Endophytic Fungal Root Colonization of Eragrostis tef in Eroded Croplands of the Ethiopian Highlands Is Limited by Low Spore Density and Fertilisation
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant and Soil Material
2.2. Experimental Set up
2.3. Harvest and Soil Sampling
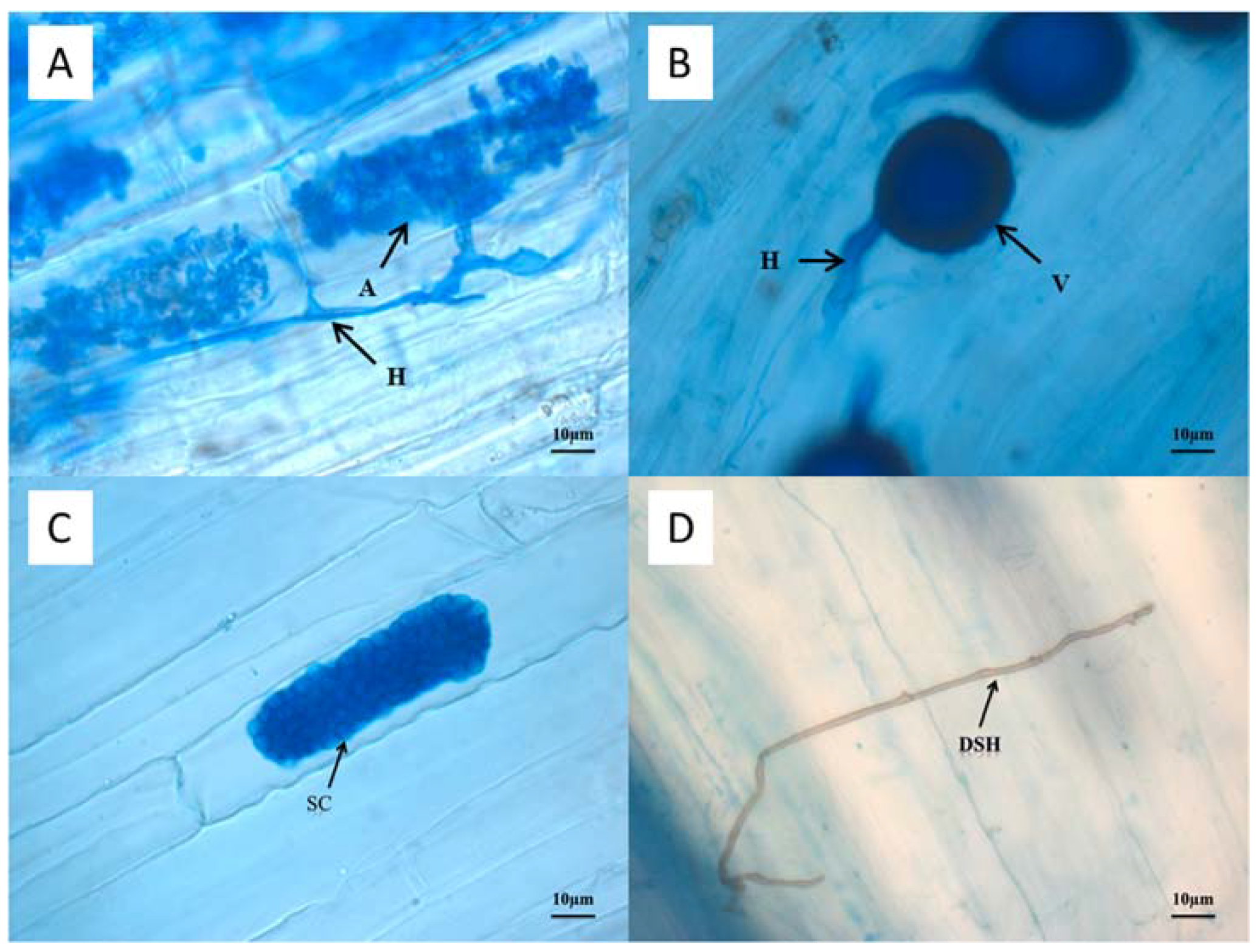
2.4. AMF and DSE Colonization Rates and AMF Spore Density
2.5. Statistical Analysis
3. Results
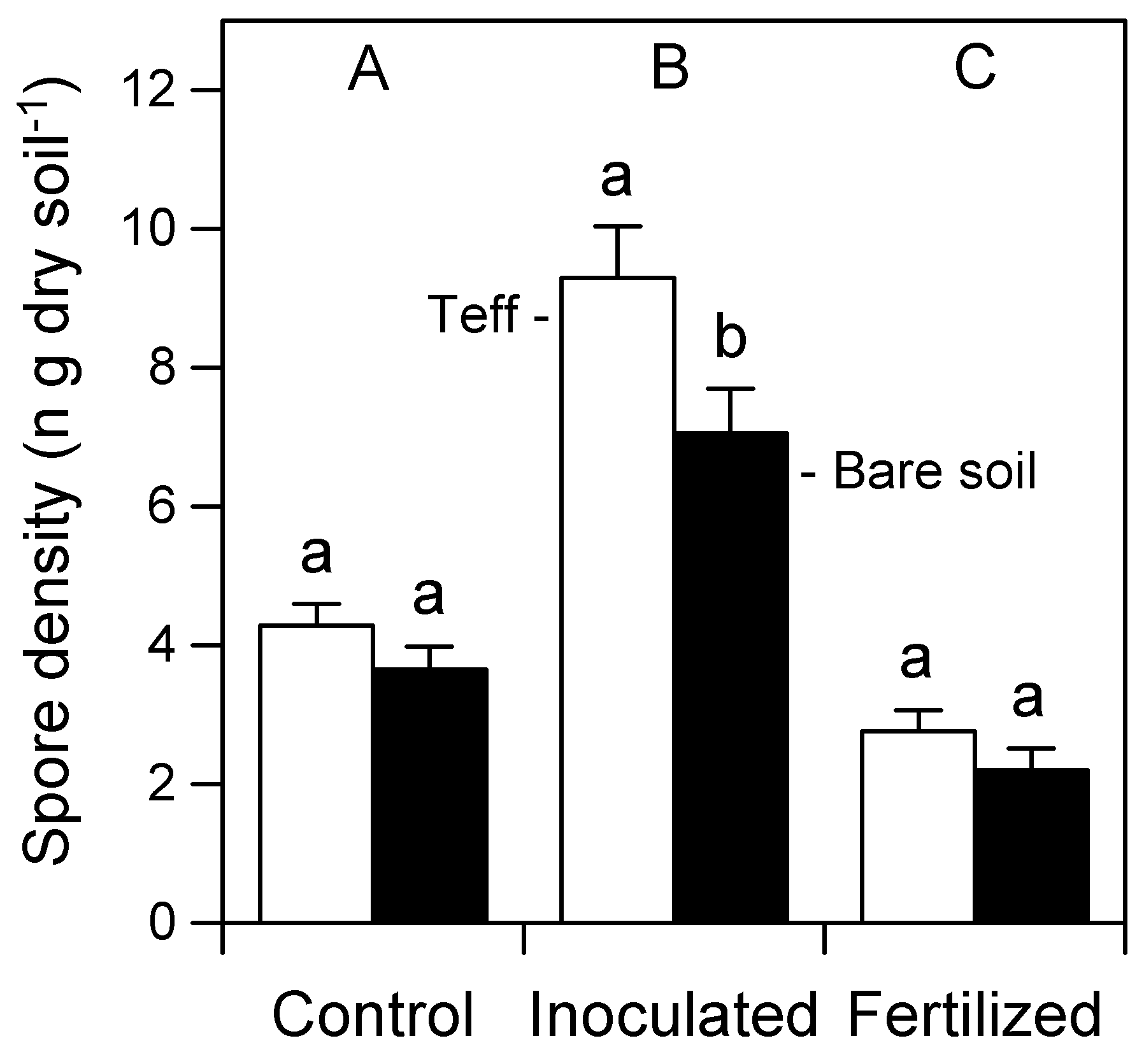
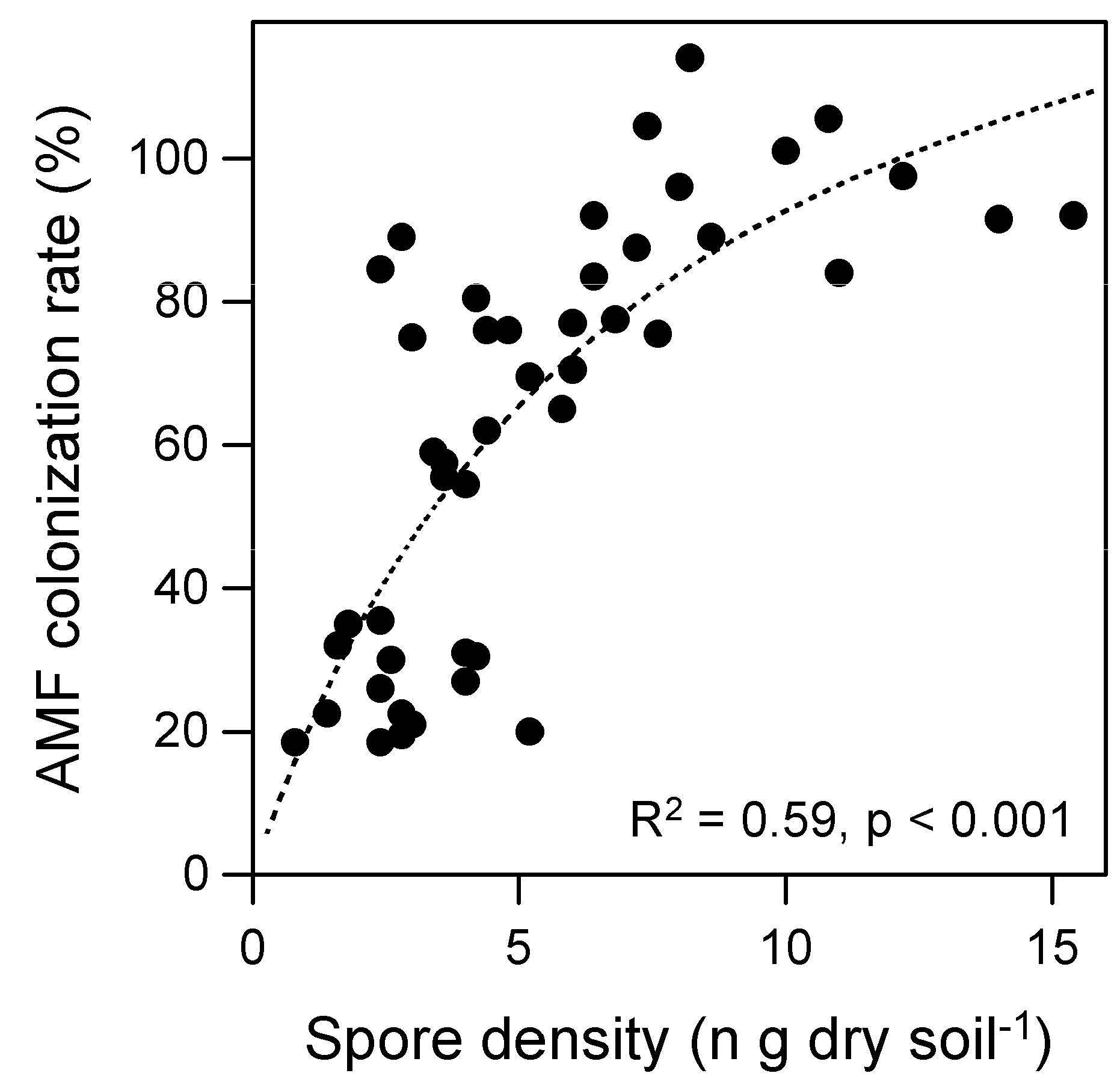
