Estimating Arsenic Mobility and Phytotoxicity Using Two Different Phosphorous Fertilizer Release Rates in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Soil Analysis
2.3. Phytotoxicity Test with Bok Choy
2.4. Statistical Analysis
3. Results
3.1. Basic Properties of Soil and Amendsments
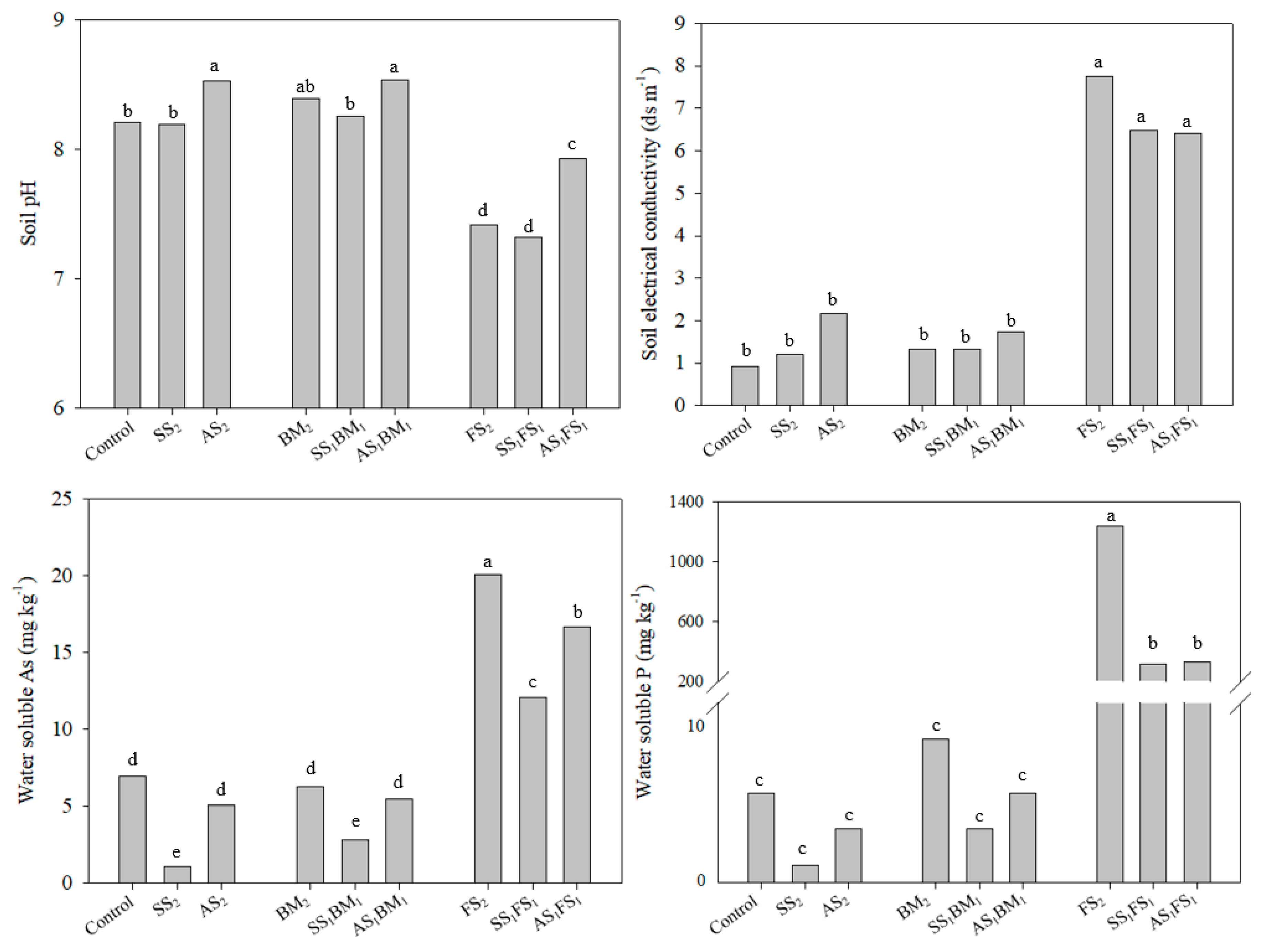
3.2. Effects of the Amendments on Soil Chemical Characteristics
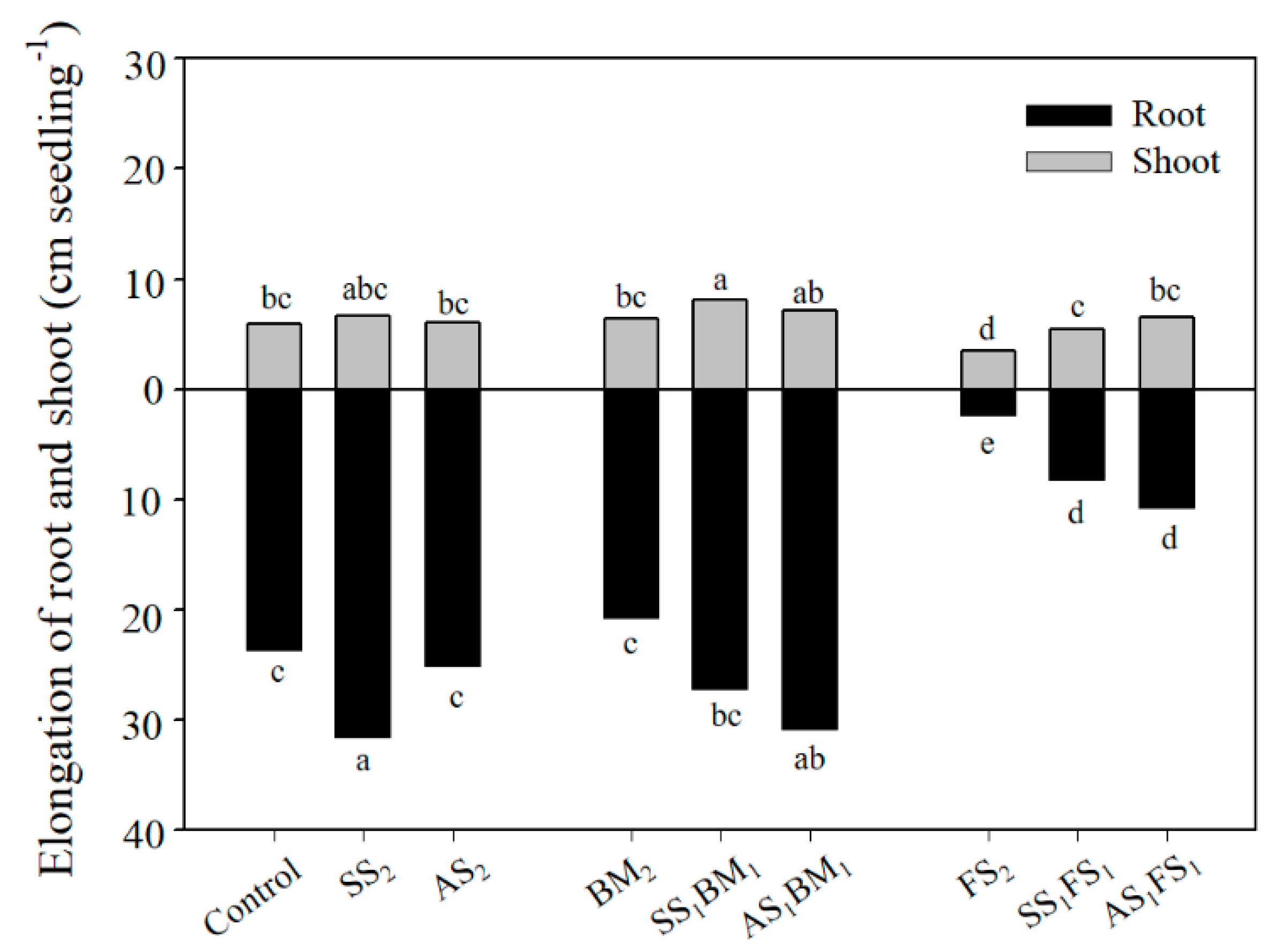
3.3. Elongation of Bok Choy Roots and Shoots
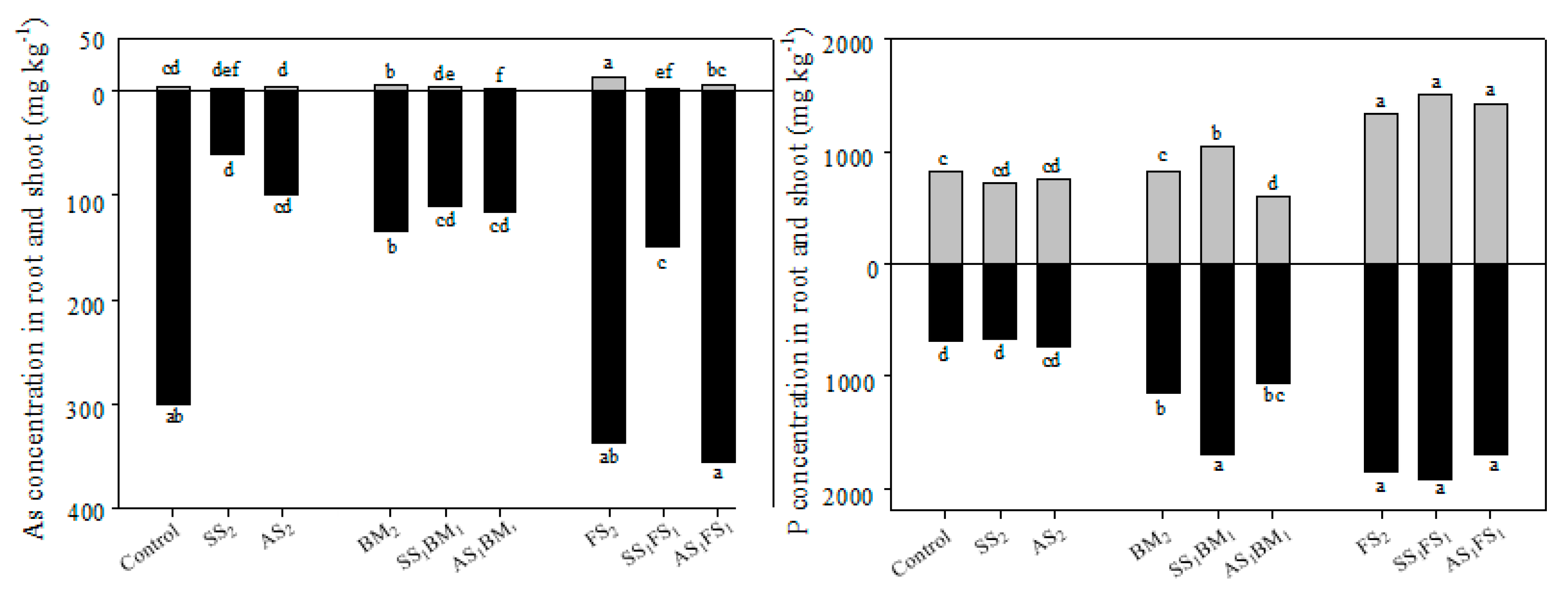
3.4. As and P Uptakes by Bok Choyelongation of Bok Choy Roots and Shoots
3.5. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fitz, W.J.; Wenzel, W.W. Arsenic transformations in the soil-rhizosphere-plant system: Fundamentals and potential application to phytoremediation. J. Biotechnol. 2002, 99, 259–278. [Google Scholar] [CrossRef]
- Smith, E.; Naidu, R.; Alston, A.M. Chemistry of inorganic arsenic in soils: II. Effect of phosphorus, sodium, and calcium on arsenic sorption. J. Environ. Qual. 2002, 31, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Rahman, M.M. Arsenic contamination of Bangladesh paddy field soils: Implications for rice contribution to arsenic consumption. Environ. Sci. Technol. 2003, 37, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.G.; Naidu, R.; Alston, A. Arsenic in the Soil Environment: A review. Adv. Agron. 1998, 64, 149–195. [Google Scholar]
- Tokunaga, S.; Hakuta, T. Acid washing and stabilization of an artificial arsenic-contaminated soil. Chemosphere 2002, 46, 31–38. [Google Scholar] [CrossRef]
- Baek, K.; Kim, D.H.; Park, S.W.; Ryu, B.G.; Bajargal, T.; Yang, J.S. Electrolyte conditioning-enhanced electrokinetic remediation of arsenic-contaminated mine tailing. J. Hazard. Mater. 2009, 161, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Lee, B.T.; Kim, K.W. Arsenic stabilization in mine tailings using nano-sized magnetite and zero valent iron with the enhancement of mobility by surface coating. J. Geochem. Explor. 2012, 113, 124–129. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, E.Y.; Park, H.; Yun, J.; Kim, J.-G. In situ stabilization of arsenic and metal-contaminated agricultural soil using industrial by-products. Geoderma 2011, 161, 1–7. [Google Scholar] [CrossRef]
