Variation in Stripe Rust Resistance and Morphological Traits in Wild Emmer Wheat Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Field Evaluation of Stripe Rust Resistance and Morphological Traits
2.3. Analysis of Presence/Absence Polymorphism in Yr15 and Yr36
2.4. Statistical Analysis
3. Results
3.1. Variation in Stripe Rust Resistance
3.2. Variation in Morphological Traits
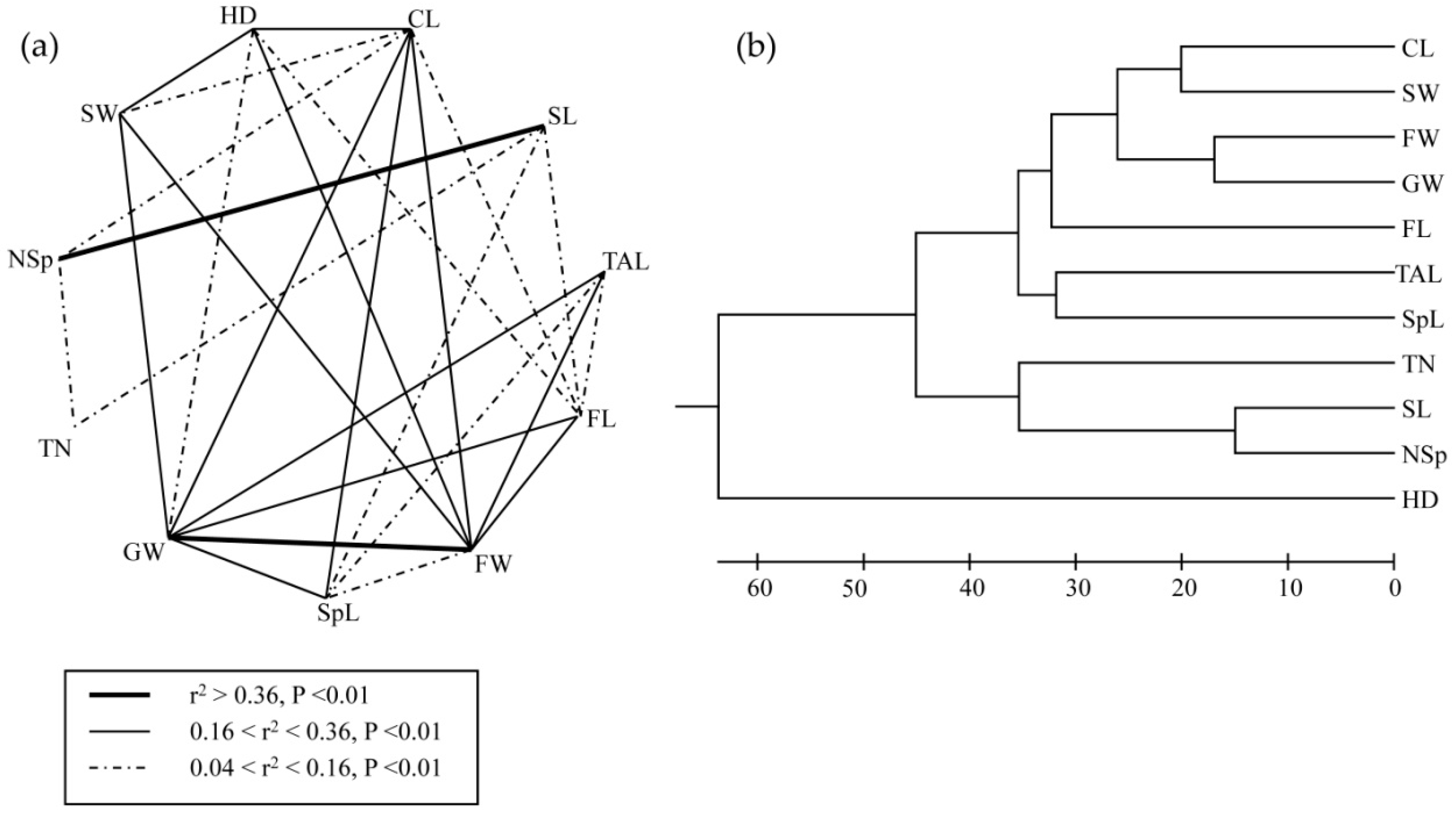
3.3. Associations between Morphological Traits and Ecogeographical Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- McFadden, E.; Sears, E. The origin of Triticum spelta and its free-threshing hexaploid relatives. J. Hered. 1946, 37, 107–116. [Google Scholar] [CrossRef]
- Harlan, J.R.; Zohary, D. Distribution of wild wheats and barley. Science 1966, 153, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Zohary, D.; Hopf, M. Domestication of plants in the old world. J. Appl. Ecol. 1993, 130, 365–372. [Google Scholar]
- Oppenheimer, H.R. Ecological relationship of wild emmer in Israel and A. Aaronsohn’s contribution to the theory of the origin of cultivated wheat. Genet. Agrar. 1963, 17, 249–258. [Google Scholar]
- Feldman, M.; Sears, E.R. The wild gene resources of wheat. Sci. Am. 1981, 244, 102–112. [Google Scholar] [CrossRef]
- Ozkan, H.; Willcox, G.; Graner, A.; Salamini, F.; Kilian, B. Geographic distribution and domestication of wild emmer wheat (Triticum dicoccoides). Genet. Resour. Crop Evol. 2011, 58, 11–53. [Google Scholar] [CrossRef]
- Uauy, C.; Brevis, J.C.; Dubcovsky, J. The high grain protein content gene Gpc-B1 accelerates senescence and has pleiotropic effects on protein content in wheat. J. Exp. Bot. 2006, 57, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Saranga, Y.; Yazici, A.; Fahima, T.; Ozturk, L.; Cakmak, I. Grain zinc, iron and protein concentrations and zinc-efficiency in wild emmer wheat under contrasting irrigation regimes. Plant Soil 2008, 306, 57–67. [Google Scholar] [CrossRef]
- Bonfil, D.J.; Kafkafi, U. Wild wheat adaptation in different soil ecosystems as expressed in the mineral concentration of the seeds. Euphytica 2000, 114, 123–134. [Google Scholar] [CrossRef]
- Peleg, Z.; Fahima, T.; Abbo, S.; Krugman, T.; Nevo, E.; Yakir, D.; Saranga, Y. Genetic diversity for drought resistance in wild emmer wheat and its ecogeographical associations. Plant Cell Environ. 2005, 28, 176–191. [Google Scholar] [CrossRef]