3.1. AMF Spore Density in Soil
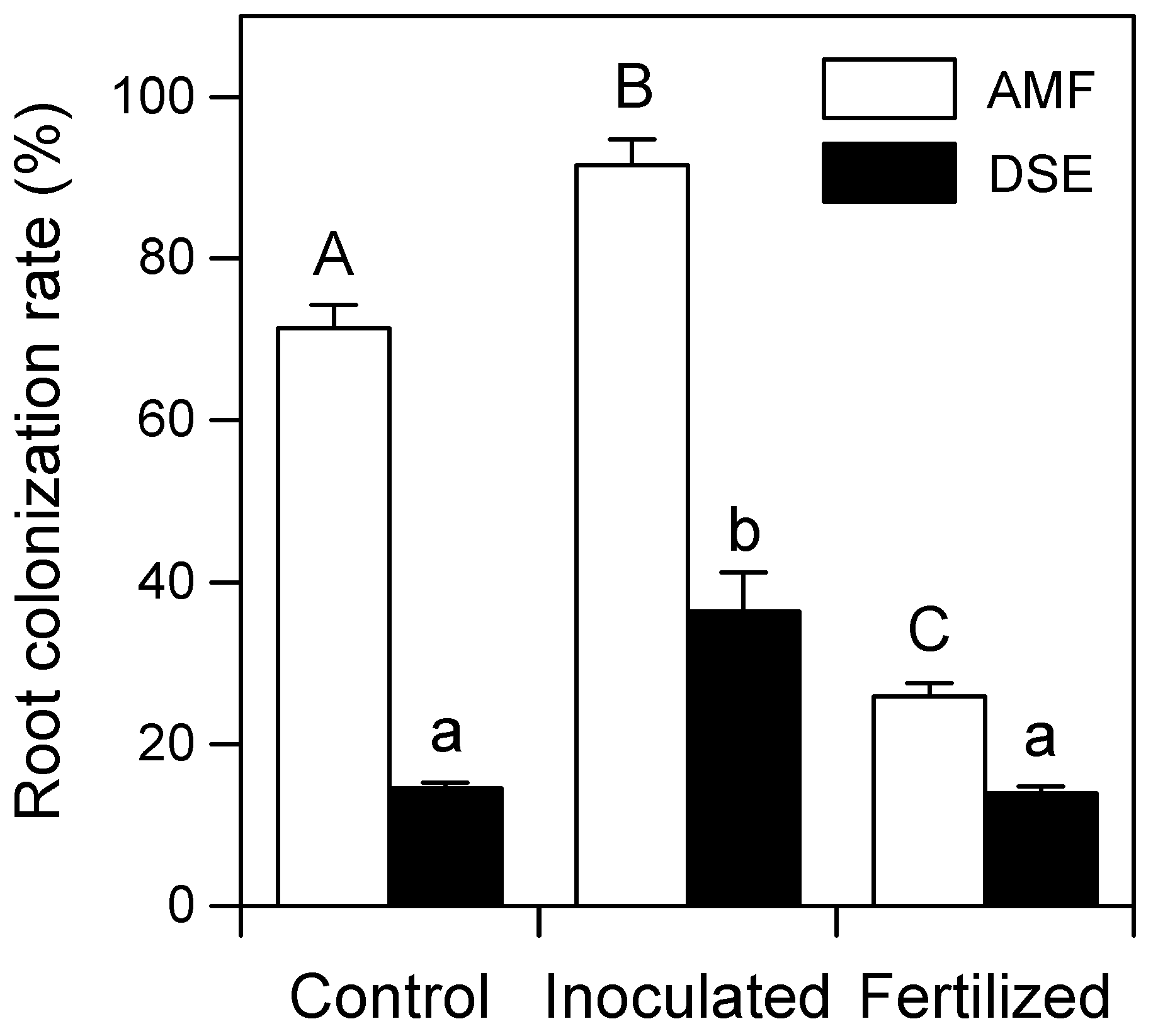
3.2. AMF and DSE Root Colonization Rates
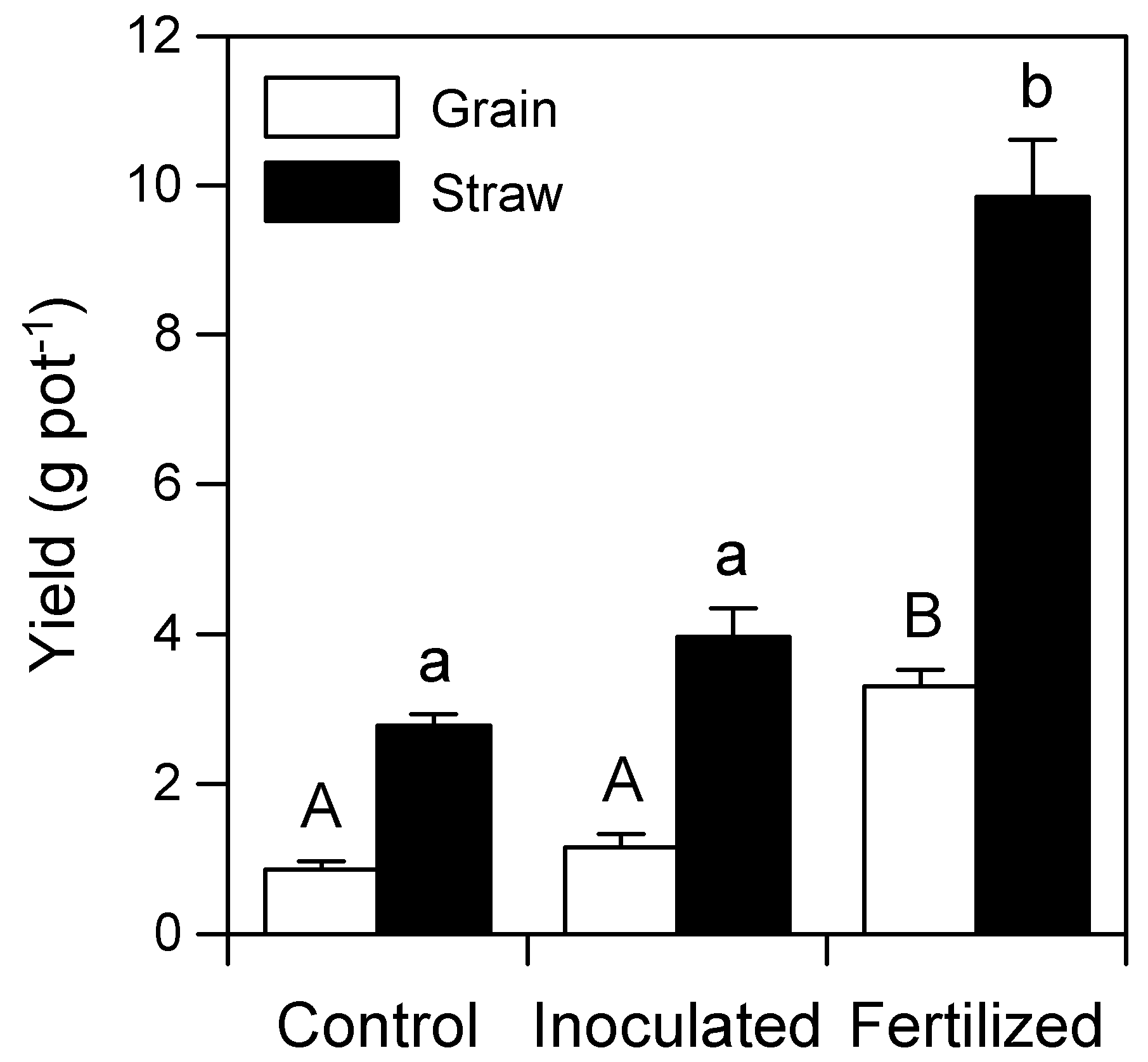
3.3. Teff Biomass and Grain Yield
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taffesse, A.; Dorosh, P.; Gemessa, S.A. Crop Production in Ethiopia: Regional Patterns and Trends; International Food Policy Research Institute: Washington, DC, USA, 2011; Volume 16, pp. 53–83. [Google Scholar]
- CSA. The Federal Democratic Republic of Ethiopia Central Statistical Agency Agricultural Sample Survey—Report on Area and Production of Major Crops, vol. I. 2017; Federal Democratic Republic of Ethiopia Central Statistical Agency: Addis Adeba, Ethiopia, 2017.
