1. Introduction
The winter oilseed rape (
Brassica napus subsp.
napus) is a commonly grown crop in the Czech Republic. During the years monitored, 2015–2017, its annual planting area accounted for more than 15% of the total arable area, making winter oilseed rape the third most grown crop in our country. In the period 2015–2017, its planting area increased from 366180 ha with a seed production of 1256 thousand tonnes, to 394262 ha with a seed production of 1146 thousand tonnes. In the Czech Republic, 99% of oilseed rape sowing area occupies the winter form of oilseed rape, while the remaining 1% belongs to the spring form [
1]. Breeding of winter oilseed rape is carried out in the Czech Republic by several breeding stations that cooperate within the framework of the Czech Rape Association. In Czech breeding programmes, particular emphasis is placed on oilseed rape seed yields with a high oil content and low content of glucosinolates, and beyond that, resistance to fungal diseases, winter tolerance, and tolerance to drought. The aim of the cooperation is to create prospective Czech varieties with the required yield, quality, and cultivational parameters, while maintaining favourable prices for Czech farmers [
2].
An inseparable part of oilseed rape breeding is to obtain genotypes that are resistant to fungal diseases, as winter oilseed rape is one of the most treated field crops grown in the Czech Republic with regard to crop protection. The most severe fungal diseases of oilseed rape are phoma stem canker, caused by a complex of the pathogens
Leptosphaeria maculans (Desm.) Ces. et de Not. and
Leptosphaeria biglobosa [
3] and Sclerotinia stem rot, caused by the polyphagous plant pathogen
Sclerotinia sclerotiorum (Lib.) de Bary [
4].
Phoma stem canker is a serious disease of oilseed rape in Australia, Canada, and Europe, causing seedling death, lodging, or early senescence [
5,
6]. Where the disease occurs, usual yield losses at harvest are <10%, although they can be as high as 30–50% [
5]. Phoma stem canker is the most important fungal diseases of winter oilseed rape in all areas of its cultivation in the Czech Republic. Its occurrence, depending on external conditions, fluctuates in individual years. Usual yield losses reach about 10%. In some years and in some places, the damage can reach up to 30–50% [
7].
Sclerotinia sclerotiorum is one of the non-specific plant pathogens [
4]. In the Czech Republic, this pathogen attacks many agronomic field crops such as potatoes, oilseed rape, poppy, sunflower, and various vegetable crops [
8]. Annual yield losses caused by Sclerotinia stem rot account for 10–20%, but in years of heavy outbreaks, it can cause yield losses of 30–50%, though in some years it hardly appears at all [
7]. The increasing harmfulness of Sclerotinia stem rot has also been confirmed by Prokinová, where the frequency of disease incidence in the Czech Republic varied considerably in individual stands. Oilseed rape growths were found with infestation levels of 0% to 80% [
9].
In addition to assessing the importance of selected fungal pathogens in the oilseed rape, our attention was also focused on plant damage caused by phytoplasmas. Although these deforming damages occur in crops sporadically, their harmfulness has increased with the growth of crop areas, as demonstrated by long-term monitoring in Poland [
10]. Olivier & Galca observed similar results in Canada, where the disease was considered to be of little importance, with an overall incidence of less than 1% in most fields. However, in 2007, the percentage of canola plants (
Brassica napus L. and
B. rapa L.,
Brassicaceae) showing symptoms ranged from traces to 12% depending on the Canadian location, with a provincial average of 2% [
11]. In the Czech Republic, plants infected by phytoplasma are severely damaged and do not provide any yield. Because of the unique occurrence, the damage is not yet economically significant [
7].
Phytoplasmas are plant pathogens consisting of more than 50 phylogenetic groups that cause devastating diseases in various crops worldwide [
12]. Phytoplasmas are simple prokaryotic organisms without a solid cell wall. Phytoplasma size is very variable, ranging from 200 to 500 nm, with elongated shapes up to 800 nm. They can be observed only by electron microscopy in insect vectors or in plant sieves [
13]. They are obligate parasites restricted to the phloem tissue of the host plant and are transmitted from plant to plant, mostly by leafhoppers (
Hemiptera:
Cicadellidae). They reproduce within the tissues of their insect vectors and are transferred to new host plants in salivary secretions during feeding [
12]. Phytoplasmas induce a number of major crop diseases. Their host spectrum is broad, so not only are they considered to be pathogens significantly reducing yield and quality, they also have a negative impact on propagation areas [
13]. They cause various symptoms, including yellows and leaf foliage chlorosis, a proliferation of shoots and roots, dwarfism, virescence, phyllody, flower sterility, or the death of shoots and even whole plants [
14]. Current phytoplasmic qualification according to IRPCM (International Research Project for Comparative Mycoplasmology) distinguishes 15 groups based on 16S rDNA analysis [
15].
