRef]
- Schuerger, A.C.; Brown, C.S.; Stryjewski, E.C. Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light. Ann. Bot. 1997, 79, 273–282. [Google Scholar] [CrossRef]
- Tsukaya, H. Leaf shape: Genetic controls and environmental factors. Int. J. Dev. Biol. 2004, 49, 547–555. [Google Scholar] [CrossRef]
- Kwon, J.K.; Lee, J.H.; Choi, Y.H.; Yu, I.H.; Hwang, G.C. Effects of UV-B and growth inhibitor on overgrowth retardation and growth and yield after planting in fruit-vegetable plug seedlings. Prot. Hortic. Plant Fact. 2003, 12, 252–258. [Google Scholar]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Lötscher, M.; Nösberger, J. Branch and root formation in Trifolium repens is influenced by the light environment of unfolded leaves. Oecologia 1997, 111, 499–504. [Google Scholar] [CrossRef]
- Wan, C.G.; Sosebee, R.E. Tillering responses to red: Far-red light ratio during different phenological stages in Eragrostis curvula. Environ. Exp. Bot. 1998, 40, 247–254. [Google Scholar] [CrossRef]
- Linkosalo, T.; Lechowicz, M.J. Twilight far-red treatment advances leaf bud burst of silver birch (Betula pendula). Tree Physiol. 2006, 26, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, S.A.; Krishnareddy, S.R.; Kebrom, T.H.; Casal, J.J. Phytochrome regulation of branching in Arabidopsis. Plant Physiol. 2010, 152, 1914–1927. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, C.C.; Trupkin, S.A.; Ghiglione, H.; Slafer, G.; Casal, J.J. Low red/far-red ratios delay spike and stem growth in wheat. J. Exp. Bot. 2010, 61, 3151–3162. [Google Scholar] [CrossRef]
- Reed, J.W.; Nagpal, P.; Poole, D.S.; Furuya, M.; Chory, J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 1993, 5, 147–157. [Google Scholar] [CrossRef]
- Devlin, P.F.; Patel, S.R.; Whitelam, G.C. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell 1998, 10, 1479–1487. [Google Scholar] [CrossRef]
- Kozuka, T.; Horiguchi, G.; Kim, G.T.; Ohgishi, M.; Sakai, T.; Tsukaya, H. The different growth responses of the Arabidopsis thaliana leaf blade and the petiole during shade avoidance are regulated by photoreceptors and sugar. Plant Cell Physiol. 2005, 46, 213–223. [Google Scholar] [CrossRef]
- Keller, M.M.; Jaillais, Y.; Pedmale, U.V.; Moreno, J.E.; Chory, J.; Ballaré, C.L. Cryptochrome 1 and phytochrome B control shade-avoidance responses in Arabidopsis via partially independent hormonal cascades. Plant J. 2011, 67, 195–207. [Google Scholar] [CrossRef]
- Givnish, T.J. Adaptation to sun and shade: A whole-plant perspective. Aust. J. Plant Physiol. 1988, 15, 63–92. [Google Scholar] [CrossRef]
- Smith, H.; Whitelam, G.C. The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant Cell Environ. 1997, 20, 840–844. [Google Scholar] [CrossRef]
- Yano, S.; Terashima, I. Separate localization of light signal perception for sun or shade type chloroplast and palisade tissue differentiation in Chenopodium album. Plant Cell Physiol. 2001, 42, 1303–1310. [Google Scholar] [CrossRef]
- Cha, M.K.; Cho, J.H.; Cho, Y.Y. Growth of leaf lettuce as affected by light quality of LED in closed-type plant factory system. Prot. Hortic. Plant Fact. 2013, 12, 291–297. [Google Scholar] [CrossRef]
- Cookson, S.J.; Granier, C. A dynamic analysis of the shade-induced plasticity in Arabidopsis thaliana rosette leaf development reveals new components of the shade-adaptative response. Ann. Bot. 2005, 97, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Oh, M.M. Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hort. Environ. Biotechnol. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Victório, C.P.; Leal-Costa, M.V.; Schwartz Tavares, E.; Machado Kuster, R.; Salgueiro Lage, C.L. Effects of supplemental UV-A on the development, anatomy and metabolite production of Phyllanthus tenellus cultured in vitro. Photochem. Photobiol. 2011, 87, 685–689. [Google Scholar] [CrossRef]