- Peleg, Z.; Fahima, T.; Krugman, T.; Abbo, S.; Yakir, D.; Korol, A.B.; Saranga, Y. Genomic dissection of drought resistance in durum wheat x wild emmer wheat recombinant inbreed line population. Plant Cell Environ. 2009, 32, 758–779. [Google Scholar] [CrossRef] [PubMed]
- Krugman, T.; Chague, V.; Peleg, Z.; Balzergue, S.; Just, J.; Korol, A.B.; Nevo, E.; Saranga, Y.; Chalhoub, B.; Fahima, T. Multilevel regulation and signalling processes associated with adaptation to terminal drought in wild emmer wheat. Funct. Integr. Genom. 2010, 10, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Krugman, T.; Peleg, Z.; Quansah, L.; Chague, V.; Korol, A.B.; Nevo, E.; Saranga, Y.; Fait, A.; Chalhoub, B.; Fahima, T. Alteration in expression of hormone-related genes in wild emmer wheat roots associated with drought adaptation mechanisms. Funct. Integr. Genom. 2011, 11, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, R.; Xie, W.L.; Peleg, Z.; Saranga, Y.; Dinoor, A.; Fahima, T. Identification and mapping of PmG16, a powdery mildew resistance gene derived from wild emmer wheat. Theor. Appl. Genet. 2010, 121, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yan, J.; Sela, H.; Manisterski, J.; Lewinsohn, D.; Nevo, E.; Fahima, T. Pathogen race determines the type of resistance response in the stripe rust-Triticum dicoccoides pathosystem. Physiol. Plant. 2010, 139, 269–279. [Google Scholar] [PubMed]
- Anikster, Y.; Manisterski, J.; Long, D.L.; Leonard, K.J. Leaf rust and stem rust resistance in Triticum dicoccoides populations in Israel. Plant Dis. 2005, 89, 55–62. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Alimari, A.; Steiner, B.; Buerstmayr, H. Genetic mapping of QTL for resistance to fusarium head blight spread (type 2 resistance) in a Triticum dicoccoides x Triticum durum backcross-derived population. Theor. Appl. Genet. 2013, 126, 2825–2834. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Raats, D.; Sela, H.; Klymiuk, V.; Lidzbarsky, G.; Feng, LH.; Krugman, T.; Fahima, T. Evolution and adaptation of wild emmer wheat populations to biotic and abiotic stresses. Annu. Rev. Phytopathol. 2016, 54, 279–301. [Google Scholar] [CrossRef] [PubMed]
- Sela, H.; Ezrati, S.; Ben-Yehuda, P.; Manisterski, J.; Akhunov, E.; Dvorak, J.; Breiman, A.; Korol, A. Linkage disequilibrium and association analysis of stripe rust resistance in wild emmer wheat (Triticum turgidum ssp. dicoccoides) population in Israel. Theor. Appl. Genet. 2014, 127, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- The, T.T.; Nevo, E.; McIntosh, R.A. Responses of Israeli wild emmers to selected Australian pathotypes of Puccinia species. Euphytica 1993, 71, 75–81. [Google Scholar] [CrossRef]