The objectives of this study were: (i) to compare the resistance to fungal diseases, such as phoma stem canker and Sclerotinia stem rot, of tested genotypes of winter oilseed rape at monitored sides; (ii) to determinate the Leptosphaeria sp. species causing phoma leaf spots on oilseed rape at monitored sites in autumn season; and (iii) to determine the phytoplasma groups from samples taken from infected oilseed rape plants with phyllody symptoms.
2. Materials and Methods
2.1. Field Trials with New Genotypes of Winter Oilseed Rape
During the monitored period 2015 to 2017, small-plot field trials were established at selected localities in Rapotín (the Olomouc region), Opava (the Moravian-Silesian region), Chlumec nad Cidlinou (the Hradec Králové region), and Lužany (the Pilsen region) to ensure as large a range of comparable data as possible. In 2017, the field trials established in Chlumec nad Cidlinou did not emerge due to the drought conditions after sowing; therefore, the data was collected from a new locality, Lužany. In the field trials, 48 new genotypes of winter oilseed rape plus two reference varieties, selected from the list of recommended varieties by the Central Institute for Supervising and Testing in Agriculture (Brno, Czech Republic) with very good resistance to fungal diseases, were tested for their economic characteristics. The collection of new winter oilseed rape genotypes differed every year consisting only of pure lines. The oilseed rape genotypes were selected from a wide breeding program of the Czech breeding institutes, one third of the genotypes originated from Opava breeding program, second third from Slapy near Tábor, and last third from Chlumec nad Cidlinou. Reference varieties were also oilseed rape lines, where the selection of reference varieties coincided with the reference varieties used in the state variety tests with oilseed rape in that year of testing. The list of used reference varieties is shown in
Table 1.
The field trials were carried out as complete fully randomized blocks of small plot experiments with a minimum field plot size of 10 m
2, with all trials being established in three replicates. This field trial design was derived from the methodology for testing the utility value of cruciferous oilseed (Central Institute for Supervising and Testing in Agriculture, Czech Republic) [
16]. For sowing of seeds, a direct drill machine was used and the seed rate was 700,000 germinate seeds per hectare. The trial plots were treated with herbicides and insecticides only, in accordance with a local practice, to ensure the best vitality of the tested genotypes. No fungicides were applied to show the resistance of tested genotypes against the fungal diseases. The list of used pesticides treated on field trials with winter oilseed rape shows
Supplementary Table S8.
Assessments were made of resistance to phoma stem canker (caused by pathogens Leptosphaeria maculans, L. biglobosa) and to Sclerotinia stem rot (caused by the pathogen Sclerotinia sclerotiorum). In the growth stage BBCH 83-85, one hundred plants, randomly chosen per plot, were assessed for disease incidence (percentage of plants affected) and the severity of both fungal diseases.
Phoma stem canker severity (crown cankers and phoma stem lesions) was scored on a 1–5 scale according to the methodology described in EPPO standard PP 1/78(3) (European and Mediterranean Plant Protection Organization, Paris, France; hereinafter EPPO) for the evaluation of root, stem, foliar, and pod diseases of oilseed rape: Level 1—no infection, no necrotic areas. Level 2—spots are present, which do not deeply necrotize the stem or the root neck. About 25% of the root neck is corky or 25% of the stem shows symptoms. Level 3—corky appearance of the root neck is obvious. The root neck is only one-sided. The spots on the stem are deeper. A total of 50% of the root neck and/or 50% of the stem have symptoms. The plant is still green. Level 4—root neck strongly and deeply corky and/or deeply penetrating spots on the stem (spots may be dried out or may be soft). About 75% of the root neck and/or 75% of the stem are with symptoms. Pycnidia usually visible. The plant starts to die during BBCH 79–81. Level 5—root neck strongly and deeply corky, with only small or no connection to the root, and/or extensive and deeply penetrating spots at the stem. About 100% of the root neck and/or 100% of the stem with symptoms. The plant is prematurely ripening or already dead [
17].