- Liang, Q.Q.; Zhao, D.Y. Immobilization of arsenate in a sandy loam soil using starch-stabilized magnetite nanoparticles. J. Hazard. Mater. 2014, 271, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Lien, H.L.; Koel, B.E.; Zhang, W.X. Iron nanoparticles for environmental clean-up: Recent developments and future outlook. Environ. Sci. Process. Impacts 2013, 15, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M. Arsenic chemistry in soils: An overview of thermodynamic predictions and field observations. Water Air Soil Pollut. 1997, 93, 117–136. [Google Scholar] [CrossRef]
- Komárek, M.; Vaněk, A.; Ettler, V. Chemical stabilization of metals and arsenic in contaminated soils using oxides—A review. Environ. Pollut. 2013, 172, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.P.; Alloway, B.J.; Lepp, N.W.; Singh, B.; Bochereau, F.J.; Penny, C. Field trials to assess the uptake of arsenic by vegetables from contaminated soils and soil remediation with iron oxides. Sci. Total Environ. 2003, 311, 19–33. [Google Scholar] [CrossRef]
- Mench, M.; Vangronsveld, J.; Beckx, C.; Ruttens, A. Progress in assisted natural remediation of an arsenic contaminated agricultural soil. Environ. Pollut. 2006, 144, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.W.; Dunham, S.J.; Dennis, P.G.; Zhao, F.J.; McGrath, S.P. Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud. Environ. Pollut. 2006, 142, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Koo, N.; Lee, S.H.; Kim, J.G. Arsenic mobility in the amended mine tailings and its impact on soil enzyme activity. Environ. Geochem. Health 2012, 34, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Min, H.G.; Lee, S.H.; Kim, J.G. The Effects of Various Amendments on Trace Element Stabilization in Acidic, Neutral, and Alkali Soil with Similar Pollution Index. PLoS ONE 2016, 11, e0166335. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Min, H.G.; Koo, N.; Park, J.; Lee, S.H.; Bak, G.I.; Kim, J.G. The effectiveness of spent coffee grounds and its biochar on the amelioration of heavy metals-contaminated water and soil using chemical and biological assessments. J. Environ. Manag. 2014, 146, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.S.; Kim, J.Y.; Lee, J.S.; Ko, J.I.; Kim, K.W. Arsenic immobilization in water and soil using acid mine drainage sludge. Appl. Geochem. 2013, 35, 1–6. [Google Scholar] [CrossRef]