- Van Silfhout, C.H. Identification and Characterization of Resistance to Yellow Rust and Powdery Mildew in Wild Emmer Wheat and Their Transfer to Bread Wheat. Ph.D. Thesis, Wageningen University, Wageningen, The Netherland, 23 November 1989. [Google Scholar]
- Gerechter-Amitai, Z.K.; van Silfhout, C.H. Race-specificity of temperature-sensitive genes for resistance to Puccinia striiformis in Triticum dicoccoides. Euphytica 1989, 43, 7–14. [Google Scholar] [CrossRef]
- Yaniv, E.; Raats, D.; Ronin, Y.; Korol, A.B.; Grama, A.; Bariana, H.; Dubcovsky, J.; Schulman, A.H.; Fahima, T. Evaluation of marker-assisted selection for the stripe rust resistance gene Yr15, introgressed from wild emmer wheat. Mol. Breed. 2015, 35, 43. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.H.; Fahima, T.; Roder, M.S.; Li, Y.C.; Dahan, A.; Grama, A.; Ronin, Y.I.; Korol, A.B.; Nevo, E. Microsatellite tagging of the stripe-rust resistance gene YrH52 derived from wild emmer wheat, Triticum dicoccoides, and suggestive negative crossover interference on chromosome 1B. Theor. Appl. Genet. 1999, 98, 862–872. [Google Scholar] [CrossRef]
- Marais, G.F.; Pretorius, Z.A.; Wellings, C.R.; McCallum, B.; Marais, A.S. Leaf rust and stripe rust resistance genes transferred to common wheat from Triticum dicoccoides. Euphytica 2005, 143, 115–123. [Google Scholar] [CrossRef]
- Dadkhodaie, N.A.; Karaoglou, H.; Wellings, C.R.; Park, R.F. Mapping genes Lr53 and Yr35 on the short arm of chromosome 6B of common wheat with microsatellite markers and studies of their association with Lr36. Theor. Appl. Genet. 2011, 122, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.L.; Uauy, C.; Distelfeld, A.; Blechl, A.; Epstein, L.; Chen, X.M.; Sela, H.; Fahima, T.; Dubcovsky, J. A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 2009, 323, 1357–1360. [Google Scholar] [CrossRef]
- Uauy, C.; Brevis, J.C.; Chen, X.M.; Khan, I.; Jackson, L.; Chicaiza, O.; Distelfeld, A.; Fahima, T.; Dubcovsky, J. High-temperature adult-plant (HTAP) stripe rust resistance gene Yr36 from Triticum turgidum ssp. dicoccoides is closely linked to the grain protein content locus Gpc-B1. Theor. Appl. Genet. 2005, 112, 97–105. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Wang, C.Y.; Wang, Y.J.; Zhou, X.L.; Lv, S.K.; Liu, X.L.; Kang, Z.S.; Ji, W.Q. Molecular mapping and marker development for the Triticum dicoccoides-derived stripe rust resistance gene YrSM139-1B in bread wheat cv. Shaanmai 139. Theor. Appl. Genet. 