Sclerotinia stem rot severity was scored on 1–5 scale derived from the same methodology described in EPPO standard PP 1/78(3): Level 1—no infection. Level 2—up to 25% infection of the stem, but strength of stem unaffected. Level 3—25–50% infection of the stem, but strength of stem unaffected. Level 4—50 to 75% infection, stem weakened. Level 5—75 to 100% infection, stem weakened, or plant is already dead [
17]. The mean severity score for evaluated plants was calculated for both monitored fungal diseases.
The data was statistically analysed using TIBCO StatisticaTM (TIBCO Software Inc., Palo Alto, CA, USA) and two-way ANOVA in MS Excel (Microsoft Office, Microsoft, Redmont, WA, USA).
2.2. Identification of the Species L. Maculans and L. Biglobosa
During the monitored period 2015 to 2017, the spotted leaves infected with the fungal pathogen Leptosphaeria spp. were collected from experimental small-plot field trials and from winter oilseed rape fields in autumn season, as a part of Leptosphaeria spp. monitoring in the Czech Republic. For a control, oilseed rape plants were grown under sterile conditions in the glass house to exclude latent infection in asymptomatic plants grown on the field.
Molecular detections of
Leptosphaeria sp. (without specific determination of race) were performed using markers for minisatellite locus
Lema 37 (GeneBank accession no. AX916774) using primers
Lema 37F (5´-TCT TGG CTT GGC TTT GTC TT-3´) and
Lema 37R (5´-ACA TTG GCT CGG AAA CA TTC-3´) [
18]. The size of this locus varies from 157 to 283 bp, according to
Leptosphaeria race.
Identification of
Leptosphaeria species (
L. maculans and/or
L. biglobosa) was performed using another pair of specific primers
ERIC1R: (5´-ATG TAA GCT CCT GGG GAT TCA C-3´) and
ERIC2: (5´-AAG TAA GTG ACT GGG GTG AGC G-3´) [
19,
20]. For PCR analyses, DNA was isolated from healthy and/or diseased plant tissues, using a commercial isolation kit, Kurabo QuickGene DNA tissue kit S (Kurabo, Japan). PCR amplification was performed in the thermal cycler, Mastercykler Gradient (Eppendorf, Germany), in a 20 μL reaction mixture containing: 50 ng genomic DNA, 10 pmol of each primer, 100 μmol.dm
−3 of each dNTP, 1 U Dream Taq polymerase (Thermo Fisher Scientific, Waltham, MA, USA) in a PCR reaction buffer (1× Dream Taq PCR buffer). Cycling parameters were the following: 10 min denaturation step at 94 °C was followed by 35 cycles of 30 s at 94 °C, 45 s at 52 °C, and 90 s at 72 °C. The products of PCR were resolved on 1.5% agarose gels (Serva, Heidelberg, Germany) and visualized using EtBr/UV.
2.3. Determination of Phytoplasma Groups
The infected plants with phytoplasma symptoms were collected from experimental small-plot field trials and from winter oilseed rape fields in the Olomouc, Hradec Králové, and Moravian-Silesian regions. Molecular detection of phytoplasmas was carried out using markers for the highly conserved 16S rRNA gene, using primers P1/P6. The size of amplified DNA fragment was approximately 1500 bp. Then, nested PCR was performed using R16F2n/R16R2 primers to increase detection sensitivity and specificity [
21]. The size of nested-PCR DNA fragment was 1200 bp. The oligonucleotide sequences of primers were: P1 (5´-AAG AGT TTG ATC CTG GCT CAG GAT T-3´), P6 (5´-CGG TAG GGA TAC CTT GTT ACG ACT TA-3´), R16F2n (5´-GAA ACG ACT GCT AAG ACT GG-3´), and R16R2 (5´-TGA CGG GCG GTG TGT ACA AAC CCC G-3´) [
21]. For PCR analyses, DNA was isolated from healthy and/or diseased plant tissue, using the commercial isolation kit, Kurabo QuickGene DNA tissue kit S (Kurabo, Japan).
PCR amplification was performed in a Mastercykler Gradient thermal cycler (Eppendorf, Germany) in a 20 μL reaction mixture containing: 50 ng genomic DNA, 10 pmol of each primer, 100 μmol/dm3 of each dNTP, 1 U Dream Taq polymerase (Thermo Scientific) in PCR reaction buffer (1× Dream Taq PCR buffer). Cycling parameters were the following: a 5 min denaturation step at 94 °C, followed by 35 cycles of 30 s at 94 °C, 40 s at 55 °C, and 1 min at 72 °C. In the nested PCR, the product from PCR with P1/P6 primers was diluted 100 times in deionized water and then used as a template for PCR with R16F2n/R2 primers. The products of PCR were resolved on 1.5% agarose gels (Serva, Heidelberg, Germany) and visualized using EtBr/UV. All amplified products were isolated and sequenced to confirm the accuracy of the PCR and to identify phytoplasma race.