- Ketema, S. Tef. Eragrostis Tef (Zucc.) Trotter; International Plant Genetic Resources Institute: Addis Adeba, Ethiopia, 1997. [Google Scholar]
- Tefera, H.; Belay, G. Eragrostis tef (zuccagni) trotter. In Prota 1: Cereals and Pulses/Céréales et Légumes Secs. [cd-rom]; Brink, M., Belay, G., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources végétales de l’Afrique tropicale): Wageningen, The Netherlands, 2006. [Google Scholar]
- Mezemir, S. Probiotic potential and nutritional importance of teff (Eragrostis tef (zucc) trotter) enjerra—A review. Afr. J. Food Agric. Nutr. Dev. 2015, 15, 9964–9981. [Google Scholar]
- Cheng, A.; Mayes, S.; Dalle, G.; Demissew, S.; Massawe, F. Diversifying crops for food and nutrition security—A case of teff. Biol. Rev. 2015, 92, 188–198. [Google Scholar] [CrossRef]
- Laekemariam, F.; Gidago, G.; Taye, W. Participatory seeding rates evaluation on teff (Eragrostis teff (zucc.) trotter) using seed spreader in wolaita, south ethiopia: Farmers evaluation and economic analysis. Adv. Life Sci. Technol. 2012, 5, 37–42. [Google Scholar]
- Degu, H.D.; Ohta, M.; Fujimura, T. Drought tolerance of eragrostis tef and development of roots. Int. J. Plant Sci. 2008, 169, 768–775. [Google Scholar] [CrossRef]
- Tefera, M.M. Land-use/land-cover dynamics in nonno district, central ethiopia. J. Sustain. Dev. Afr. 2011, 13, 123–141. [Google Scholar]
- UNEP. Adaptation to Climate-Change Induced Water Stress in the Nile Basin: A Vulnerability Assessment Report; Division of Early Warning and Assessment (DEWA), United Nations Environment Programme: Nairobi, Kenya, 2013. [Google Scholar]
- Oicha, T.; Cornelis, W.M.; Verplancke, H.; Nyssen, J.; Govaerts, B.; Behailu, M.; Haile, M.; Deckers, J. Short-term effects of conservation agriculture on vertisols under tef (Eragrostis tef (zucc.) trotter) in the northern ethiopian highlands. Soil Till. Res. 2010, 106, 294–302. [Google Scholar] [CrossRef]
- Haregeweyn, N.; Tsunekawa, A.; Nyssen, J.; Poesen, J.; Tsubo, M.; Meshesha, D.T.; Schütt, B.; Adgo, E.; Tegegne, F. Soil erosion and conservation in ethiopia: A review. Prog. Phys. Geog. 2015, 39, 750–774. [Google Scholar] [CrossRef]
- Assefa, D.; Rewald, B.; Sandén, H.; Rosinger, C.; Abiyu, A.; Yitaferu, B.; Godbold, D.L. Deforestation and land use strongly effect soil organic carbon and nitrogen stock in northwest ethiopia. Catena 2017, 153, 89–99. [Google Scholar] [CrossRef]
- Balesh, T.; Zapta, F.; Aune, J.; Situala, B. N fertilization, soil type and cultivars effects on n use efficiency in tef (Eragrostis tef (zucc.) trotter). Nutr. Cycl. Agroecosyst. 2005, 71, 203–211. [Google Scholar]
- Habtegebrial, K.; Singh, B.R.; Haile, M. Impact of tillage and nitrogen fertilization on yield, nitrogen use efficiency of tef (eragrostis tef (zucc.) trotter) and soil properties. Soil Till. Res. 2007, 94, 55–63. [Google Scholar] [CrossRef]
- Quinton, J.N.; Govers, G.; Van Oost, K.; Bardgett, R.D. The impact of agricultural soil erosion on biogeochemical cycling. Nat. Geosci. 2010, 3, 311–314. [Google Scholar] [CrossRef]
- Asefa, F.; Debela, A.; Mohammed, M. Evaluation of teff [Eragrostis tef (zuccagni) trotter] responses to different rates of npk along with zn and b in didessa district, southwestern ethiopia. World Appl. Sci. J. 2014, 32, 2245–2249. [Google Scholar]
- Lee, H. Teff, a rising global crop: Current status of teff production and value chain. Open Agric. J. 2018, 12, 194–206. [Google Scholar] [CrossRef]
- Bender, S.F.; van der Heijden, M.G. Soil biota enhance agricultural sustainability by improving crop yield, nutrient uptake and reducing nitrogen leaching losses. J. Appl. Ecol. 2015, 52, 228–239. [Google Scholar] [CrossRef]
