Effect of Vermicompost Application on Bioactive Properties and Antioxidant Potential of MD2 Pineapple Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Sample Extraction
2.3. Phytochemical Analysis
2.4. Determination of Chlorophyll a and b
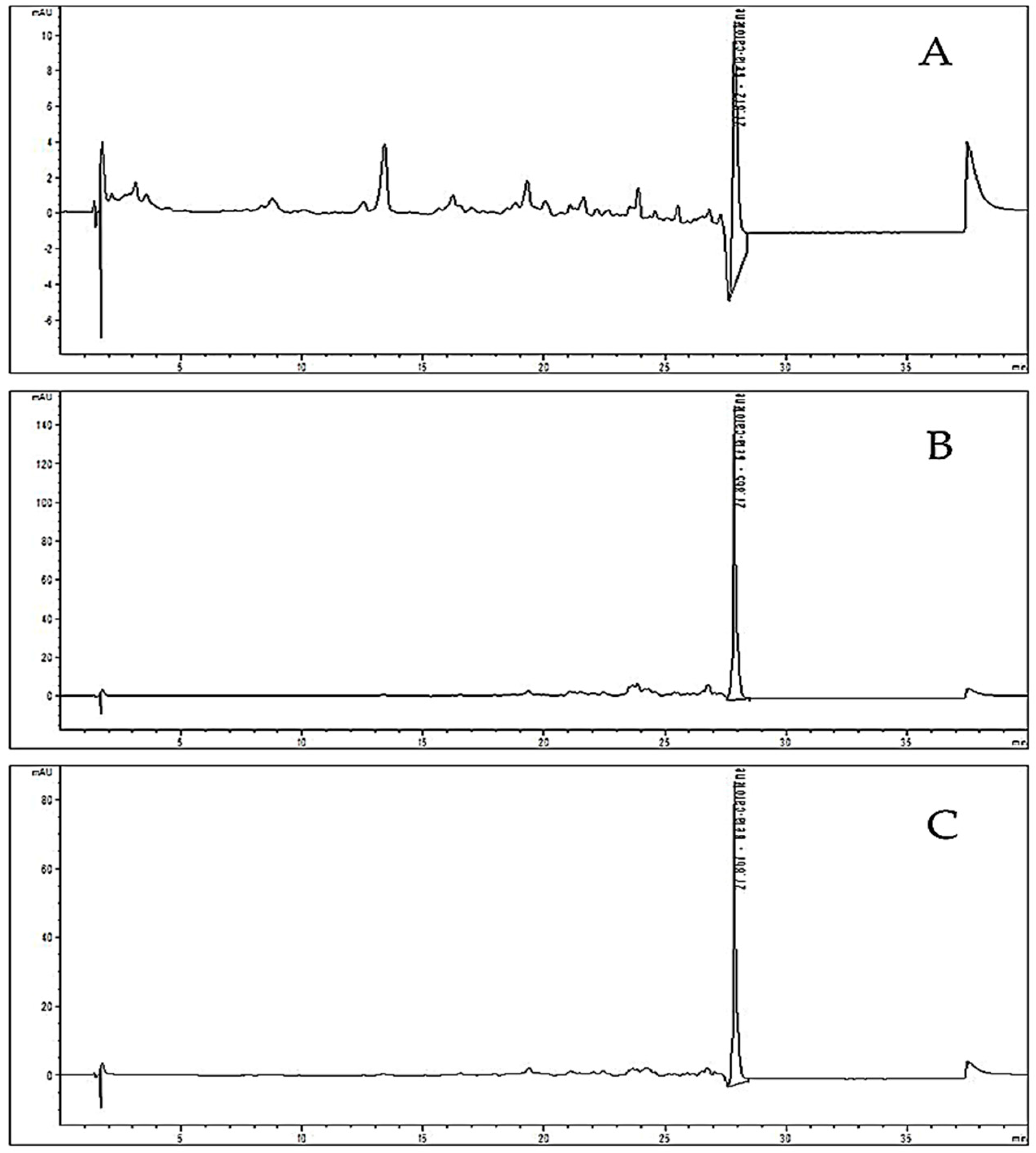
2.5. Chromatographic Determination of Carotenes
2.6. Total Phenolic Content (TPC)
2.7. DPPH Radical Scavenging Assay
2.8. ABTS Radical Scavenging Assay
2.9. FRAP Assay
2.10. Statistical Analysis
3. Results
3.1. Detection and Quantification of Bioactive Compounds in Fruit Samples
3.2. Antioxidant Activity of Fruit Extracts
3.3. Correlation Analysis with NPK Contents in the D-Leaves of MD2 Pineapple Plant
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Firoozabady, E.; Gutterson, N. Cost-effective in vitro propagation methods for pineapple. Plant Cell Rep. 2003, 21, 844–850. [Google Scholar] [PubMed]
- Loeillet, D.; Dawson, C.; Paqui, T. Fresh pineapple market: From the banal to the vulgar. Acta Hortic. 2011, 902, 587–594. [Google Scholar] [CrossRef]
- Wardy, W.; Saalia, F.K.; Steiner-Asiedu, M.; Budu, A.S.; Sefa-Dedeh, S. A comparison of some physical, chemical and sensory attributes of three pineapple (Ananas comosus) varieties grown in Ghana. Afr. J. Food Sci. 2009, 3, 94–99. [Google Scholar]
- Malezieux, E.; Bartholomew, D.P. Plant nutrition. In The Pineapple: Botany, Production, and Uses; Bartholomew, D.P., Paull, R.E., Rohrbach, K.G., Eds.; CAB International: Wallingford, UK, 2003; pp. 143–165. [Google Scholar]
- Song, X.; Liu, M.; Wu, D.; Griffiths, B.S.; Jiao, J.; Li, H.; Hu, F. Interaction matters: Synergy between vermicompost and PGPR agents improves soil quality, crop quality and crop yield in the field. Appl. Soil Ecol. 2015, 89, 25–34. [Google Scholar] [CrossRef]