- Ko, M.S.; Kim, J.Y.; Park, H.S.; Kim, K.W. Field assessment of arsenic immobilization in soil amended with iron rich acid mine drainage sludge. J. Clean. Prod. 2015, 108, 1073–1080. [Google Scholar] [CrossRef]
- Lee, H.; Kim, D.; Kim, J.; Ji, M.K.; Han, Y.S.; Park, Y.T.; Yun, H.S.; Choi, J. As(III) and As(V) removal from the aqueous phase via adsorption onto acid mine drainage sludge (AMDS) alginate beads and goethite alginate beads. J. Hazard. Mater. 2015, 292, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Kumpiene, J.; Bert, V.; Dimitriou, I.; Eriksson, J.; Friesl-Hanl, W.; Galazka, R.; Herzig, R.; Janssen, J.; Kidd, P.; Mench, M.; et al. Selecting chemical and ecotoxicological test batteries for risk assessment of trace element-contaminated soils (phyto)managed by gentle remediation options (GRO). Sci. Total Environ. 2014, 496, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Quintela-Sabaris, C.; Marchand, L.; Kidd, P.S.; Friesl-Hanl, W.; Puschenreiter, M.; Kumpiene, J.; Muller, I.; Neu, S.; Janssen, J.; Vangronsveld, J.; et al. Assessing phytotoxicity of trace element-contaminated soils phytomanaged with gentle remediation options at ten European field trials. Sci. Total Environ. 2017, 599, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Min, H.-G.; Lee, S.-H.; Kim, J.-G. A Comparative Study on Poaceae and Leguminosae Forage Crops for Aided Phytostabilization in Trace-Element-Contaminated Soil. Agronomy 2018, 8, 105. [Google Scholar] [CrossRef]
- Cundy, A.B.; Bardos, R.; Church, A.; Puschenreiter, M.; Friesl-Hanl, W.; Müller, I.; Neu, S.; Mench, M.; Witters, N.; Vangronsveld, J. Developing principles of sustainability and stakeholder engagement for “gentle” remediation approaches: The European context. J. Environ. Manag. 2013, 129, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Kapustka, L.A.; Bowers, K.; Isanhart, J.; Martinez-Garza, C.; Finger, S.; Stahl, R.G., Jr.; Stauber, J. Coordinating ecological restoration options analysis and risk assessment to improve environmental outcomes. Integr. Environ. Assess. Manag. 2016, 12, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Tordoff, G.M.; Baker, A.J.; Willis, A.J. Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 2000, 41, 219–228. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, Z.; Fu, S.; Mendez, A.; Gasco, G.; Paz-Ferreiro, J. Combining phytoextraction and biochar addition improves soil biochemical properties in a soil contaminated with Cd. Chemosphere 2015, 119, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Courtney, R.; Mullen, G. Use of Germination and Seedling Performance Bioassays for Assessing Revegetation Strategies on Bauxite Residue. Water Air Soil Pollut. 2009, 197, 15–22. [Google Scholar] [CrossRef]
- Koo, N.; Kim, M.-S.; Hyun, S.; Kim, J.-G. Effects of the Incorporation of Phosphorus and Iron into Arsenic-Spiked Artificial Soils on Root Growth of Lettuce using Response Surface Methodology. Commun. Soil Sci. Plant Anal. 2013, 44, 1259–1271. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.Q.; Shiralipour, A. Effects of compost and phosphate amendments on arsenic mobility in soils and arsenic uptake by the hyperaccumulator, Pteris vittata L. Environ. Pollut. 2003, 126, 157–167. [Google Scholar] [CrossRef]
- Chen, T.; Wei, C.; Huang, Z.; Huang, Q.; Lu, Q.; Fan, Z. Arsenic hyperaccumulator Pteris vittata L. and its arsenic accumulation. Chin. Sci. Bull. 2002, 47, 902–905. [Google Scholar] [CrossRef]
- Woolson, E.; Axley, J.; Kearney, P. The Chemistry and Phytotoxicity of Arsenic in Soils: II. Effects of Time and Phosphorus 1. Soil Sci. Soc. Am. J. 1973, 37, 254–259. [Google Scholar] [CrossRef]
- Kim, M.-S.; Min, H.-G.; Kim, J.-G.J.E.; Infrastructure, R. Effects of Phosphorus and Iron on the Phytotoxicity of Lettuce (Lactuca sativa L.) in Arsenic-contaminated Soil. Ecol. Resilient Infrastruct. 2018, 5, 1–10. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis Part 3—Chemical Methods; Sparks, D.L., Ed.; SSSA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Tsang, S.; Phu, F.; Baum, M.M.; Poskrebyshev, G.A. Determination of phosphate/arsenate by a modified molybdenum blue method and reduction of arsenate by S2O42−. Talanta 2007, 71, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Loeppert, R.; Inskeep, W. Iron. In Methods of Soil Analysis. Part 3; Sparks, D., Ed.; SSSA: Madison, WI, USA, 1996; pp. 639–664. [Google Scholar]