- Carpenter, F.L.; Mayorga, S.P.; Quintero, E.G.; Schroeder, M. Land-use and erosion of a costa rican ultisol affect soil chemistry, mycorrhizal fungi and early regeneration. Forest Ecol. Manag. 2001, 144, 1–17. [Google Scholar] [CrossRef]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Godbold, D.; Sharrock, R. Mycorrhizas. In Trees, Crops and Soil Fertility: Concepts and Research Methods; Schroth, G., Sinclair, F.L., Eds.; CABI: Cambridge, UK, 2003. [Google Scholar]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.-M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fert. Soils 2003, 37, 1–16. [Google Scholar]
- Sharif, M.; Saud, S.; Burni, T.; Afzal, M.; Khan, F.; Khan, M.J.; Wahid, F. Effect of arbuscular mycorrhizal fungal inoculation in combination with different organic fertilizers on maize crop in eroded soils. Pak. J. Bot. 2012, 44, 1427–1432. [Google Scholar]
- Mamo, T.; Killham, K. Effect of soil liming and vesicular-arbuscular-mycorrhizal inoculation on the growth and micronutrient content of the teff plant. Plant Soil 1987, 102, 257–259. [Google Scholar] [CrossRef]
- Jumpponen, A. Dark septate endophytes–are they mycorrhizal? Mycorrhiza 2001, 11, 207–211. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Fernando, A.A.; Currah, R.S. A comparative study of the effects of the root endophytes Leptodontidium orchidicola and Phialocephala fortinii (fungi imperfecti) on the growth of some subalpine plants in culture. Can. J. Bot. 1996, 74, 1071–1078. [Google Scholar] [CrossRef]
- Vergara, C.; Araujo, K.E.C.; Alves, L.S.; Souza, S.R.D.; Santos, L.A.; Santa-Catarina, C.; da Silva, K.; Pereira, G.M.D.; Xavier, G.R.; Zilli, J.É. Contribution of dark septate fungi to the nutrient uptake and growth of rice plants. Braz. J. Microbiol. 2018, 49, 67–78. [Google Scholar] [CrossRef]
- Mandyam, K.; Jumpponen, A. Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud. Mycol. 2005, 53, 173–189. [Google Scholar] [CrossRef]
- Jumpponen, A.; Trappe, J.M. Dark septate endophytes: A review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Li, L.; Zhao, Z.-W. The colonization of plants by dark septate endophytes (dse) in the valley-type savanna of yunnan, southwest China. Af. J. Microbiol Res. 2011, 5, 5540–5547. [Google Scholar] [CrossRef]
- Assefa, K.; Aliye, S.; Belay, G.; Metaferia, G.; Tefera, H.; Sorrells, M.E. Quncho: The first popular tef variety in ethiopia. Int. J. Agric. Sustain. 2011, 9, 25–34. [Google Scholar] [CrossRef]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piché, Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microb. 1998, 64, 5004–5007. [Google Scholar]
- Vierheilig, H.; Schweiger, P.; Brundrett, M. An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots. Physiol. Plantarum 2005, 125, 393–404. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.; Miller, M.; Evans, D.; Fairchild, G.; Swan, J. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Brundrett, M.; Bougher, N.; Dell, B.; Grove, T. Working with Mycorrhizas in Forestry and Agriculture; Australian Centre for International Agricultural Research: Canberra, Australia, 1996. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Birhane, E.; Aregawi, K.; Giday, K. Changes in arbuscular mycorrhiza fungi spore density and root colonization of woody plants in response to exclosure age and slope position in the highlands of tigray, northern ethiopia. J. Arid Environ. 2017, 142, 1–10. [Google Scholar] [CrossRef]
- Raznikiewicz, H.; Carlgren, K.; Maartensson, A. Impact of phosphorus fertilization and liming on the presence of arbuscular mycorrhizal spores in a swedish long-term field experiment. Swed. J. Agric. Res. (Sweden) 1994, 24, 157–164. [Google Scholar]
- Egerton-Warburton, L.M.; Allen, E.B. Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecol. Appl. 2000, 10, 484–496. [Google Scholar] [CrossRef]