- Zucco, M.A.; Walters, S.A.; Chong, S.-K.; Klubek, B.P.; Masabni, J.G. Effect of soil type and vermicompost applications on tomato growth. Int. J. Recycl. Org. Waste Agric. 2015, 4, 135–141. [Google Scholar] [CrossRef]
- Yousefi, A.A.; Sadeghi, M. Effect of vermicompost and urea chemical fertilizers on yield and yield components of wheat (Triticum aestivum) in the field condition. Int. J. Agric. Crop Sci. 2014, 7, 1227–1230. [Google Scholar]
- Kmeťová, M.; Kováčik, P. The impact of vermicompost application on the yield parameters of maize (Zea mays L.) observed in selected phenological growth stages (BBCH-scale). Acta Fytotech. Zootech. 2014, 17, 100–108. [Google Scholar] [CrossRef]
- Ayyobi, H.; Olfati, J.-A.; Peyvast, G.-A. The effects of cow manure vermicompost and municipal solid waste compost on peppermint (Mentha piperita L.) in Torbat-e-jam and Rasht regions of Iran. Int. J. Recycl. Org. Waste Agric. 2014, 3, 147–153. [Google Scholar] [CrossRef]
- Lazar, M.; Mitre, V.; Tripon, F.A.; Badiu, D.-E. The potential use of vermicompost in orchards. Bull. UASVM Hortic. 2014, 71, 56–58. [Google Scholar]
- Mahmud, M.; Abdullah, R.; Yaacob, J.S. Effect of vermicompost amendment on nutritional status of sandy loam soil, growth performance, and yield of pineapple (Ananas comosus var. MD2) under field conditions. Agronomy 2018, 8, 183. [Google Scholar] [CrossRef]
- Malaysia Pineapple Industrial Board (MPIB). Pengurusan Tanaman Nanas Pelbagai Kultivar. 2018; p. 18. Available online: http://www.youblisher.com/p/44324-Pengurusan-Tanaman-Nanas-Pelbagai-Kultivar/ (accessed on 19 August 2018).