3. Results
3.1. Phoma Stem Canker and Sclerotinia Stem Rot Infestation of Winter Oilseed Rape on Monitored Localities
The average incidence of phoma stem canker epidemics on monitored localities differed significantly every year. The highest disease incidence was recorded in 2015 in Chlumec nad Cidlinou (mean incidence score 31.00%), in 2016 in Opava (mean incidence score 29.33%), and in 2017 in Rapotín (mean incidence score 14.22%). The same trend was observed for phoma stem canker severity, when mean disease severity score significantly differed too and the highest severity corresponded to the incidence results for all experimental sides (mean severity scores for Chlumec nad Cidlinou, Opava, and Rapotín were 22.38%, 20.78%, and 8.44%, respectively). As well as the mean incidence and the mean severity score of Sclerotinia stem rot differed significantly in monitored years. The highest incidence of Sclerotinia stem rot epidemics was recorded in 2015 in Chlumec nad Cidlinou (41.17%), in 2016 in Opava (21.20%), and in 2017 in Lužany (8.06%). The inicial data for the health state of tested oilseed rape genotypes recorded in 2015 to 2017 are listed in
Supplementary Tables S1–S3. Maximal mean severity scores for the most infected localities were 36.85% in Chlumec nad Cidllinou, 18.84% in Opava, and 7.15% in Lužany. Obtained results of mean incidence and mean severity scores shows
Table 2.
As the new oilseed rape genotypes came from three different breeding programs (Opava, Slapy, and Chlumec nad Cidlinou), the effect of the origin on the resistance to the fungal diseases was evaluated.
a) Phoma Stem Canker Infestation
In 2015 for all monitored localities, the results of phoma stem canker incidence of tested genotypes of winter oilseed rape of different origin did significantly differ. The severity results of phoma stem canker epidemics significantly differed only in Rapotín and in Chlumec nad Cidlinou. In Opava, tested genotypes of different origin were affected equally. The average results of phoma stem canker infestation of new genotypes of different origin showed that genotypes originated from Chlumec nad Cidlinou had a better resistance to phoma stem canker in Rapotín and Chlumec nad Cidlinou, and genotypes from Slapy breeding station had a better resistance to phoma stem canker in Lužany. The level of phoma stem canker infestation of reference varieties CS Slaki and Rescator in 2015 did significantly differ too. In Opava and in Chlumec nad Cidlinou, localities with the higher phoma stem canker severity, cultivar CS Slaki was the least affected genotype in the tested collection of oilseed rape genotypes. In 2016, phoma stem canker infestation of genotypes of different origin did differ only in Opava, where the highest infestation was recorded that year. From the collection of genotypes of different origin, in Rapotín and in Opava, the highest resistance to phoma stem canker were recorded on genotypes originated from Chlumec nad Cidlinou, and in Chlumec nad Cidlinou the best resistance to phoma stem canker had genotypes that originated from Opava. In comparison to the reference varieties, in Rapotín and Opava, new tested genotypes were more affected with phoma stem canker than Arabella, but less affected than Arot. In Chlumec nad Cidlinou, new genotypes were more infected than both reference varieties. In 2017, phoma stem canker infestation of genotypes of different origin significantly differed in Rapotín and in Lužany. In both cases, the most affected genotypes originated from Opava. In Opava, there were no recorded significant differences between the average results of phoma stem canker infestation. In comparison to the reference varieties, new tested genotypes were more affected with phoma stem canker than both reference varieties in Rapotín in all cases. In Lužany, phoma stem canker infestation of reference varieties was comparable only with new genotypes from Slapy, and significantly more affected genotypes originated from Opava and Chlumec nad Cidlinou. Obtained results of phoma stem canker infestation of tested oilseed rape genotypes in the monitored period is shown
Table 3. Examples of phoma stem canker infestation of winter oilseed rape show
Figures S1 and S2.
b) Sclerotinia Stem Rot Infestation
In 2015, the results of Sclerotinia stem rot incidence and the severity of tested oilseed rape genotypes originated from three breeding programmes showed significant differences for two localities, in Rapotín and in Chlumec nad Cidlinou. In both cases, the oilseed rape genotypes originated from Slapy showed significantly better resistance against Sclerotinia stem rot. In comparison with the reference varieties, the average infestation of new genotypes tested in Rapotín corresponded to the infestation of the reference varieties CS Slaki and Rescator with a slight difference (mean severity score 3.60a and 2.70ab, respectively). In Chlumec nad Cidlinou, the reference variety CS Slaki was at least infected genotype with Sclerotinia stem rot (mean severity score 15.00c) unlike the second refence variety Rescator, where the Sclerotinia stem rot mean severity score belonged to the most affected results (50.00a). In Opava, no significant differences of Sclerotinia stem rot average infestation between the tested genotypes of different origin were observed.