- ISO. Soil quality—Extraction of trace elements soluble in aqua regia. In ISO 11466; International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Rodriguez, R.R.; Basta, N.T.; Casteel, S.W.; Armstrong, F.P.; Ward, D.C. Chemical extraction methods to assess bioavailable arsenic in soil and solid media. J. Environ. Qual. 2003, 32, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Sikora, F.J.; Howe, P.S.; Hill, L.E.; Reid, D.C.; Harover, D.E. Comparison of colorimetric and ICP determination of phosphorus in Mehlich3 soil extracts. Commun. Soil Sci. Plant Anal. 2005, 36, 875–887. [Google Scholar] [CrossRef]
- Bernstein, L. Effects of Salinity and Sodicity on Plant-Growth. Annu. Rev. Phytopathol. 1975, 13, 295–312. [Google Scholar] [CrossRef]
- Hettiarachchi, G.; Pierzynski, G.; Ransom, M. In situ stabilization of soil lead using phosphorus. J. Environ. Qual. 2001, 30, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Duraisamy, V.P. Role of inorganic and organic soil amendments on immobilisation and phytoavailability of heavy metals: A review involving specific case studies. Aust. J. Soil Res. 2003, 41, 533–555. [Google Scholar] [CrossRef]
- Miretzky, P.; Fernandez-Cirelli, A. Phosphates for Pb immobilization in soils: A review. Environ. Chem. Lett. 2008, 6, 121–133. [Google Scholar] [CrossRef]
- Kwak, J.-H.; Park, K.; Chang, P.-C.; Liu, W.; Kim, J.-Y.; Kim, K.-W. Influence of phosphate and citric acid on the phytoextraction of as from contaminated soils. Int. J. Environ. Waste Manag. 2013, 11, 1–12. [Google Scholar] [CrossRef]
- Bolland, M.D.A.; Glencross, R.N.; Gilkes, R.J.; Kumar, V. Agronomic Effectiveness of Partially Acidulated Rock Phosphate and Fused Calcium-Magnesium Phosphate Compared with Superphosphate. Fertil. Res. 1992, 32, 169–183. [Google Scholar] [CrossRef]
- Davies, N.A.; Hodson, M.E.; Black, S. Changes in toxicity and bioavailability of lead in contaminated soils to the earthworm Eisenia fetida (Savigny 1826) after bone meal amendments to the soil. Environ. Toxicol. Chem. 2002, 21, 2685–2691. [Google Scholar] [CrossRef]