- Kalaiselvi, M.; Ravikumar, G.; Gomathi, D.; Uma, C. In vitro free radical scavenging activity of Ananas comosus (L.) Merrill peel. Int. J. Pharm. Pharm. Sci. 2012, 4, 604–609. [Google Scholar]
- Solihah, M.A.; Wan Rosli, W.I.; Nurhanan, A.R. Phytochemicals screening and total phenolic content of Malaysian Zea mays hair extracts. Int. Food Res. J. 2012, 19, 1533–1538. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-vis spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Sporns, P., Eds.; John Wiley and Sons: New York, NY, USA, 2001; pp. F4.3.1–F4.3.8. [Google Scholar]
- Othman, R.; Zaifuddin, M.F.; Hassan, M.N. Potential sources for lipid soluble food colorants from selected Malaysian traditional vegetables. Malays. J. Anal. Sci. 2015, 19, 268–274. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Samad, M.A.; Hashim, S.H.; Simarani, K.; Yaacob, J.S. Antibacterial properties and effects of fruit chilling and extract storage on antioxidant activity, total phenolic and anthocyanin content of four date palm (Phoenix dactylifera) cultivars. Molecules 2016, 21, 419. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Marikkar, J.; Tan, S.; Salleh, A.; Azrina, A.; Shukri, M. Evaluation of banana (Musa sp.) flowers of selected varieties for their antioxidative and anti-hyperglycemic potentials. Int. Food Res. J. 2016, 23, 1988–1995. [Google Scholar]
- Crosby, K.; Jifon, J.; Leskovar, D. Agronomy and the nutritional quality of vegetables. In Improving the Health-Promoting Properties of Fruit and Vegetable Products; Tomas-Barberan, F.A., Gil, M.I., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2008; pp. 392–407. [Google Scholar]
- Jagathan, I.B.; Crozier, A. Overview of health-promoting compounds in fruit and vegetables. In Improving the Health-Promoting Properties of Fruit and Vegetable Products; Tomas-Barberan, F.A., Gil, M.I., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2008; pp. 3–10. [Google Scholar]
- Gunwantrao, B.B.; Bhausaheb, S.K.; Ramrao, B.S.; Subhash, K.S. Antimicrobial activity and phytochemical analysis of orange (Citrus aurantium L.) and pineapple (Ananas comosus (L.) Merr.) peel extract. Ann. Phytomed. 2016, 5, 156–160. [Google Scholar] [CrossRef]
- Agnes, J.X.; Anusuya, A. Phytochemical screening and in vitro antioxidant activity of Ananas Comosus. J. Res. Pharm. Pharmacother. 2016, 5, 162–169. [Google Scholar]
- Kaur, A.; Singh, B.; Ohri, P.; Wang, J.; Wadhwa, R.; Kaul, S.C.; Pati, P.K.; Kaur, A. Organic cultivation of ashwagandha with improved biomass and high content of active withanolides: Use of vermicompost. PLoS ONE 2018, 13, e0194314. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Downey, P.J.; Levine, L.H.; Musgrave, M.E.; McKeon-Bennett, M.; Moane, S. Effect of hypergravity and phytohormones on isoflavonoid accumulation in soybean (Glycine max L.) callus. Microgravity Sci. Technol. 2013, 25, 9–15. [Google Scholar] [CrossRef]
- Zong-min, M.; Ning, Y.; Shu-yun, L.; Hong, H. Nitrogen requirements for vegetative growth, flowering, seed production, and ramet growth of Paphiopedilum armeniacum (orchid). HortScience 2012, 47, 585–588. [Google Scholar] [CrossRef]