In 2016, the results of Sclerotinia stem rot incidence and severity of tested oilseed rape genotypes of different origin showed significant differences in two localities, in Opava and in Chlumec nad Cidlinou, when the highest infestation was observed in Opava that year. On both localities, the most resistant genotypes originated from the breeding station Slapy. In Rapotín, there were no observed significant differences of Sclerotinia stem rot infestation between tested genotypes of different origin except the reference varieties. The most infested genotype, recorded in Rapotín, was the reference variety Arabella, despite the fact that the reference variety Arot was not infected at all. Similar results of infestation of the reference varieties were observed in Opava and Chlumec nad Cidlinou, where reference variety Arabella was the most infected genotype with Sclerotinia stem rot from the tested collection that year.
In 2017, the small differences of Sclerotinia stem rot severity between genotypes of different origin were observed only in Lužany, where the tested genotypes from Opava and Slapy were more infected with Sclerotinia stem rot than the rest of genotypes from tested collection. In Rapotín and Opava, the epidemic severity of tested genotypes did not significantly differ. Obtained results of epidemic severity showed that the least-affected genotypes of the tested collection originated from Chlumec nad Cidlinou. The results of infestation of winter oilseed rape genotypes with Sclerotinia stem rot is shown in
Table 4. Examples of Sclerotinia stem rot infestation of winter oilseed rape show
Figures S3 and S4.
c) Effect of Year and Locality on Incidence and Severity of Phoma Stem Canker and Sclerotinia Stem Rot Epidemics
Analysis of variance (two-way ANOVA test, alpha level 0.05) of phoma stem canker incidence and severity recorded on winter oilseed rape genotypes in experimental field trials in Rapotín, Opava, Chlumec nad Cidlinou, and Lužany in three consecutive years (2015, 2016, and 2017) was performed. The inicial data for two-way ANOVA tests of phoma stem canker epidemics (incidence, resp. severity) show
Supplementary Tables S4, resp. S5. Obtained results showed that both factors (year and locality) had a significant effect on the results of phoma stem canker incidence and severity. Also, the F-value for interaction effect was higher than its F-critical value, so we can conclude that both year and locality had a combined effect on phoma stem canker epidemics data (
Table 5 and
Table 6).
The same tests (two-way ANOVA test, alpha level 0.05) were performed for Sclerotinia stem rot incidence and severity. The inicial data for two-way ANOVA tests of Sclerotinia stem rot epidemics (incidence, resp. severity) show
Supplementary Tables S6, resp. S7. The results confirmed that even in this case both factors (year and locality) had a significant effect on Sclerotinia stem rot incidence and severity; a significant interaction effect was confirmed too (
Table 7 and
Table 8).