- Barth, E.; Sass, B.; Chattopadhyay, S. Evaluation of blast furnace slag as a means of reducing metal availability in a contaminated sediment for beneficial use purposes. Soil Sediment Contam. 2007, 16, 281–300. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Gratao, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the life of heavy metal-stressed plants a little easier. Funct. Plant Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef]
- Lee, S.H.; Ji, W.; Lee, W.S.; Koo, N.; Koh, I.H.; Kim, M.S.; Park, J.S. Influence of amendments and aided phytostabilization on metal availability and mobility in Pb/Zn mine tailings. J. Environ. Manag. 2014, 139, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lottermoser, B.G.; Ashley, P.M. Trace element uptake by Eleocharis equisetina (spike rush) in an abandoned acid mine tailings pond, northeastern Australia: Implications for land and water reclamation in tropical regions. Environ. Pollut. 2011, 159, 3028–3035. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Hong, C.O.; Lee, B.H.; Kim, P.J. Effect of steel-making slag as a soil amendment on arsenic uptake by radish (Raphanus sativa L.) in an upland soil. Biol. Fertil. Soils 2010, 46, 617–623. [Google Scholar] [CrossRef]
- Gunes, A.; Pilbeam, D.J.; Inal, A. Effect of arsenic–phosphorus interaction on arsenic-induced oxidative stress in chickpea plants. Plant Soil 2009, 314, 211–220. [Google Scholar] [CrossRef]
- Asher, C.J.; Reay, P.F. Arsenic Uptake by Barley Seedlings. Aust. J. Plant Physiol. 1979, 6, 459–466. [Google Scholar] [CrossRef]
- Meharg, A.A.; Macnair, M.R. Relationship between plant phosphorus status and the kinetics of arsenate influx in clones of deschampsia cespitosa (L.) beauv. that differ in their tolerance to arsenate. Plant Soil 1994, 162, 99–106. [Google Scholar] [CrossRef]
- Yadav, G.; Srivastava, P.K.; Singh, V.P.; Prasad, S.M. Light intensity alters the extent of arsenic toxicity in Helianthus annuus L. seedlings. Biol. Trace Elem. Res. 2014, 158, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Berti, W.R.; Cunningham, S.D. Phytostabilization of metals. In Phytoremediation of Toxic Metals: Using Plants to Clean-Up the Environment; Raskin, I., Ed.; John Wiley and Sons: New York, NY, USA, 2000; pp. 71–88. [Google Scholar]
- Pigna, M.; Cozzolino, V.; Violante, A.; Meharg, A.A. Influence of Phosphate on the Arsenic Uptake by Wheat (Triticum durum L.) Irrigated with Arsenic Solutions at Three Different Concentrations. Water Air Soil Pollut. 2009, 197, 371–380. [Google Scholar] [CrossRef]
