Assessment of Ultrasound Assisted Extraction as an Alternative Method for the Extraction of Anthocyanins and Total Phenolic Compounds from Maqui Berries (Aristotelia chilensis (Mol.) Stuntz)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Chemicals and Reagents
2.3. Ultrasound-Assisted Extraction Procedure
2.4. Identification of Anthocyanins
2.5. Detection of Anthocyanins
2.6. Total Phenolic Content (TPC)
2.7. Response Surface Regression Analysis
2.8. Statistical Analysis
3. Results and Discussion
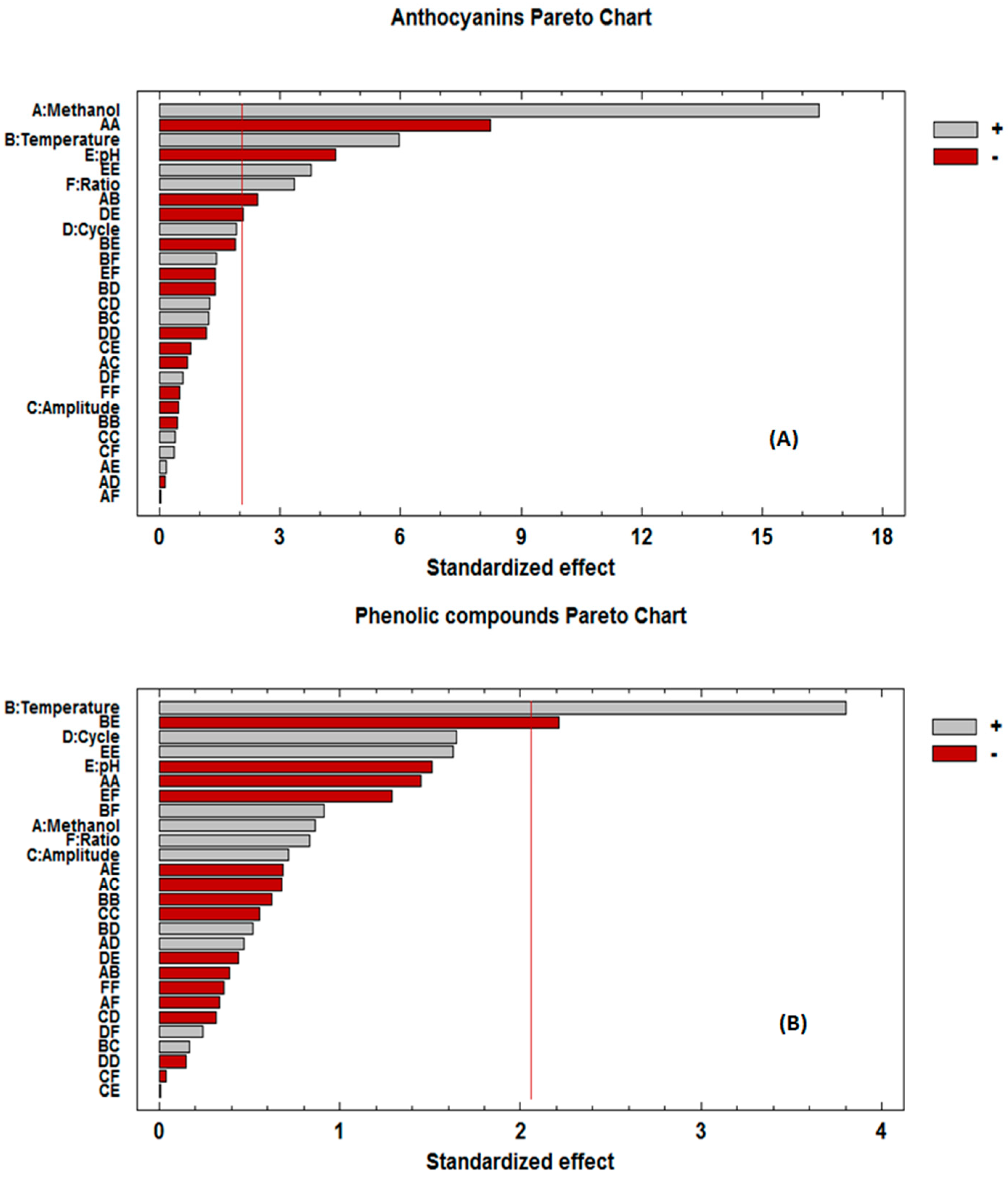
3.1. Fitting the Model of the Extraction Process
3.2. Optimal Conditions
3.3. Extraction Kinetics
3.4. Repeatability and Intermediate Precision
3.5. Application to Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Belhachat, D.; Mekimene, L.; Belhachat, M.; Ferradji, A.; Aid, F. Application of response surface methodology to optimize the extraction of essential oil from ripe berries of Pistacia lentiscus using ultrasonic pretreatment. J. Appl. Res. Med. Aromat. Plants 2018, 9, 132–140. [Google Scholar] [CrossRef]
- Fredes, C.; Robert, P. The powerful colour of the maqui (Aristotelia chilensis [Mol.] Stuntz) fruit. J. Berry Res. 2014, 4, 175–182. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Mena, P.; García-Viguera, C.; Moreno, D.A. A novel beverage rich in antioxidant phenolics: Maqui berry (Aristotelia chilensis) and lemon juice. LWT Food Sci. Technol. 2012, 47, 279–286. [Google Scholar] [CrossRef]
- Fredes, C.; Yousef, G.G.; Robert, P.; Grace, M.H.; Lila, M.A.; Gómez, M.; Gebauer, M.; Montenegro, G. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [Mol.] Stuntz) from different geographical regions in Chile. J. Sci. Food Agric. 2014, 94, 2639–2648. [Google Scholar] [CrossRef]
- Brauch, J.E.; Buchweitz, M.; Schweiggert, R.M.; Carle, R. Detailed analyses of fresh and dried maqui (Aristotelia chilensis (Mol.) Stuntz) berries and juice. Food Chem. 2016, 190, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, S.; Vasudeva, N. Review on antioxidants and evaluation procedures. Chin. J. Integr. Med. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Georgantzi, C.; Lioliou, A.E.; Paterakis, N.; Makris, D.P. Combination of Latic Acid-Based Deep Eutectic Solvents (DES) with β-Cyclodextrin: Performance Screening Using Ultrasound-Assisted Extraction of Polyphenols from Selected Native Greek Medicinal Plants. Agronomy 2017, 7, 54. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Alcalde-Eon, C.; Muñoz, O.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanins in berries of Maqui (Aristotelia chilensis (Mol.) Stuntz). Phytochem. Anal. 2006, 17, 8–14. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Campos-Requena, V. Antioxidant Compound Extraction from Maqui (Aristotelia chilensis [Mol] Stuntz) Berries: Optimization by Response Surface Methodology. Antioxidants 2017, 6, 10. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernandez-Lopez, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (Aristotelia chilensis). LWT Food Sci. Technol. 2015, 64, 1057–1062. [Google Scholar] [CrossRef]