- Barreiro, R.; Guiamét, J.J.; Beltrano, J.; Montaldi, E.R. Regulation of the photosynthetic capacity of primary bean leaves by the red:far-red ratio and photosynthetic photon flux density of incident light. Physiol. Plant 1992, 85, 97–101. [Google Scholar] [CrossRef]
- Pushnik, J.C.; Miller, G.W.; Jolley, V.D.; Brown, J.C.; Davis, T.D.; Barnes, A.M. Influences of ultra-violet (UV)-blue light radiation on the growth of cotton. II. Photosynthesis, leaf anatomy, and iron reduction. J. Plant Nutr. 1987, 10, 2283–2297. [Google Scholar] [CrossRef]
- Kasperbauer, M.J.; Peaslee, D.E. Morphology and photosynthetic efficiency of tobacco leaves that received end-of-day red or far red light during development. Plant Physiol. 1973, 52, 440–442. [Google Scholar] [CrossRef]
- Boardman, N.K. Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Physiol. 1977, 28, 355–377. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Takaki, M.; Azevedo, R.A. Plant pigments: The many faces of light perception. Acta Physiol. Plant 2011, 33, 241–248. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Son, K.H.; Kim, E.Y.; Oh, M.M. Growth and development of cherry tomato seedlings grown under various combined ratios of red to blue LED lights and fruit yield and quality after transplanting. Prot. Hortic. Plant Fact. 2018, 27, 54–63. [Google Scholar] [CrossRef]
- Lee, H.B.; An, S.K.; Lee, S.Y.; Kim, K.S. Vegetative growth characteristics of Phalaenopsis and Doritaenopsis plants under different artificial lighting sources. Hortic. Sci. Technol. 2017, 35, 21–29. [Google Scholar]
- Teramura, A.H.; Ziska, L.H.; Sztein, A.E. Changes in growth and photosynthetic capacity of rice with increased UV-B radiation. Physiol. Plant 1991, 83, 373–380. [Google Scholar] [CrossRef]
- Kim, H.Y. Effect of UV-B radiation on growth and pigments in gourd (Lagenaria siceraria) plant. J. NERI 2004, 9, 39–43. [Google Scholar]
- Kim, H.C.; Cho, Y.H.; Ku, Y.G.; Bae, J.H. Seedling qualities of hot pepper according to seedling growth periods and growth and yield after planting. Korean J. Hortic. Sci. Technol. 2015, 33, 839–844. [Google Scholar] [CrossRef]
- Kim, H.M.; Kang, J.H.; Jeong, B.R.; Hwang, S.J. Light quality and photoperiod affect growth of sowthistle (Ixeris dentata Nakai) in a closed-type plant production system. Korean J. Hortic. Sci. Technol. 2016, 34, 67–76. [Google Scholar] [CrossRef]
- Kitaya, Y.; Niu, G.; Kozai, T.; Ohashi, M. Photosynthetic photon flux, photoperiod, and CO2 concentration affect growth and morphology of lettuce plug transplants. HortScience 1998, 33, 988–991. [Google Scholar] [CrossRef]
- Kang, S.B.; Jang, H.I.; Lee, I.B.; Park, J.M.; Moon, D.K. Effect of waterlogging condition on the photosynthesis of ‘Campbell Early’ grapevine. Korean J. Hortic. Sci. Technol. 2008, 26, 372–379. [Google Scholar]
- Choi, Y.H.; Kwon, J.K.; Lee, J.H.; Kang, N.J.; Cho, M.W.; Kang, J.S. Effect of night and daytime temperatures on growth and yield of paprika ‘Fiesta’ and ‘Jubilee’. J. Bio-Envrion. Control 2004, 13, 226–232. [Google Scholar]
- Calatayud, A.; Roca, D.; Martinez, P.F. Spatial–temporal variations in rose leaves under water stress conditions studied by chlorophyll fluorescence imaging. Plant Physiol. Biochem. 2006, 44, 564–573. [Google Scholar] [CrossRef]
- Demmig, B.; Björkman, O. Comparison of the effect of excessive light on chlorophyll fluorescence (77 K) and photon yield of O2 evolution in leaves of higher plants. Planta 1987, 171, 171–184. [Google Scholar] [CrossRef]