- Crous, K.Y.; Reich, P.B.; Hunter, M.D.; Ellsworth, D.S. Maintenance of leaf N controls the photosynthetic CO2 response of grassland species exposed to 9 years of free-air CO2 enrichment. Glob. Chang. Biol. 2010, 16, 2076–2088. [Google Scholar] [CrossRef]
- Brunetto, G.; Melo, G.W.B.D.; Toselli, M.; Quartieri, M.; Tagliavini, M. The role of mineral nutrition on yields and fruit quality in grapevine, pear and apple. Rev. Bras. Frutic. 2015, 37, 1089–1104. [Google Scholar] [CrossRef]
- Taber, H.; Perkins-Veazie, P.; Li, S.; White, W.; Rodermel, S.; Xu, Y. Enhancement of tomato fruit lycopene by potassium is cultivar dependent. HortScience 2008, 43, 159–165. [Google Scholar] [CrossRef]
- Afzal, I.; Hussain, B.; Basra, S.M.A.; Ullah, S.H.; Shakeel, Q.; Kamran, M. Foliar application of potassium improves fruit quality and yield of tomato plants. Acta Sci. Pol.-Hortorum Cultus 2015, 14, 3–13. [Google Scholar]
- Almeselmani, M.; Pant, R.; Singh, B. Potassium level and physiological response and fruit quality in hydroponically grown tomato. Int. J. Veg. Sci. 2009, 16, 85–99. [Google Scholar] [CrossRef]
- Serio, F.; Leo, J.; Parente, A.; Santamaria, P. Potassium nutrition increases the lycopene content of tomato fruit. J. Hortic. Sci. Biotechnol. 2007, 82, 941–945. [Google Scholar] [CrossRef]
- Trudel, M.; Ozbun, J. Relationship between chlorophylls and carotenoids of ripening tomato fruit as influenced by potassium nutrition. J. Exp. Bot. 1970, 21, 881–886. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef]
- Almeida, M.M.B.; de Sousa, P.H.M.; Arriaga, Â.M.C.; Prado, G.M.; Maia, G.A.; de Carvalho Magalhães, C.E.; de Lemos, T.L.G. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res. Int. 2011, 44, 2155–2159. [Google Scholar] [CrossRef]
- Yuris, A.; Siow, L.-F. A comparative study of the antioxidant properties of three pineapple (Ananas comosus L.) varieties. J. Food Stud. 2014, 3, 40–56. [Google Scholar] [CrossRef]
- Lu, X.-H.; Sun, D.-Q.; Wu, Q.-S.; Liu, S.-H.; Sun, G.-M. Physico-chemical properties, antioxidant activity and mineral contents of pineapple genotypes grown in China. Molecules 2014, 19, 8518–8532. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- De Oliveira, A.C.; Valentim, I.B.; Silva, C.A.; Bechara, E.J.H.; de Barros, M.P.; Mano, C.M.; Goulart, M.O.F. Total phenolic content and free radical scavenging activities of methanolic extract powders of tropical fruit residues. Food Chem. 2009, 115, 469–475. [Google Scholar] [CrossRef]
- Haripyaree, A.; Guneshwor, K.; Damayanti, M. Evaluation of antioxidant properties of phenolic extracted from Ananas comosus L. Not. Sci. Biol. 2010, 2, 68–71. [Google Scholar] [CrossRef]
- Yusof, Z.; Ramasamy, S.; Mahmood, N.; Yaacob, J. Vermicompost supplementation improves the stability of bioactive anthocyanin and phenolic compounds in clinacanthus nutans lindau. Molecules 2018, 23, 1345. [Google Scholar] [CrossRef]
| Chemical Constituents | Control | Chemical Fertilizer | Vermicompost |
|---|---|---|---|
| Phenol | + | + | + |
