Optimizing Crop Load for New Apple Cultivar: “WA38”
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Fruit Grading and Quality Analysis
2.3. Leaf Area Quantification
2.4. Leaf-to-Fruit Ratio Calculations
- Total leaf area per tree (TLA) (sum of five collections) = ∑ LA (m2) (collection 1 to collection 5);
- Total no. of leaves per tree (TLN) = ∑ total no. of leaves in sample (collection 1 to collection 5);
- Leaf-area-to-fruit (LA:F) ratio = TLA/number of fruits per tree [27];
- Leaf-to-fruit ratio (L:F) = TLN/number of fruits per tree [27];
- Specific leaf area (SLA) = average leaf area (cm2)/average leaf fresh weight (g) [39].
2.5. Leaf and Fruit Mineral Analysis
2.6. Return Bloom
2.7. Statistical Analysis
3. Results
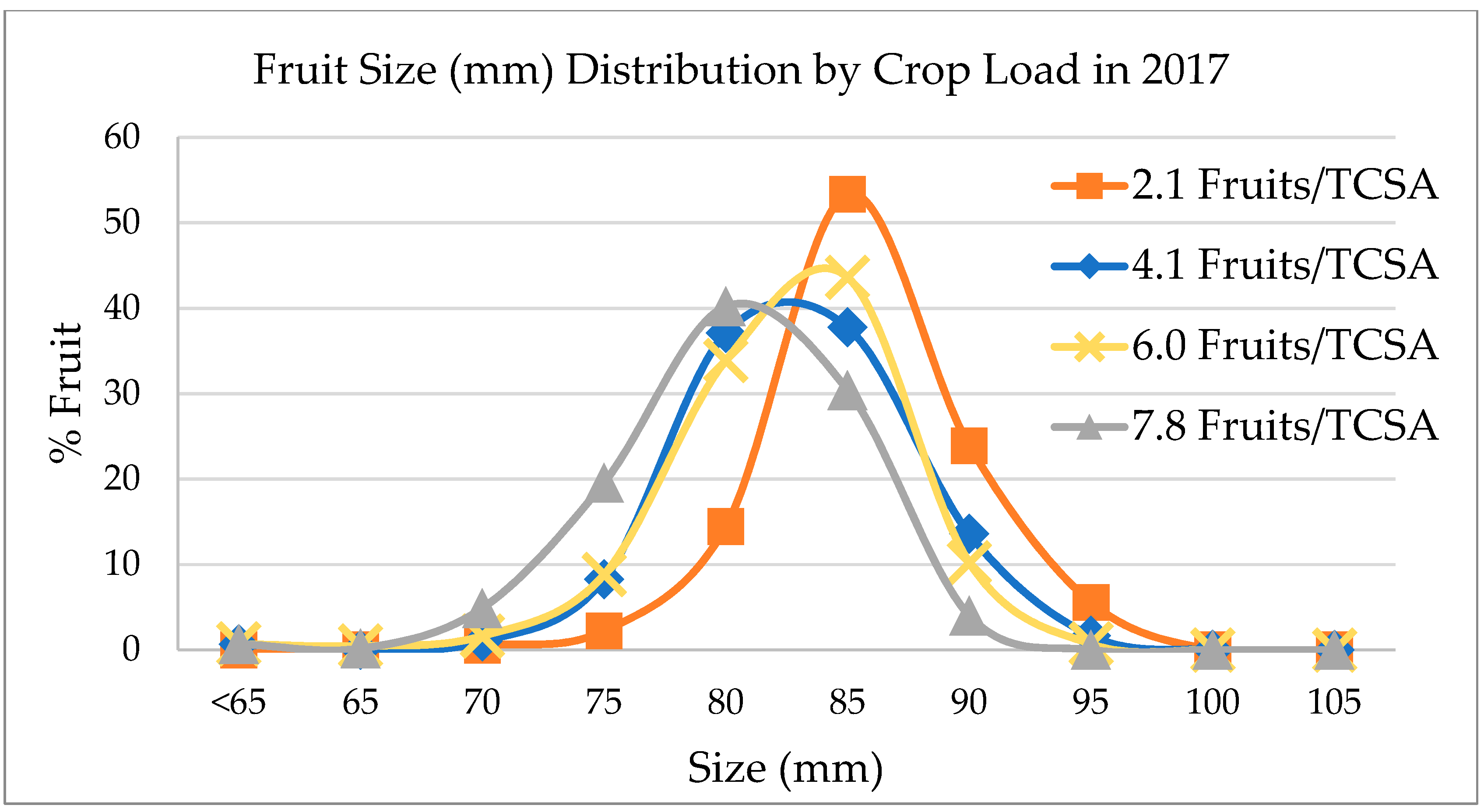
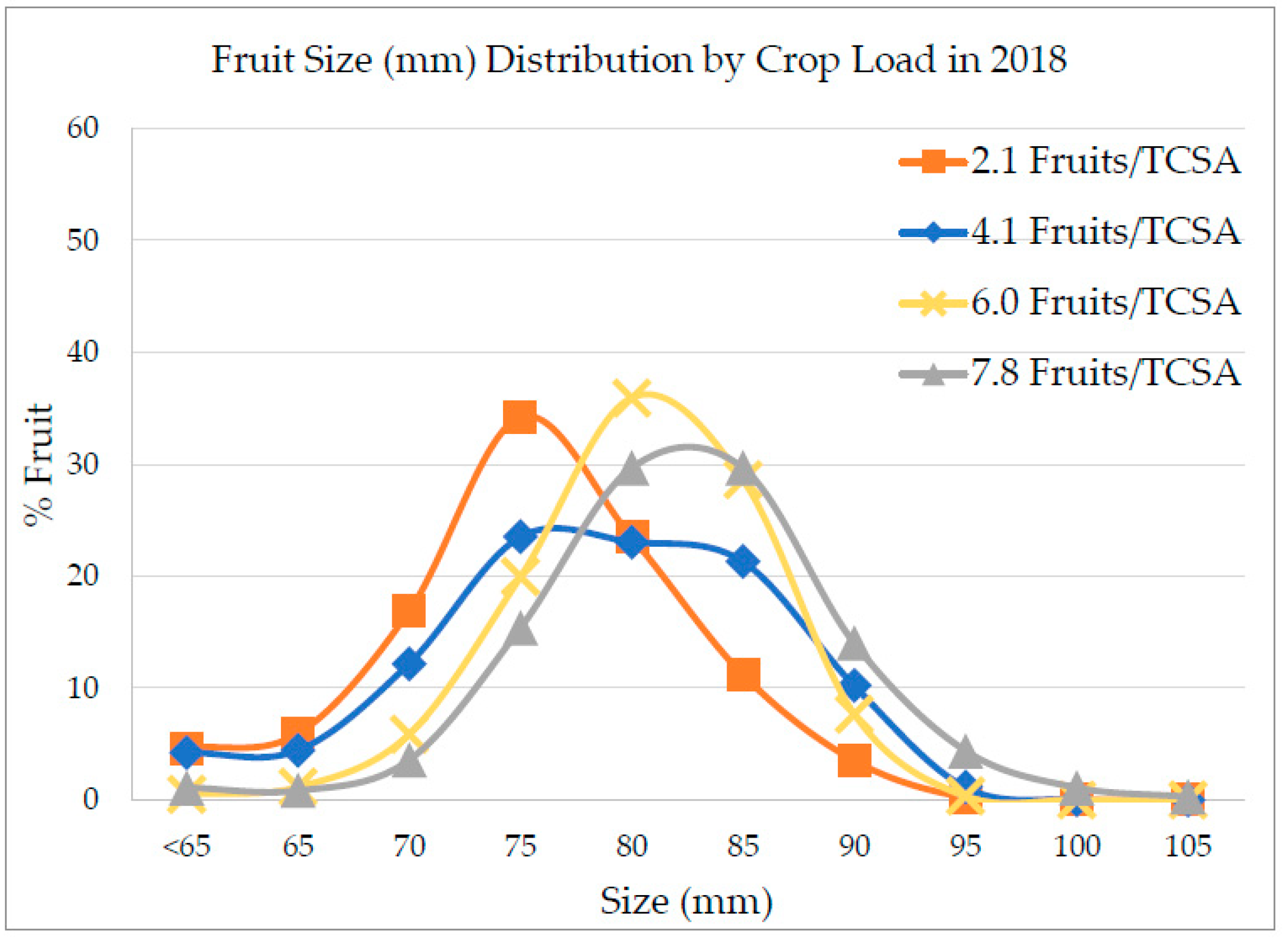
3.1. Crop Load Effect on Yield and Fruit Size
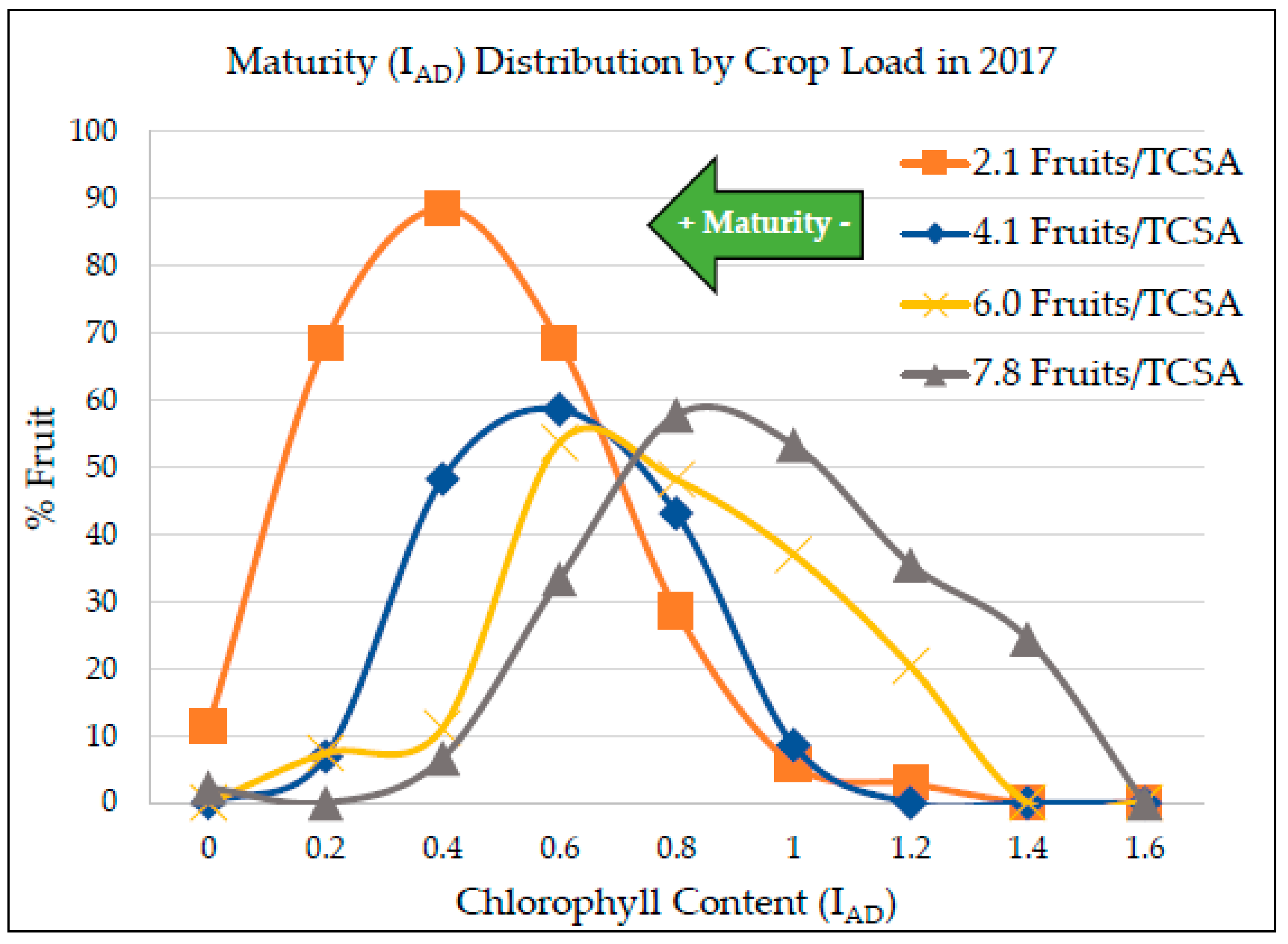
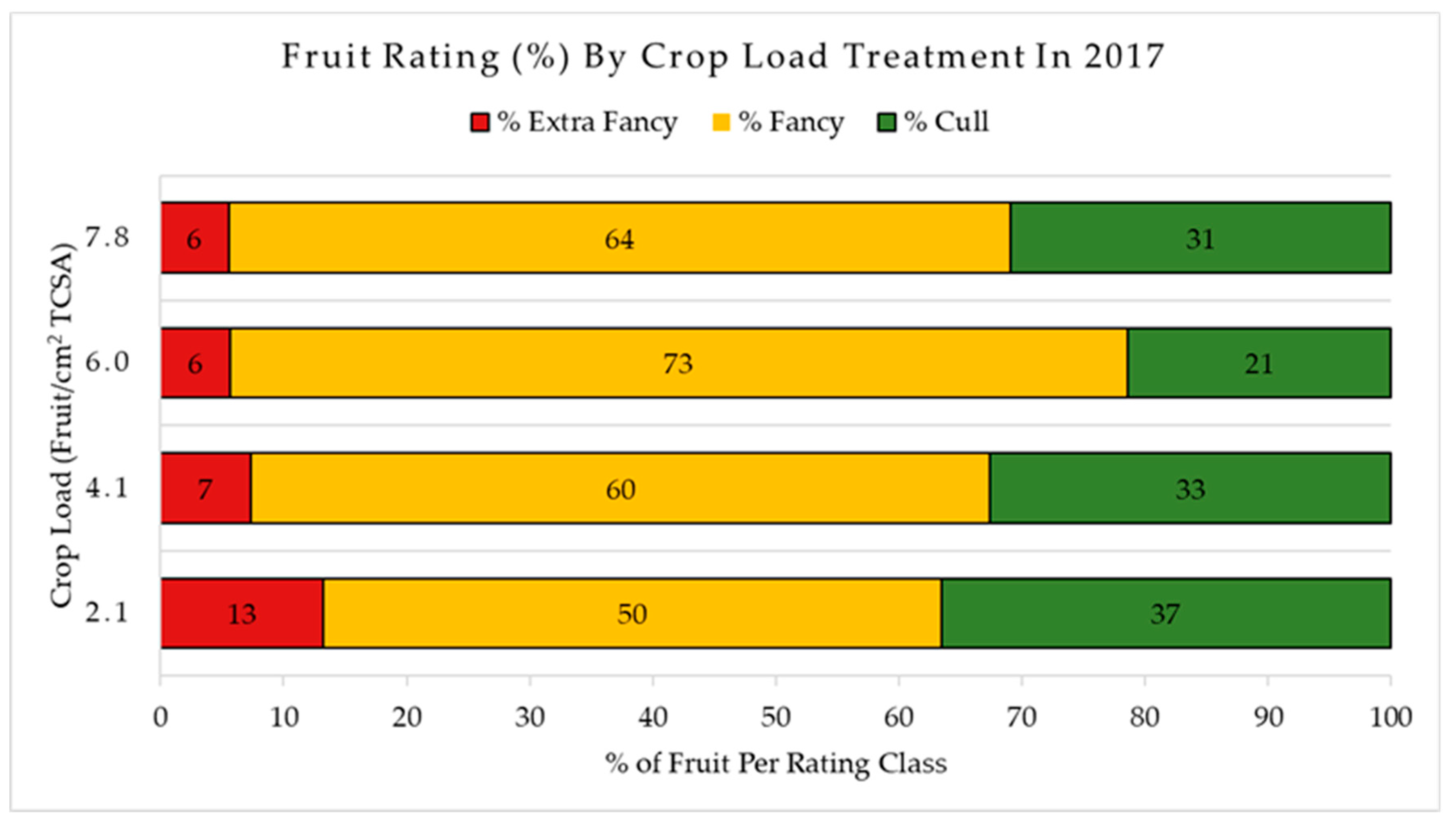
3.2. Crop Load Effect on Fruit Quality
3.3. Crop Load Effect on Vegetative Parameters
3.4. Crop Load Effect on Leaf and Fruit Mineral Composition
3.5. Crop Load Effect on Return Bloom
4. Discussion