- Bhadalung, N.N.; Suwanarit, A.; Dell, B.; Nopamornbodi, O.; Thamchaipenet, A.; Rungchuang, J. Effects of long-term np-fertilization on abundance and diversity of arbuscular mycorrhizal fungi under a maize cropping system. Plant Soil 2005, 270, 371–382. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, X.; Guo, R.; Guo, J. Response of am fungi spore population to elevated temperature and nitrogen addition and their influence on the plant community composition and productivity. Sci. Rep. 2016, 6, 24749. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Valentine, A.J.; Mortimer, P.E.; Kleinert, A.; Kang, Y.; Benedito, V.A. Carbon metabolism and costs of arbuscular mycorrhizal associations to host roots. In Symbiotic Endophytes; Aroca, R., Ed.; Springer: Berlin, Germany, 2013; pp. 233–252. [Google Scholar]
- Li, L.F.; Zhang, Y.; Zhao, Z.W. Arbuscular mycorrhizal colonization and spore density across different land-use types in a hot and arid ecosystem, southwest China. J. Plant Nutr. Soil Sc. 2007, 170, 419–425. [Google Scholar] [CrossRef]
- Tchabi, A.; Coyne, D.; Hountondji, F.; Lawouin, L.; Wiemken, A.; Oehl, F. Arbuscular mycorrhizal fungal communities in sub-saharan savannas of benin, west africa, as affected by agricultural land use intensity and ecological zone. Mycorrhiza 2008, 18, 181–195. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Zhao, Z. Arbuscular mycorrhizas in a valley-type savanna in southwest China. Mycorrhiza 2004, 14, 323–327. [Google Scholar]
- Delelegn, Y.T.; Purahong, W.; Blazevic, A.; Yitaferu, B.; Wubet, T.; Sandén, H.; Godbold, D. Changes in land use alter soil quality and aggregate stability in the highlands of northern ethiopia. Sci. Rep. 2017, 7, 13602. [Google Scholar] [CrossRef]
- Řezáčová, V.; Konvalinková, T.; Jansa, J. Carbon fluxes in mycorrhizal plants. In Mycorrhiza-Eco-Physiology, Secondary Metabolites, Nanomaterials, 4th ed.; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Lenoir, I.; Fontaine, J.; Sahraoui, A.L.-H. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef]
- Pozo, M.J.; Azcón-Aguilar, C. Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef]
- Zhang, Q.; Gong, M.; Yuan, J.; Hou, Y.; Zhang, H.; Wang, Y.; Hou, X. Dark septate endophyte improves drought tolerance in sorghum. Int. J. Agric. Biol. 2017, 19, 53–60. [Google Scholar] [CrossRef]
- Newsham, K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011, 190, 783–793. [Google Scholar] [CrossRef]
- Rho, H.; Hsieh, M.; Kandel, S.L.; Cantillo, J.; Doty, S.L.; Kim, S.-H. Do endophytes promote growth of host plants under stress? A meta-analysis on plant stress mitigation by endophytes. Microb. Ecol. 2018, 75, 407–418. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Getachew, G.; Rewald, B.; Godbold, D.L.; Sandén, H. Endophytic Fungal Root Colonization of Eragrostis tef in Eroded Croplands of the Ethiopian Highlands Is Limited by Low Spore Density and Fertilisation. Agronomy 2019, 9, 73. https://doi.org/10.3390/agronomy9020073
Getachew G, Rewald B, Godbold DL, Sandén H. Endophytic Fungal Root Colonization of Eragrostis tef in Eroded Croplands of the Ethiopian Highlands Is Limited by Low Spore Density and Fertilisation. Agronomy. 2019; 9(2):73. https://doi.org/10.3390/agronomy9020073
Chicago/Turabian StyleGetachew, Gezahagn, Boris Rewald, Douglas L. Godbold, and Hans Sandén. 2019. "Endophytic Fungal Root Colonization of Eragrostis tef in Eroded Croplands of the Ethiopian Highlands Is Limited by Low Spore Density and Fertilisation" Agronomy 9, no. 2: 73. https://doi.org/10.3390/agronomy9020073
APA StyleGetachew, G., Rewald, B., Godbold, D. L., & Sandén, H. (2019). Endophytic Fungal Root Colonization of Eragrostis tef in Eroded Croplands of the Ethiopian Highlands Is Limited by Low Spore Density and Fertilisation. Agronomy, 9(2), 73. https://doi.org/10.3390/agronomy9020073