- Zúñiga, G.E.; Tapia, A.; Arenas, A.; Contreras, R.A.; Zúñiga-Libano, G. Phytochemistry and biological properties of Aristotelia chilensis a Chilean blackberry: A review. Phytochem. Rev. 2017, 16, 1081–1094. [Google Scholar] [CrossRef]
- Brauch, J.E.; Reuter, L.; Conrad, J.; Vogel, H.; Schweiggert, R.M.; Carle, R. Characterization of anthocyanins in novel Chilean maqui berry clones by HPLC–DAD–ESI/MSnand NMR-spectroscopy. J. Food Compos. Anal. 2017, 58, 16–22. [Google Scholar] [CrossRef]
- Cespedes, C.L.; Pavon, N.; Dominguez, M.; Alarcon, J.; Balbontin, C.; Kubo, I.; El-Hafidi, M.; Avila, J.G. The chilean superfruit black-berry Aristotelia chilensis (Elaeocarpaceae), Maqui as mediator in inflammation-associated disorders. Food Chem. Toxicol. 2016, 108, 438–450. [Google Scholar] [CrossRef]
- Manning, L. Food fraud: Policy and food chain. Curr. Opin. Food Sci. 2016, 10, 16–21. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; Huisman, W.; Luning, P.A. Food fraud vulnerability and its key factors. Trends Food Sci. Technol. 2017, 67, 70–75. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Castejón, N.; Luna, P.; Señoráns, F.J. Alternative oil extraction methods from Echium plantagineum L. seeds using advanced techniques and green solvents. Food Chem. 2018, 244, 75–82. [Google Scholar] [CrossRef]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.T. Influence of Extraction Conditions on Ultrasound-Assisted Recovery of Bioactive Phenolics from Blueberry Pomace and Their Antioxidant Activity. Molecules 2018, 23, 1685. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-Assisted Extraction of Natural Products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Ali, A.; Lim, X.Y.; Chong, C.H.; Mah, S.H.; Chua, B.L. Optimization of ultrasound-assisted extraction of natural antioxidants from Piper betle using response surface methodology. LWT Food Sci. Technol. 2018, 89, 681–688. [Google Scholar] [CrossRef]
- Duan, L.L.; Jiang, R.; Shi, Y.; Duan, C.Q.; Wu, G.F. Optimization of ultrasonic-assisted extraction of higher fatty acids in grape berries (seed-free fruit sections). Anal. Methods 2016, 8, 6208–6215. [Google Scholar] [CrossRef]
- Nguyen, T.N.T.; Phan, L.H.N.; Le, V.V.M. Enzyme-assisted and ultrasound-assisted extraction of phenolics from mulberry (Morus alba) fruit: Comparison of kinetic parameters and antioxidant level. Int. Food Res. J. 2014, 21, 1937–1940. [Google Scholar]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Yue, P.X. Optimization of Ultrasound-Assisted Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, E.; Abedi, M. Efficient Ultrasound-Assisted Extraction of Cichoric Acid from Echinacea purpurea Root. Pharm. Chem. J. 2017, 51, 471–475. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Ross, K.A.; Beta, T.; Arntfield, S.D. A comparative study on the phenolic acids identified and quantified in dry beans using HPLC as affected by different extraction and hydrolysis methods. Food Chem. 2009, 113, 336–344. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Chen, S.X.; Li, K.K.; Pubu, D.; Jiang, S.P.; Chen, B.; Chen, L.R.; Yang, Z.; Ma, C.; Gong, X.J. Optimization of Ultrasound-Assisted Extraction, HPLC and UHPLC-ESI-Q-TOF-MS/MS Analysis of Main Macamides and Macaenes from Maca (Cultivars of Lepidium meyenii Walp). Molecules 2017, 22, 2196. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qu, J.; Luo, S.; Feng, S.; Li, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Optimization of Ultrasound-Assisted Extraction of Flavonoids from Olive (Olea europaea) Leaves, and Evaluation of Their Antioxidant and Anticancer Activities. Molecules 2018, 23, 2513. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Chandra-Pradhan, R.; Mishra, S.; Rout, P.K. Quantification and Concentration of Anthocyanidin from Indian Blackberry (Jamun) by Combination of Ultra- and Nano-filtrations. Food Bioprocess Technol. 2018, 11, 2194–2203. [Google Scholar] [CrossRef]
- Tardugno, R.; Pozzebon, M.; Beggio, M.; Del Turco, P.; Pojana, G. Polyphenolic profile of Cichorium intybus L. endemic varieties from the Veneto region of Italy. Food Chem. 2018, 266, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Friščić, M.; Bucar, F.; Hazler-Pilepic, K. LC-PDA-ESI-MSn analysis of phenolic and iridoid compounds from Globularia spp. J. Mass Spectrom. 2016, 51, 1211–1236. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Covarrubias-Cárdenas, A.G.; Martínez-Castillo, J.I.; Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; García-Cruz, N.U.; Pacheco, N. Antioxidant Capacity and UPLC-PDA ESI-MS Phenolic Profile of Stevia rebaudiana Dry Powder Extracts Obtained by Ultrasound Assisted Extraction. Agronomy 2018, 8, 170. [Google Scholar] [CrossRef]
- Jakobek, L.; Boc, M.; Barron, A.R. Optimization of Ultrasonic-Assisted Extraction of Phenolic Compounds from Apples. Food Anal. Methods 2015, 8, 2612–2625. [Google Scholar] [CrossRef]
- Haya, S.; Bentahar, F.; Trari, M. Optimization of polyphenols extraction from orange peel. J. Food Meas. Charact. 2019, 13, 614–621. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandao, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.C.; Chin, N.L.; Yusof, Y.A. A Box-Behnken Design for Determining the Optimum Experimental Condition of Cake Batter Mixing. Food Bioprocess Technol. 2012, 5, 972–982. [Google Scholar] [CrossRef]