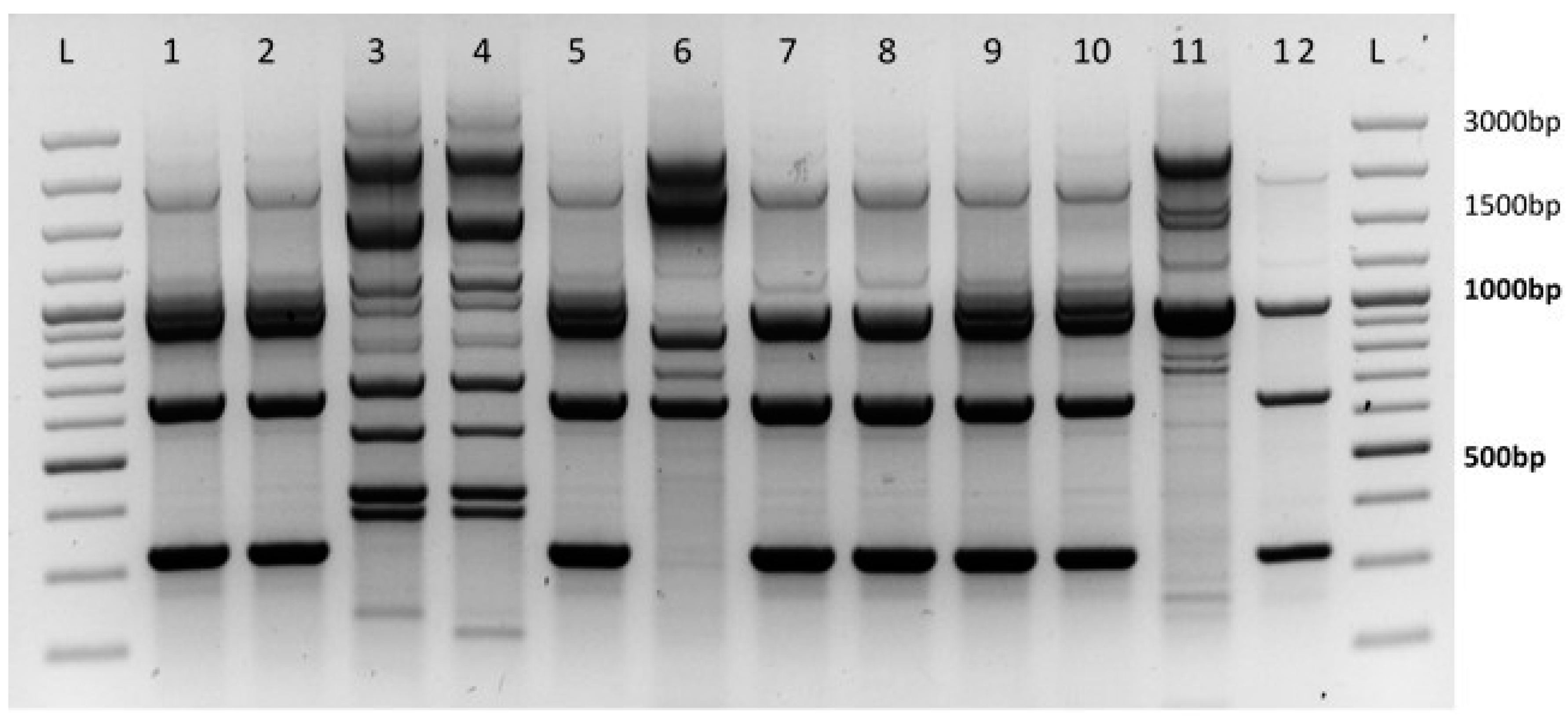
3.2. Determination of the Leptosphaeria Species
Between 2015 to 2017, more than 400 samples of
Leptosphaeria spp. were analyzed. In the course of monitoring the harmfulness of
Leptosphaeria spp., infected leaves were sampled from 20 districts in the nine Czech regions, particularly from the Hradec Králové region, Zlín region, Moravian-Silesian region, Prague region, Pilsen region, Olomouc region, South Moravian region, Vysočina region, and the Central Bohemia region. In all regions, infection caused by
Leptosphaeria maculans was prevalent. The results of previous analyses showed that the portion of leaves infected with
Leptosphaeria maculans was 98% while the remaining 2% belonged to
Leptosphaeria biglobosa (
Figure 1).
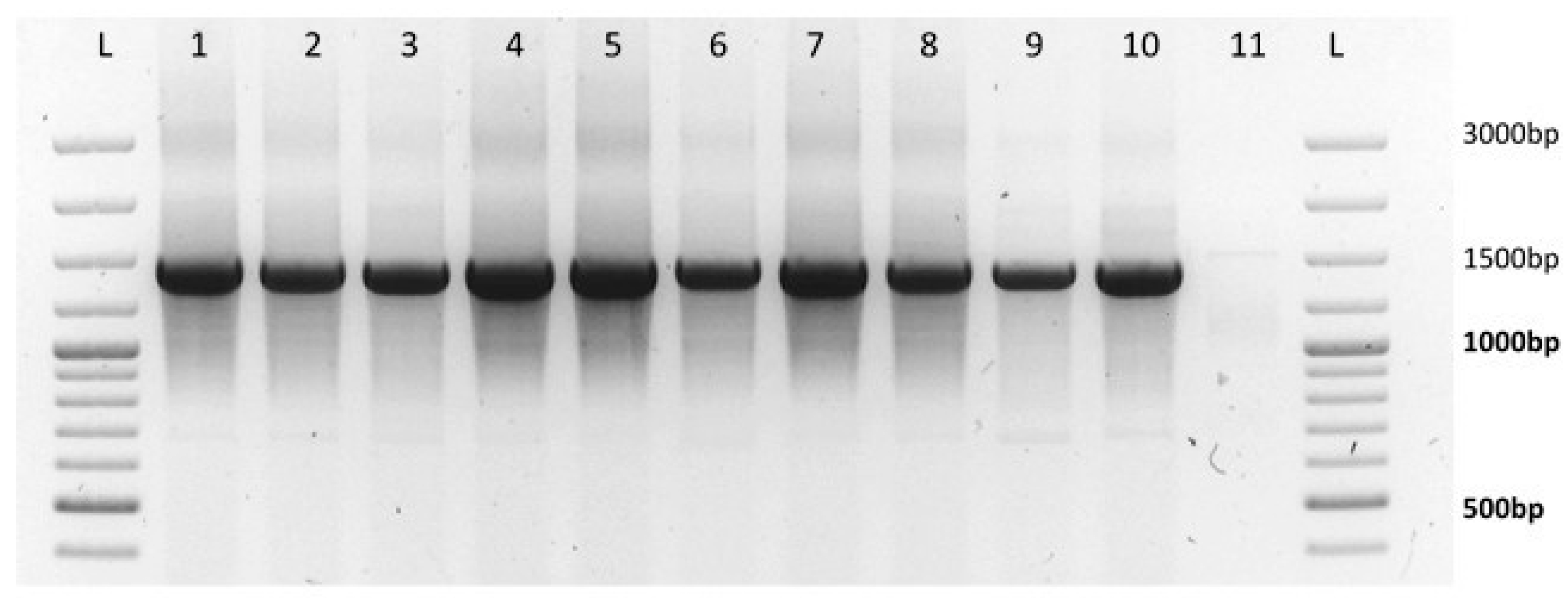
3.3. Determination of the Phytoplasma Groups
In the monitored crops of oilseed rape, about 0–1% of plants annually were found with phytoplasma symptoms. Subsequently the molecular detection of phytoplasmas was performed using nested-PCR and the derived products were sent for sequencing. The obtained nucleotide sequences were analyzed and compared with known sequences in the NCBI database BLAST (National Center for Biotechnology Information, U.S. National Library of Medicine, Bethesda, Rockville, MD, USA). The results confirmed, in all cases, the presence of phytoplasma isolates from the 16SrI Aster Yellows group. Some minor differences in sequences were found among phytoplasma isolates (
Figure 2).
4. Discussion
In Europe and North America,
L. maculans often co-exists with
L. biglobosa [
22,
23].
Leptosphaeria biglobosa is associated with upper stem lesions; whilst generally not damaging, they can cause serious losses in countries like Poland with high summer temperatures [
24], and
L. maculans is considered more damaging, causing stem base canker [