- Schreiber, U.; Bilger, W. Progress in Chlorophyll Fluorescence Research: Major Developments during the Past Years in Retrospect; Progress in Botany/Fortschritte der Botanik; Springer: Berlin/Heidelberg, Germany, 1993; pp. 151–173. [Google Scholar]
- Long, S.P.; Humphries, S.; Ealkowski, P.G. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Biol. 1994, 45, 633. [Google Scholar] [CrossRef]
- Zhang, D.H.; Wang, Q.P.; Xue, Z.Y. Photoluminescence of ZnO films excited with light of different wavelength. Appl. Surf. Sci. 2003, 207, 20–25. [Google Scholar] [CrossRef]
- Ouzounis, T.; Fretté, X.; Ottosen, C.O.; Rosenqvist, E. Spectral effects of LEDs on chlorophyll fluorescence and pigmentation in Phalaenopsis ‘Vivien’ and ‘Purple Star’. Physiol. Plant. 2015, 154, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Park, S.Y.; Oh, M.M. Growth and cell division of lettuce plants under various ratios of red to far-red light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 186–194. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Bjorn, L.O.; Bornman, J.F.; Flint, S.D.; Kulandaivelu, G.; Teramura, A.H.; Tevini, M. Effects of increased solar ultraviolet radiation on terrestrial ecosystems. Photochem. Photobiol. 1998, 46, 40–52. [Google Scholar] [CrossRef]
- Musil, C.F.; Chimphango, S.B.; Dakora, F.D. Effects of elevated ultraviolet-B radiation on native and cultivated plants of southern Africa. Ann. Bot. 2002, 90, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, K.; Ramanujam, M.P. Improvement of biomass partitioning, flowering and yield by triadimefon in UV-B stressed Vigna radiata (L.) Wilczek. Biol. Plant. 2004, 48, 145–148. [Google Scholar] [CrossRef]

| Chemical | Concentration (mg·L−1) | Chemical | Concentration (mg·L−1) |
|---|---|---|---|
| Ca(NO3)2·4H2O | 1274.4 | Fe-EDTA | 6.20 |
| KNO3 | 525.2 | H3BO3 | 1.84 |
| KH2PO4 | 204.0 | CuSO4·5H2O | 0.16 |
| MgSO4·7H2O | 590.4 | MnSO4·5H2O | 2.19 |
| NH4NO3 | 96.0 | Na2MoO4·2H2O | 0.10 |
| K2SO4 | 348.0 | ZnSO4·7H2O | 1.41 |
| Light Quality z | Fresh Weight (g) | Dry Weight (g) | ||||
|---|---|---|---|---|---|---|
| Leaf | Stem | Total | Leaf | Stem | Total | |
| FL | 1.39 d y | 1.69 de | 3.08 d | 0.12 d | 0.06 c | 0.18 d |
| Dini | 0.63 e | 0.76 f | 1.39 e | 0.05 e | 0.03 d | 0.08 e |
| R3B7 | 2.20 ab | 1.53 de | 3.74 c | 0.19 ab | 0.07 c | 0.27 bc |
| G20 | 1.76 c | 1.47 e | 3.23 cd | 0.15 bcd | 0.07 c | 0.22 cd |
| G40 | 1.66 cd | 1.79 d | 3.45 cd | 0.14 cd | 0.07 c | 0.21 cd |
| UV 0.4 | 1.99 bc | 1.43 e | 3.42 cd | 0.17 bcd | 0.06 c | 0.23 bc |
| UV 0.6 | 1.97 bc | 1.72 de | 3.69 c | 0.17 bc | 0.07 c | 0.23 c |
| UV 0.8 | 1.90 bc | 1.66 de | 3.55 cd | 0.16 bcd | 0.07 c | 0.22 c |
| Fr1 | 2.37 a | 3.92 a | 6.29 a | 0.23 a | 0.20 a | 0.43 a |
| Fr2 | 1.88 c | 3.04 b | 4.92 b | 0.16 bcd | 0.13 b | 0.29 b |
| Fr3 | 2.02 b | 2.59 c | 4.61 b | 0.18 bc | 0.11 c | 0.29 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.M.; Hwang, S.J. The Growth and Development of ‘Mini Chal’ Tomato Plug Seedlings Grown under Various Wavelengths Using Light Emitting Diodes. Agronomy 2019, 9, 157. https://doi.org/10.3390/agronomy9030157
Kim HM, Hwang SJ. The Growth and Development of ‘Mini Chal’ Tomato Plug Seedlings Grown under Various Wavelengths Using Light Emitting Diodes. Agronomy. 2019; 9(3):157. https://doi.org/10.3390/agronomy9030157
Chicago/Turabian StyleKim, Hye Min, and Seung Jae Hwang. 2019. "The Growth and Development of ‘Mini Chal’ Tomato Plug Seedlings Grown under Various Wavelengths Using Light Emitting Diodes" Agronomy 9, no. 3: 157. https://doi.org/10.3390/agronomy9030157
APA StyleKim, H. M., & Hwang, S. J. (2019). The Growth and Development of ‘Mini Chal’ Tomato Plug Seedlings Grown under Various Wavelengths Using Light Emitting Diodes. Agronomy, 9(3), 157. https://doi.org/10.3390/agronomy9030157