- He, Q.; Du, B.; Xu, B. Extraction Optimization of Phenolics and Antioxidants from Black Goji Berry by Accelerated Solvent Extractor Using Response Surface Methodology. Appl. Sci. 2018, 8, 1905. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J.; Peñas, E. Response surface optimization of germination conditions to improve the accumulation of bioactive compounds and the antioxidant activity in quinoa. Int. J. Food Sci. Technol. 2018, 53, 516–524. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Li, Q.; Jin, W.; Chen, W.; Han, J.; Zhang, Y. Simultaneous Optimization for Ultrasound-Assisted Extraction and Antioxidant Activity of Flavonoids from Sophora flavescens Using Response Surface Methodology. Molecules 2019, 24, 112. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.P.D.F.; Pereira, A.L.D.; Barbero, G.F.; Martínez, J. Recovery of anthocyanins from residues of Rubus fruticosus, Vaccinium myrtillus and Eugenia brasiliensis by ultrasound assisted extraction, pressurized liquid extraction and their combination. Food Chem. 2017, 231, 1–10. [Google Scholar] [CrossRef]
- Ruenroengklin, N.; Zhong, J.; Duan, X.; Yang, B.; Li, J.; Jiang, Y. Effects of Various Temperatures and pH Values on the Extraction Yield of Phenolics from Litchi Fruit Pericarp Tissue and the Antioxidant Activity of the Extracted Anthocyanins. Int. J. Mol. Sci. 2008, 9, 1333–1341. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Heffels, P.; Weber, F.; Schieber, A. Influence of Accelerated Solvent Extraction and Ultrasound-Assisted Extraction on the Anthocyanin Profile of Different Vaccinium Species in the Context of Statistical Models for Authentication. J. Agric. Food Chem. 2015, 63, 7532–7538. [Google Scholar] [CrossRef]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016, 197, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Stability of 40 Phenolic Compounds during Ultrasound-Assisted Extraction (UAE). AIP Conf. Proc. 2016, 1755, 080009. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.J.; Qian, L.S.; Sun, W.J.; Zhang, J.S.; Yang, Y.; Li, N.; Zhuang, H.N.; We, D. Ultrasound-Assisted Extraction of Polysaccharides from Volvariella volvacea: Process Optimization and Structural Characterization. Molecules 2018, 23, 1706. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, K.; Ah-Hen, K.S.; Vega-Gálvez, A.; Vásquez, V.; Quispe-Fuentes, I.; Rojas, P.; Lemus-Mondaca, R. Changes in bioactive components and antioxidant capacity of maqui, Aristotelia chilensis [Mol] Stuntz, berries during drying. LWT Food Sci. Technol. 2016, 65, 537–542. [Google Scholar] [CrossRef]
- AOAC. AOAC Peer Verified Methods Program. In Manual on Policies and Procedures; AOAC International: Rockville, MD, USA, 1998. [Google Scholar]