2016, 129, 369–376. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Xie, J.Z.; Guo, L.; Zhang, D.Y.; Li, G.Q.; Fang, T.L.; Chen, Y.X.; Li, J.; Wu, Q.H.; Lu, P.; et al. Molecular mapping of YrTZ2, a stripe rust resistance gene in wild emmer accession TZ-2 and its comparative analyses with Aegilops tauschii. J. Integr. Agr. 2018, 17, 1267–1275. [Google Scholar] [CrossRef]
- Klymiuk, V.; Yaniv, E.; Huang, L.; Raats, D.; Fatiukha, A.; Chen, S.; Feng, L.; Frenkel, Z.; Krugman, T.; Lidzbarsky, G.; et al. Cloning of the wheat Yr15 resistance gene sheds light on the plant tandem kinase-pseudokinase family. Nat. Commun. 2018, 9, 3735. [Google Scholar] [CrossRef]
- Nevo, E.; Golenberg, E.; Beiles, A.; Brown, A.H.D.; Zohary, D. Genetic diversity and environmental associations of wild wheat, Triticum dicoccoides, in Israel. Theor. Appl. Genet. 1982, 62, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Nevo, E.; Beiles, A.; Gutterman, Y.; Storch, N.; Kaplan, D. Genetic-resources of wild cereals in Israel and Vicinity. 1. Phenotypic variation within and between populations of wild wheat, Triticum dicoccoides. Euphytica 1984, 33, 717–735. [Google Scholar] [CrossRef]
- Kato, K.; Mori, Y.; Beiles, A.; Nevo, E. Geographical variation in heading traits in wild emmer wheat, Triticum dicoccoides. 1. Variation in vernalization response and ecological differentiation. Theor. Appl. Genet. 1997, 95, 546–552. [Google Scholar] [CrossRef]
- Kato, K.; Tanizoe, C.; Beiles, A.; Nevo, E. Geographical variation in heading traits in wild emmer wheat, Triticum dicoccoides. II. Variation in heading date and adaptation to diverse eco-geographical conditions. Hereditas 1998, 128, 33–39. [Google Scholar] [CrossRef]
- Huang, L.; Sela, H.; Feng, L.; Chen, Q.; Krugman, T.; Yan, J.; Dubcovsky, J.; Fahima, T. Distribution and haplotype diversity of WKS resistance genes in wild emmer wheat natural populations. Theor. Appl. Genet. 2016, 129, 921–934. [Google Scholar] [CrossRef]
- Line, R.F.; Qayoum, A. Virulence, Aggressiveness, Evolution, and Distribution of Races of Puccinia striiformis (the Causes of Stripe Rust of Wheat) in North America, 1968–1987; Technical Bulletin 1788; United State Department of Agriculture: Washington, DC, USA, 1992.
- Rogers, S.O.; Bendich, A.J. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol. Biol. 1985, 5, 69–76. [Google Scholar] [CrossRef]