| Flavonoids I | + | ++ | +++ |
| Flavonoids II | + | + | + |
| Tannins | ++ | +++ | ++ |
| Alkaloids I | - | - | - |
| Alkaloids II | - | - | - |
| Alkaloids III | - | - | - |
| Bioactive Compounds | Control | Chemical Fertilizer | Vermicompost |
|---|---|---|---|
| Chlorophyll a (µg/g) | 1.055 ± 0.078 c | 2.438 ± 0.038 a | 1.866 ± 0.000 b |
| Chlorophyll b (µg/g) | 3.194 ± 0.096 c | 7.203 ± 0.073 a | 5.496 ± 0.000 b |
| Total Chlorophyll (µg/g) | 4.249 ± 0.041 c | 9.640 ± 0.040 a | 7.362 ± 0.000 b |
| Chlorophyll a/b ratio | 0.332 ± 0.035 a | 0.339 ± 0.009 a | 0.340 ± 0.000 a |
| Total phenolics (mg GAE/g DE) | 5.983 ± 0.001 b | 8.895 ± 0.002 a | 4.859 ± 0.001 c |
| β-carotene (µg/g DW) | 2.32 ± 0.17 c | 44.21 ± 0.69 a | 28.43 ± 2.41 b |
| Antioxidant Assay | Ascorbic Acid (Standard) | Control | Chemical Fertilizer | Vermicompost |
|---|---|---|---|---|
| DPPH, IC50 (mg/mL) | 0.034 ± 0.002 c | 5.133 ± 0.101 a | 2.909 ± 0.050 b | 3.239 ± 0.213 b |
| ABTS, IC50 (mg/mL) | 0.057 ± 0.004 d | 8.393 ± 0.100 a | 5.777 ± 0.130 c | 7.290 ± 0.188 b |
| FRAP (mg FE/g dE) | 36.198 ± 4.398 a | 0.368 ± 0.005 b | 0.402 ± 0.017 b | 0.276 ± 0.020 b |
| Parameters | Chl a | Chl b | Total Chl | TPC | β-Carotene | DPPH | ABTS | FRAP | Total N | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Chl a | 1 | |||||||||
| Chl b | 0.984 ** | 1 | ||||||||
| Total Chl | 0.991 ** | 0.999 ** | 1 | |||||||
| TPC | 0.620 | 0.634 | 0.632 | 1 | ||||||
| β-carotene | 0.984 ** | 0.990 ** | 0.992 ** | 0.587 | 1 | |||||
| DPPH | −0.927 ** | −0.937 ** | −0.937 ** | −0.371 | −0.961 ** | 1 | ||||
| ABTS | −0.964 ** | −0.963 ** | −0.966 ** | −0.747 * | −0.949 ** | 0.862 ** | 1 | |||
| FRAP | 0.146 | 0.158 | 0.155 | 0.805 ** | 0.100 | 0.129 | −0.291 | 1 | ||
| Total N | 0.656 | 0.699 * | 0.690 * | 0.158 | 0.670 * | −0.679 * | −0.580 | −0.110 | 1 | |
| P | 0.548 | 0.589 | 0.581 | 0.567 | 0.576 | −0.524 | −0.613 | 0.064 | 0.138 | 1 |
| K | 0.761 * | 0.778 * | 0.776 * | 0.561 | 0.720 * | −0.636 | −0.692 * | 0.370 | 0.504 | 0.226 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmud, M.; Ramasamy, S.; Othman, R.; Abdullah, R.; Yaacob, J.S. Effect of Vermicompost Application on Bioactive Properties and Antioxidant Potential of MD2 Pineapple Fruits. Agronomy 2019, 9, 97. https://doi.org/10.3390/agronomy9020097
Mahmud M, Ramasamy S, Othman R, Abdullah R, Yaacob JS. Effect of Vermicompost Application on Bioactive Properties and Antioxidant Potential of MD2 Pineapple Fruits. Agronomy. 2019; 9(2):97. https://doi.org/10.3390/agronomy9020097
Chicago/Turabian StyleMahmud, Mawiyah, Sujatha Ramasamy, Rashidi Othman, Rosazlin Abdullah, and Jamilah Syafawati Yaacob. 2019. "Effect of Vermicompost Application on Bioactive Properties and Antioxidant Potential of MD2 Pineapple Fruits" Agronomy 9, no. 2: 97. https://doi.org/10.3390/agronomy9020097
APA StyleMahmud, M., Ramasamy, S., Othman, R., Abdullah, R., & Yaacob, J. S. (2019). Effect of Vermicompost Application on Bioactive Properties and Antioxidant Potential of MD2 Pineapple Fruits. Agronomy, 9(2), 97. https://doi.org/10.3390/agronomy9020097