- Fracassetti, D.; Del Bo, C.; Simonetti, P.; Gardana, C.; Klimis-Zacas, D.; Ciappellano, S. Effect of Time and Storage Temperature on Anthocyanin Decay and Antioxidant Activity in Wild Blueberry (Vaccinium angustifolium) Powder. J. Agric. Food Chem. 2013, 61, 2999–3005. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Bary, S.; Zhou, W. Changes in the color, chemical stability and antioxidant capacity of thermally treated anthocyanin aqueous solution over storage. Food Chem. 2016, 192, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.C.; Guiné, R.P.F.; Gonçalves, F.J.A. Evaluation of phenolic compounds, antioxidant activity and bioaccessibility in white crowberry (Corema album). J. Food Meas. Charact. 2017, 11, 1936–1946. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; Espada-Bellido, E.; González de Peredo, A.V.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of Microwave-Assisted Extraction for the Recovery of Bioactive Compounds from the Chilean Superfruit (Aristotelia chilensis (Mol.) Stuntz). Agronomy 2018, 8, 240. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Prieto, M.A.; Vazquez, J.A.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Recovery of bioactive compounds from Arbutus unedo L. fruits: Comparative optimization study of maceration/microwave/ultrasound extraction techniques. Food Res. Int. 2018, 109, 455–471. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerate-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef]
- Verbeyst, L.; Van Crombruggen, K.; Van der Plancken, I.; Hendrickx, M.; Van Loey, A. Anthocyanin degradation kinetics during thermal and high-pressure treatments of raspberries. J. Food Eng. 2011, 105, 513–521. [Google Scholar] [CrossRef]
- Corrales, M.; Butz, P.; Tauscher, B. Anthocyanin condensation reactions under high hydrostatic pressure. Food Chem. 2008, 110, 627–635. [Google Scholar] [CrossRef]
- Kovacevic, D.B.; Maras, M.; Barba, F.J.; Granato, D.; Roohinejad, S.; Mallikarjunan, K.; Montesano, D.; Lorenzo, J.M.; Putnik, P. Innovative technologies for the recovery of phytochemicals from Stevia rebaudiana Bertoni leaves: A review. Food Chem. 2018, 268, 513–521. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Maullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]

| Run | Factors | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| Solvent X1 | Temp.* X2 | Amplitude X3 | Cycle X4 | pH X5 | Ratio X6 | Total Anthocyanins (mg g−1) | Total Phenolic Compounds (mg g−1) | |
| 1 | 50 | 40 | 30 | 0.45 | 2 | 10 | 34.21 | 37.10 |
| 2 | 50 | 40 | 70 | 0.45 | 2 | 10 | 34.48 | 38.49 |
| 3 | 50 | 40 | 30 | 0.45 | 7 | 10 | 31.29 | 44.45 |
| 4 | 50 | 40 | 70 | 0.45 | 7 | 10 | 31.00 | 47.68 |
| 5 | 50 | 40 | 30 | 0.45 | 2 | 20 | 38.17 | 50.36 |
| 6 | 50 | 40 | 70 | 0.45 | 2 | 20 | 40.00 | 55.15 |
| 7 | 50 | 40 | 30 | 0.45 | 7 | 20 | 33.19 | 48.39 |
| 8 | 50 | 40 | 70 | 0.45 | 7 | 20 | 31.88 | 51.44 |
| 9 | 50 | 10 | 50 | 0.2 | 2 | 15 | 29.96 | 38.07 |
| 10 | 50 | 70 | 50 | 0.2 | 2 | 15 | 36.47 | 60.46 |
| 11 | 50 | 10 | 50 | 0.7 | 2 | 15 | 33.86 | 35.18 |
| 12 | 50 | 70 | 50 | 0.7 | 2 | 15 | 38.77 | 71.07 |