- Garcia-Vallve, S.; Palau, J.; Romeu, A. Horizontal gene transfer in glycosyl hydrolases inferred from codon usage in Escherichia coli and Bacillus subtilis. Mol. Biol. Evol. 1999, 16, 1125–1134. [Google Scholar] [CrossRef]
- Wan, A.M.; Zhao, Z.H.; Chen, X.M.; He, Z.H.; Jin, S.L.; Jia, Q.Z.; Yao, G.; Yang, J.X.; Wang, B.T.; Li, G.B.; et al. Wheat stripe rust epidemic and virulence of Puccinia striiformis f. sp. tritici in China in 2002. Plant Dis. 2004, 88, 896–904. [Google Scholar] [CrossRef]
- Han, D.J.; Wang, Q.L.; Chen, X.M.; Zeng, Q.D.; Wu, J.H.; Xue, W.B.; Zhan, G.M.; Huang, L.L.; Kang, Z.S. Emerging Yr26-virulent races of Puccinia striiformis f. tritici are threatening wheat production in the Sichuan basin, China. Plant Dis. 2015, 99, 754–760. [Google Scholar]
- Nevo, E. Molecular evolution and ecological stress at global, regional and local scales: The Israeli perspective. J. Exp. Zool. 1998, 282, 95–119. [Google Scholar] [CrossRef]
- Hoffman, A.A.; Parsons, P.A. Evolutionary Genetics and Environmental Stress; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Parsons, P.A. Environments and evolution: Interactions between stress, resource inadequacy and energetic efficiency. Biol. Rev. 2005, 80, 589–610. [Google Scholar] [CrossRef] [PubMed]
- Fahima, T.; Roder, M.S.; Wendehake, K.; Kirzhner, V.M.; Nevo, E. Microsatellite polymorphism in natural populations of wild emmer wheat, Triticum dicoccoides, in Israel. Theor. Appl. Genet. 2002, 104, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Fahima, T.; Sun, G.L.; Beharav, A.; Krugman, T.; Beiles, A.; Nevo, E. RAPD polymorphism of wild emmer wheat populations, Triticum dicoccoides, in Israel. Theor. Appl. Genet. 1999, 98, 434–447. [Google Scholar] [CrossRef]
- Li, Y.C.; Fahima, T.; Korol, A.B.; Peng, J.H.; Roder, M.S.; Kirzhner, V.; Beiles, A.; Nevo, E. Microsatellite diversity correlated with ecological-edaphic and genetic factors in three microsites of wild emmer wheat in North Israel. Mol. Biol. Evol. 2000, 17, 851–862. [Google Scholar] [CrossRef]
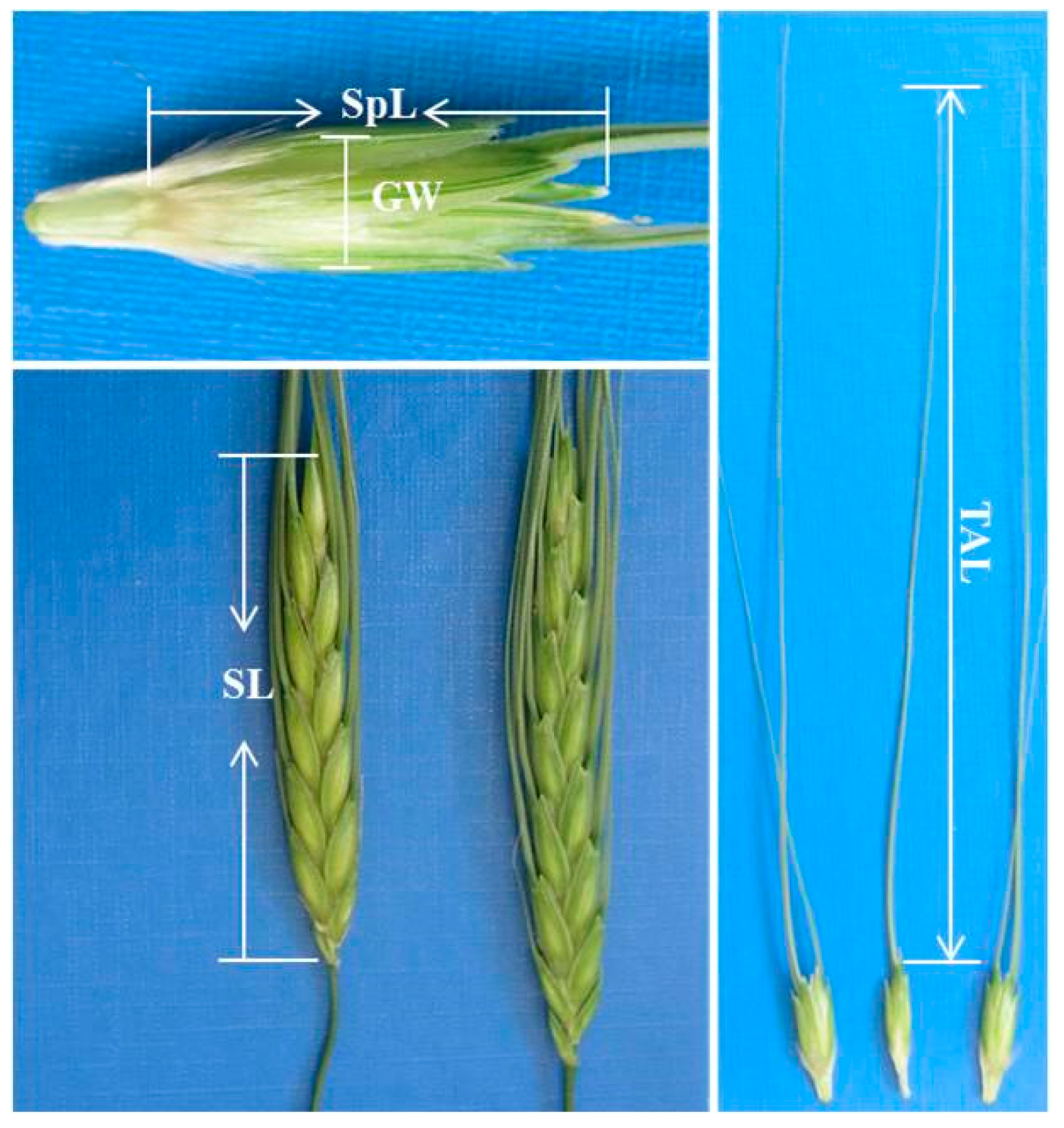
| Trait | Average | STDV | Min. | Median | Max. | ||
|---|---|---|---|---|---|---|---|
| HD | Heading date | (day) | 144.40 | 5.32 | 129.33 | 144.33 | 157.67 |
| CL | Culm length | (cm) | 100.95 | 14.69 | 53.65 | 101.70 | 128.57 |
| SL | Spike length | (cm) | 9.07 | 1.01 | 7.10 | 9.00 | 12.47 |
| TAL | Top awn length | (cm) | 14.71 | 2.21 | 8.20 | 14.43 | 19.30 |
| FL | Flag leaf length | (cm) | 18.68 | 3.68 | 9.85 | 18.30 | 28.10 |
| FW | Flag leaf width | (cm) | 1.19 | 0.21 | 0.65 | 1.17 | 1.77 |