| Stabilizer | Fertilizer | |||
|---|---|---|---|---|
| SS a | AS | BM | FS | |
| Control | ||||
| SS2 | 2% | |||
| AS2 | 2% | |||
| BM2 | 2% | |||
| FS2 | 2% | |||
| SS1BM1 | 1% | 1% | ||
| SS1FS1 | 1% | 1% | ||
| AS1BM1 | 1% | 1% | ||
| AS1FS1 | 1% | 1% | ||
| Unit | Soil | |
|---|---|---|
| pH | 8.2 | |
| EC a | ds m−1 | 0.9 |
| LOI b | % | 2.8 |
| T-P c | g kg−1 | 1.1 |
| Alox d | mg g−1 | 1.2 |
| Feox d | mg g−1 | 13.4 |
| Mnox d | mg g−1 | 2.0 |
| As e | mg kg−1 | 1853.7 |
| Cd e | mg kg−1 | 3.6 |
| Cu e | mg kg−1 | 49.3 |
| Pb e | mg kg−1 | 132.9 |
| Zn e | mg kg−1 | 212.3 |
| Unit | SS a | AS | BM | FS | |
|---|---|---|---|---|---|
| pH | 12.4 | 8.4 | 7.3 | 5.4 | |
| EC b | ds m−1 | 9.4 | 3.8 | 21.5 | 60.3 |
| LOI c | % | 0.3 | 20.0 | 44.1 | 32.8 |
| As d | mg kg−1 | - e | - | - | 10.9 |
| Cd d | mg kg−1 | 44.4 | 29.8 | - | - |
| Cu d | mg kg−1 | 18.6 | - | 1.9 | 18.4 |
| Pb d | mg kg−1 | 10.6 | 6.3 | - | 7.6 |
| Zn d | mg kg−1 | 204.2 | 966.5 | 116. 4 | 46.2 |
| RE a | WS-As b | WS-P c | |
|---|---|---|---|
| WS-As | −0.942 *** | ||
| WS-P | −0.830 * | 0.864 ** | |
| RootAs d | 0.169 ns | −0.090 ns | |
| RootP e | −0.780 * | 0.606 ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-S.; Min, H.-G.; Kim, J.-G.; Lee, S.-R. Estimating Arsenic Mobility and Phytotoxicity Using Two Different Phosphorous Fertilizer Release Rates in Soil. Agronomy 2019, 9, 111. https://doi.org/10.3390/agronomy9030111
Kim M-S, Min H-G, Kim J-G, Lee S-R. Estimating Arsenic Mobility and Phytotoxicity Using Two Different Phosphorous Fertilizer Release Rates in Soil. Agronomy. 2019; 9(3):111. https://doi.org/10.3390/agronomy9030111
Chicago/Turabian StyleKim, Min-Suk, Hyun-Gi Min, Jeong-Gyu Kim, and Sang-Ryong Lee. 2019. "Estimating Arsenic Mobility and Phytotoxicity Using Two Different Phosphorous Fertilizer Release Rates in Soil" Agronomy 9, no. 3: 111. https://doi.org/10.3390/agronomy9030111
APA StyleKim, M.-S., Min, H.-G., Kim, J.-G., & Lee, S.-R. (2019). Estimating Arsenic Mobility and Phytotoxicity Using Two Different Phosphorous Fertilizer Release Rates in Soil. Agronomy, 9(3), 111. https://doi.org/10.3390/agronomy9030111