| 13 | 50 | 10 | 50 | 0.2 | 7 | 15 | 30.73 | 38.89 |
| 14 | 50 | 70 | 50 | 0.2 | 7 | 15 | 35.54 | 46.32 |
| 15 | 50 | 10 | 50 | 0.7 | 7 | 15 | 31.35 | 41.21 |
| 16 | 50 | 70 | 50 | 0.7 | 7 | 15 | 31.12 | 44.11 |
| 17 | 25 | 40 | 30 | 0.2 | 4.5 | 15 | 21.11 | 39.51 |
| 18 | 75 | 40 | 30 | 0.2 | 4.5 | 15 | 31.95 | 37.39 |
| 19 | 25 | 40 | 70 | 0.2 | 4.5 | 15 | 21.20 | 40.57 |
| 20 | 75 | 40 | 70 | 0.2 | 4.5 | 15 | 31.22 | 38.26 |
| 21 | 25 | 40 | 30 | 0.7 | 4.5 | 15 | 21.20 | 41.96 |
| 22 | 75 | 40 | 30 | 0.7 | 4.5 | 15 | 33.66 | 46.65 |
| 23 | 25 | 40 | 70 | 0.7 | 4.5 | 15 | 25.17 | 45.97 |
| 24 | 75 | 40 | 70 | 0.7 | 4.5 | 15 | 35.10 | 39.14 |
| 25 | 50 | 10 | 30 | 0.45 | 4.5 | 10 | 30.53 | 47.25 |
| 26 | 50 | 70 | 30 | 0.45 | 4.5 | 10 | 34.19 | 37.92 |
| 27 | 50 | 10 | 70 | 0.45 | 4.5 | 10 | 26.92 | 46.70 |
| 28 | 50 | 70 | 70 | 0.45 | 4.5 | 10 | 32.26 | 45.80 |
| 29 | 50 | 10 | 30 | 0.45 | 4.5 | 20 | 31.11 | 36.72 |
| 30 | 50 | 70 | 30 | 0.45 | 4.5 | 20 | 36.91 | 42.25 |
| 31 | 50 | 10 | 70 | 0.45 | 4.5 | 20 | 27.17 | 41.08 |
| 32 | 50 | 70 | 70 | 0.45 | 4.5 | 20 | 37.19 | 41.07 |
| 33 | 25 | 10 | 50 | 0.45 | 2 | 15 | 21.56 | 35.93 |
| 34 | 75 | 10 | 50 | 0.45 | 2 | 15 | 35.88 | 44.42 |
| 35 | 25 | 70 | 50 | 0.45 | 2 | 15 | 28.78 | 51.74 |
| 36 | 75 | 70 | 50 | 0.45 | 2 | 15 | 36.10 | 56.54 |
| 37 | 25 | 10 | 50 | 0.45 | 7 | 15 | 21.79 | 35.11 |
| 38 | 75 | 10 | 50 | 0.45 | 7 | 15 | 35.34 | 37.37 |
| 39 | 25 | 70 | 50 | 0.45 | 7 | 15 | 24.80 | 47.58 |
| 40 | 75 | 70 | 50 | 0.45 | 7 | 15 | 33.68 | 46.77 |
| 41 | 25 | 40 | 50 | 0.2 | 4.5 | 10 | 20.26 | 37.54 |
| 42 | 75 | 40 | 50 | 0.2 | 4.5 | 10 | 31.89 | 39.78 |
| 43 | 25 | 40 | 50 | 0.7 | 4.5 | 10 | 19.91 | 38.76 |
| 44 | 75 | 40 | 50 | 0.7 | 4.5 | 10 | 32.76 | 48.22 |
| 45 | 25 | 40 | 50 | 0.2 | 4.5 | 20 | 20.08 | 37.56 |
| 46 | 75 | 40 | 50 | 0.2 | 4.5 | 20 | 34.21 | 39.58 |
| 47 | 25 | 40 | 50 | 0.7 | 4.5 | 20 | 23.60 | 43.52 |
| 48 | 75 | 40 | 50 | 0.7 | 4.5 | 20 | 34.09 | 47.47 |
| 49 | 50 | 40 | 50 | 0.45 | 4.5 | 15 | 33.66 | 43.97 |
| 50 | 50 | 40 | 50 | 0.45 | 4.5 | 15 | 31.30 | 46.44 |
| 51 | 50 | 40 | 50 | 0.45 | 4.5 | 15 | 34.37 | 44.78 |
| 52 | 50 | 40 | 50 | 0.45 | 4.5 | 15 | 33.64 | 45.63 |
| 53 | 50 | 40 | 50 | 0.45 | 4.5 | 15 | 29.16 | 44.08 |
| 54 | 50 | 40 | 50 | 0.45 | 4.5 | 15 | 31.90 | 46.76 |
| (A) | ||||||
| Source | Degrees of Freedom | Sum of Squares | Mean Square | F-Value | P-Value | Coefficient |
| Model | 27 | 1429.49 | 52.94 | 18.40 | 0.0000 | |
| Intercept | 1 | 32.34 | ||||
| X1 | 1 | 775.46 | 775.46 | 269.44 | 0.0000 | 5.68 |
| X2 | 1 | 102.53 | 102.53 | 35.62 | 0.0000 | 2.07 |
| X3 | 1 | 0.6324 | 0.6324 | 0.2197 | 0.6431 | −0.1623 |
| X4 | 1 | 10.63 | 10.63 | 3.69 | 0.0657 | 0.6654 |
| X5 | 1 | 55.61 | 55.61 | 19.32 | 0.0002 | −1.52 |
| X6 | 1 | 32.51 | 32.51 | 11.29 | 0.0024 | 1.16 |
| X1 × 2 | 1 | 17.06 | 17.06 | 5.93 | 0.0221 | −1.46 |