22]. Comparing two areas of Europe with severe phoma stem canker epidemics, but different pathogen populations and climate, England and Poland, Huang et al. [
24] confirmed both
L. maculans and
L. biglobosa are present in England, but
L. maculans is the predominant cause of the severe stem base cankers observed in summer before harvest. In contrast, in Poland,
L. biglobosa is the predominant cause of the mid- and upper-stem lesions observed before harvest [
25]. These results suggest that differences in the development of phoma stem canker epidemics between England and Poland relate to differences in weather patterns between the two countries rather than differences in their pathogen populations. Winter weather is usually colder in the winter oilseed rape growing regions of Poland than in such regions in England, but springs and summers are warmer in Poland than England. The main difference in the seasonal pattern of ascospore release between England (
L. maculans) and Poland (
L. biglobosa) was that the duration of ascospore release was longer in England than Poland. Furthermore, because the winter is colder in Poland than England, leaves infected early in autumn in Poland may fall off before the pathogen reaches the stem base, such that the plants generally escape development of stem base canker [
24]. Previous studies suggest that the low temperatures not only decrease the germination of ascospores and penetration of leaf surfaces, but also slow down the growth of the fungus in plants and delay the development of symptoms [
26]. Weather is the main environmental influence on plant diseases and affects disease distribution, although other factors such as changes to the host crop distribution, intensity of cropping and farming practices can also greatly affect disease severity [
27].