| SpL | Spikelet length | (mm) | 16.72 | 1.25 | 13.52 | 16.67 | 20.33 |
| GW | Empty glume width | (mm) | 5.13 | 0.66 | 3.43 | 5.00 | 7.15 |
| TN | Tiller number | 18.81 | 7.00 | 9.00 | 17.33 | 47.67 | |
| NSp | Number of spikelets per spike | 30.38 | 4.66 | 21.00 | 30.00 | 46.33 | |
| SW | Fifty seeds weight | (g) | 1.33 | 0.38 | 0.67 | 1.34 | 2.24 |
| Lat | Lon | Alt | Rn | Tjmin | Tsr | Tj | Tjmax | |
|---|---|---|---|---|---|---|---|---|
| HD | −0.088 | −0.353 ** | 0.219 * | 0.341 ** | −0.250 * | −0.439 ** | −0.109 | −0.447 ** |
| CL | 0.463 ** | 0.524 ** | −0.147 | 0.184 | −0.016 | 0.398 ** | −0.086 | 0.096 |
| SL | 0.002 | 0.004 | 0.196 | −0.005 | −0.164 | −0.021 | −0.158 | −0.132 |
| TAL | 0.203 * | 0.058 | −0.079 | 0.007 | 0.071 | −0.066 | 0.108 | 0.022 |
| FL | −0.071 | 0.110 | −0.199 * | −0.231 * | 0.227 * | 0.209 * | 0.121 | 0.282 ** |
| FW | 0.276 ** | 0.350 ** | −0.336 ** | −0.101 | 0.307 ** | 0.314 ** | 0.229 * | 0.410 ** |
| SpL | 0.210 * | 0.143 | −0.108 | −0.084 | 0.139 | 0.038 | 0.149 | 0.133 |
| GW | 0.346 ** | 0.359 ** | −0.446 ** | −0.166 | 0.400 ** | 0.270 ** | 0.300 ** | 0.413 ** |
| TN | 0.084 | 0.083 | 0.136 | 0.268 ** | −0.183 | 0.075 | −0.232 * | −0.139 |
| NSp | −0.152 | 0.007 | 0.357 ** | 0.053 | −0.322 ** | 0.091 | −0.376 ** | −0.257 * |
| SW | 0.440 ** | 0.587 ** | −0.189 | −0.026 | 0.084 | 0.507 ** | −0.026 | 0.265 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Feng, L.; He, Y.; Tang, Z.; He, J.; Sela, H.; Krugman, T.; Fahima, T.; Liu, D.; Wu, B. Variation in Stripe Rust Resistance and Morphological Traits in Wild Emmer Wheat Populations. Agronomy 2019, 9, 44. https://doi.org/10.3390/agronomy9020044
Huang L, Feng L, He Y, Tang Z, He J, Sela H, Krugman T, Fahima T, Liu D, Wu B. Variation in Stripe Rust Resistance and Morphological Traits in Wild Emmer Wheat Populations. Agronomy. 2019; 9(2):44. https://doi.org/10.3390/agronomy9020044
Chicago/Turabian StyleHuang, Lin, Lihua Feng, Yu He, Zizhong Tang, Jingshu He, Hanan Sela, Tamar Krugman, Tzion Fahima, Dengcai Liu, and Bihua Wu. 2019. "Variation in Stripe Rust Resistance and Morphological Traits in Wild Emmer Wheat Populations" Agronomy 9, no. 2: 44. https://doi.org/10.3390/agronomy9020044
APA StyleHuang, L., Feng, L., He, Y., Tang, Z., He, J., Sela, H., Krugman, T., Fahima, T., Liu, D., & Wu, B. (2019). Variation in Stripe Rust Resistance and Morphological Traits in Wild Emmer Wheat Populations. Agronomy, 9(2), 44. https://doi.org/10.3390/agronomy9020044