| X1 × 3 | 1 | 1.40 | 1.40 | 0.4851 | 0.4923 | −0.4178 |
| X1 × 4 | 1 | 0.0503 | 0.0503 | 0.0175 | 0.8958 | −0.0561 |
| X1 × 5 | 1 | 0.0783 | 0.0783 | 0.0272 | 0.8703 | 0.0989 |
| X1 × 6 | 1 | 0.0027 | 0.0027 | 0.0009 | 0.9759 | 0.0183 |
| X2 × 3 | 1 | 4.34 | 4.34 | 1.51 | 0.2304 | 0.7366 |
| X2 × 4 | 1 | 5.52 | 5.52 | 1.92 | 0.1780 | −0.8304 |
| X2 × 5 | 1 | 10.44 | 10.44 | 3.63 | 0.0680 | −0.8077 |
| X2 × 6 | 1 | 5.80 | 5.80 | 2.02 | 0.1676 | 0.8515 |
| X3 × 4 | 1 | 4.58 | 4.58 | 1.59 | 0.2181 | 0.7570 |
| X3 × 5 | 1 | 1.71 | 1.71 | 0.5942 | 0.4477 | −0.4624 |
| X3 × 6 | 1 | 0.3622 | 0.3622 | 0.1259 | 0.7256 | 0.1505 |
| X4 × 5 | 1 | 12.55 | 12.55 | 4.36 | 0.0467 | −1.25 |
| X4 × 6 | 1 | 1.03 | 1.03 | 0.3591 | 0.5542 | 0.3594 |
| X5 × 6 | 1 | 5.60 | 5.60 | 1.95 | 0.1749 | −0.8366 |
| X12 | 1 | 194.81 | 194.81 | 67.69 | 0.0000 | −4.35 |
| X22 | 1 | 0.6133 | 0.6133 | 0.2131 | 0.6482 | −0.2442 |
| X32 | 1 | 0.4469 | 0.4469 | 0.1553 | 0.6968 | 0.2084 |
| X42 | 1 | 3.92 | 3.92 | 1.36 | 0.2539 | −0.6172 |
| X52 | 1 | 41.13 | 41.13 | 14.29 | 0.0008 | 2.00 |
| X62 | 1 | 0.7366 | 0.7366 | 0.2559 | 0.6172 | −0.2676 |
| Residual | 26 | 74.83 | 2.88 | |||
| Lack of fit | 21 | 55.92 | 2.66 | 0.7042 | 0.7421 | |
| Pure error | 5 | 18.91 | 3.78 | |||
| Total | 53 | 1504.32 | ||||
| (B) | ||||||
| Source | Degrees of Freedom | Sum of Squares | Mean Square | F−Value | P−Value | Coefficient |
| Model | 27 | 1417.49 | 52.50 | 1.41 | 0.1926 | |
| Intercept | 1 | 45.28 | ||||
| X1 | 1 | 27.83 | 27.83 | 0.7467 | 0.3954 | 1.08 |
| X2 | 1 | 538.78 | 538.78 | 14.46 | 0.0008 | 4.74 |
| X3 | 1 | 19.06 | 19.06 | 0.5114 | 0.4809 | 0.8911 |
| X4 | 1 | 101.19 | 101.19 | 2.72 | 0.1114 | 2.05 |
| X5 | 1 | 85.09 | 85.09 | 2.28 | 0.1428 | −1.88 |
| X6 | 1 | 25.79 | 25.79 | 0.6920 | 0.4131 | 1.04 |
| X1 × 2 | 1 | 5.72 | 5.72 | 0.1535 | 0.6984 | −0.8455 |
| X1 × 3 | 1 | 17.15 | 17.15 | 0.4604 | 0.5035 | −1.46 |
| X1 × 4 | 1 | 8.19 | 8.19 | 0.2199 | 0.6431 | 0.7156 |
| X1 × 5 | 1 | 17.47 | 17.47 | 0.4689 | 0.4995 | −1.48 |
| X1 × 6 | 1 | 4.11 | 4.11 | 0.1103 | 0.7425 | −0.7168 |
| X2 × 3 | 1 | 1.04 | 1.04 | 0.0278 | 0.8688 | 0.3600 |
| X2 × 4 | 1 | 10.06 | 10.06 | 0.2699 | 0.6078 | 1.12 |
| X2 × 5 | 1 | 182.28 | 182.28 | 4.89 | 0.0360 | −3.38 |
| X2 × 6 | 1 | 31.00 | 31.00 | 0.8318 | 0.3701 | 1.97 |
| X3 × 4 | 1 | 3.69 | 3.69 | 0.0990 | 0.7555 | −0.6791 |
| X3 × 5 | 1 | 0.0017 | 0.0017 | 0.0000 | 0.9947 | 0.0145 |
| X3 × 6 | 1 | 0.0520 | 0.0520 | 0.0014 | 0.9705 | −0.0570 |
| X4 × 5 | 1 | 7.23 | 7.23 | 0.1940 | 0.6632 | −0.9506 |
| X4 × 6 | 1 | 2.21 | 2.21 | 0.0592 | 0.8096 | 0.5252 |
| X5 × 6 | 1 | 61.66 | 61.66 | 1.65 | 0.2097 | −2.78 |
| X12 | 1 | 78.29 | 78.29 | 2.10 | 0.1592 | −2.76 |
| X22 | 1 | 14.43 | 14.43 | 0.3873 | 0.5391 | −1.18 |
| X32 | 1 | 11.50 | 11.50 | 0.3086 | 0.5833 | −1.06 |
| X42 | 1 | 0.8004 | 0.8004 | 0.0215 | 0.8846 | −0.2790 |