In the Czech Republic, the
Leptosphaeria spp. ascospore release was first observed at the end of August, but mostly in September and October [
6,
28]. In experimental conditions, crop infection occurs from 5 to 24 °C with optimum temperatures between 10 and 20 °C and 8 to 72 h of leaf wetness. Leaf spot development is most favoured between 5 and 20 °C and at 48 hr of leaf wetness, while at optimum temperatures of 12–25 °C [
24,
29]. As the winter in the Czech Republic is cold and the oilseed rape growths are covered with snow, the disease development may continue during the winter season because the growth of the pathogen in the plant can occur even in winter when temperatures are higher than 5 °C [
24,
29]. In the spring, at the time of the oilseed rape bloom, phoma stem lesions can be found, mainly caused by autumn infections. The latent incidence of autumn phoma disease and the severity of infection at the end of vegetation is often closely related [
30].
Within Europe, the harmfulness of phoma stem canker is the most striking in Western Europe, towards the east the disease severity decreases. Since the Czech Republic is located in Central Europe, it is likely that the severity of phoma stem canker epidemics is somewhere in the middle. In Poland, the harmfulness of phoma stem canker is considerable in some years [
25]. The results of our observations have confirmed that the harmfulness of phoma stem canker epidemics varies depending on the year, region, and course of weather conditions (dry year 2017). The highest difference in phoma stem canker severity was up to 19.71 percent within the year. The highest mean severity during the monitored period reached 22.78% in Chlumec nad Cidlinou. The long-term low infestation of winter oilseed rape with phoma stem canker showed results from Rapotín. Totally low infestation on all monitored localities was recorded in year 2017 due to the lack of precipitation during the whole growing season, which negatively influenced the development of pathogens. Results of phoma stem canker infestation of tested genotypes of different origin showed the most resistant genotypes to this disease originated mostly from the breeding program in Slapy (2015 and 2017) and from the breeding program in Chlumec nad Cidlinou (2016).
The severity of Sclerotinia stem rot is extremely variable from year to year, region-to-region and even from field to field. Sclerotinia has become more serious as oilseed rape production has increased, likely due to a combination of more hectares of oilseed rape in rotations and management practices that contribute to high yields, but also produce dense canopies, which are a better microclimate for disease development. Wet weather has also favoured disease development in recent years [
31,
32].
Sclerotinia sclerotiorum is probably the most common cause of premature drying of oilseed rape plants in conditions found in the Czech Republic [
33]. According to our results, the highest severity of Sclerotinia stem rot was observed in 2015 in Chlumec nad Cidlinou (max mean severity score 45.00%) and in 2016 in Opava (max mean severity score 26.67%), both recorded on the reference variety Arabella. The highest difference between Sclerotinia stem rot severity within the year reached 33.81%, calculated for 2015. The most resistant genotypes of different origin came from the Slapy breeding station (in 2015 and 2016) and from Chlumec nad Cidlinou (in 2017).
The results of the determination of pathogens causing phoma leaf spots, sampled in autumn seasons, indicated that the dominant pathogen occurring on monitored localities was Leptosphaeria maculans. This was confirmed in 98% for the samples of infected oilseed rape leaves. The pathogen Leptosphaeria biglobosa was identified in a minority (2%) of samples taken. Sampling and determination of acquired pathogens is continuing but we do not expect a large change in the proportion of species represented. The predominance of L. maculans detected from leaf lesions confirmed results of phoma stem canker severity observed mainly on winter oilseed rape crowns and lower parts of stems.
The results of phytoplasma monitoring confirmed that phytoplasma´s occurrence in the studied oilseed rape growths was sporadic. The available literature shows phytoplasma incidence in oilseed rape ranging from 0.01% to 0.50% [
11,
34], which confirms our results. The results of molecular determination by PCR confirmed the presence of phytoplasmas from the 16SrI Aster Yellows group. In neighbouring Poland, a phytoplasma belonging to the Aster Yellows, a 16SrI-B subgroup, which causes characteristic deformations of winter oilseed rape plants, has been observed for more than a decade [
35]. Similar results were obtained by Bertaccini et al., where symptoms such as dwarfing, reddish leaves, and extensive malformations of flowers were observed in infected oilseed rape plants in southern Bohemia, caused by phytoplasmas belonging to the Aster Yellows subgroup 16SrI-B. Interestingly, a Czech phytoplasma had identical RFLP (restriction fragment length polymorphism) profiles as a phytoplasma obtained from monitored areas in Italy [
35].
The acquired knowledge of phoma stem canker and Sclerotinia stem rot epidemics will help identify more resistant breeding materials and allow us to determine their plasticity in resistance via multi-site testing. Selected oilseed rape genotypes will continue to be used in the breeding process as donors of increased resistance to the formation of new varieties. The findings will also help to determine the severity and importance of the disease to be monitored, and will enable the breeding to target the resistance in the desired direction.