| X52 | 1 | 98.81 | 98.81 | 2.65 | 0.1155 | 3.10 |
| X62 | 1 | 4.86 | 4.86 | 0.1305 | 0.7208 | −0.6876 |
| Residual | 26 | 968.87 | 37.26 | |||
| Lack of fit | 21 | 961.79 | 45.80 | 32.34 | 0.0006 | |
| Pure error | 5 | 7.08 | 1.42 | |||
| Total | 53 | 2386.36 | ||||
| (A) Total Anthocyanins | (B) Total Phenolic Compounds | |
|---|---|---|
| Percentage of methanol (%) | 61.5 | 50 |
| pH | 2.1 | 2 |
| Ratio (mL:g) | 20:0.5 | 20:0.5 |
| Temperature (°C) | 69.4 | 70 |
| Amplitude (%) | 46 | 35 |
| Cycle (s) | 0.7 | 0.7 |
| Foodstuff Made with Maqui | Total Anthocyanins (mg g−1) | Total Phenolic Compounds (mg g−1) | ||
|---|---|---|---|---|
| UAE | MAE | UAE | MAE | |
| M-1 | 2.13 ± 0.16 a | 1.73 ± 0.16 b | 6.83 ± 0.23 a | 8.22 ± 0.34 b |
| M-2 | 78.73 ± 0.67 a | 75.55 ± 3.80 a | 100.27 ± 1.44 a | 103.30 ± 0.30 a |
| M-3 | -* | -* | 10.46 ± 0.29 a | 11.45 ± 0.45 b |
| M-4 | 30.81 ± 3.13 a | 30.35 ± 3.25 a | 47.25 ± 1.44 a | 53.06 ± 1.53 b |
| M-5 | 28.39 ± 1.72 a | 27.66 ± 1.02 a | 43.40 ± 1.99 a | 49.29 ± 2.17 b |
| M-6 | 37.26 ± 2.08 a | 35.51 ± 1.40 a | 58.28 ± 1.33 a | 59.57 ± 0.70 a |
| M-7 | 23.21 ± 0.73 a | 19.89 ± 1.44 b | 50.95 ± 1.38 a | 52.13 ± 1.44 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Espinosa, M.; V. González de Peredo, A.; Ferreiro-González, M.; Carrera, C.; Palma, M.; F. Barbero, G.; Espada-Bellido, E. Assessment of Ultrasound Assisted Extraction as an Alternative Method for the Extraction of Anthocyanins and Total Phenolic Compounds from Maqui Berries (Aristotelia chilensis (Mol.) Stuntz). Agronomy 2019, 9, 148. https://doi.org/10.3390/agronomy9030148
Vázquez-Espinosa M, V. González de Peredo A, Ferreiro-González M, Carrera C, Palma M, F. Barbero G, Espada-Bellido E. Assessment of Ultrasound Assisted Extraction as an Alternative Method for the Extraction of Anthocyanins and Total Phenolic Compounds from Maqui Berries (Aristotelia chilensis (Mol.) Stuntz). Agronomy. 2019; 9(3):148. https://doi.org/10.3390/agronomy9030148
Chicago/Turabian StyleVázquez-Espinosa, Mercedes, Ana V. González de Peredo, Marta Ferreiro-González, Ceferino Carrera, Miguel Palma, Gerardo F. Barbero, and Estrella Espada-Bellido. 2019. "Assessment of Ultrasound Assisted Extraction as an Alternative Method for the Extraction of Anthocyanins and Total Phenolic Compounds from Maqui Berries (Aristotelia chilensis (Mol.) Stuntz)" Agronomy 9, no. 3: 148. https://doi.org/10.3390/agronomy9030148
APA StyleVázquez-Espinosa, M., V. González de Peredo, A., Ferreiro-González, M., Carrera, C., Palma, M., F. Barbero, G., & Espada-Bellido, E. (2019). Assessment of Ultrasound Assisted Extraction as an Alternative Method for the Extraction of Anthocyanins and Total Phenolic Compounds from Maqui Berries (Aristotelia chilensis (Mol.) Stuntz). Agronomy, 9(3), 148. https://doi.org/10.3390/agronomy9030148