4.1. Crop Load Effect on Yield and Fruit Size
4.2. Crop Load Effect on Fruit Quality
4.3. Crop Load Effect on Vegetative Parameters
4.4. Crop Load Effect on Leaf and Fruit Mineral Composition
4.5. Crop Load Effect on Return Bloom
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cosmic Crisp Plantings Beat Estimate. Available online: https://www.cosmiccrisp.com/news/2018/4/30/cosmic-crisp-plantings-beat-estimate (accessed on 10 January 2019).
- Evans, K.M.; Barritt, B.H.; Konishi, B.S.; Brutcher, L.J.; Ross, C.F. ‘WA 38’apple. HortScience 2012, 47, 1177–1179. [Google Scholar] [CrossRef]
- Lespinasse, J.M.; Chol, P.; Dupin, J.; Terenne, E. La conduite du Pommier: Types de Fructification, Incidence sur la conduite de l’arbre; Brochure INVUFLEC: Paris, France, 1977; p. 80. [Google Scholar]
- Musacchi, S.; Greene, D. Innovations in apple tree cultivation to manage crop load and ripening. In Achieving Sustainable Cultivation of Apples; Evans, K., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; pp. 195–237. [Google Scholar]
- Mohammadi, A.; Mahmoudi, M.J.; Rezaee, R. Vegetative and reproductive responses of some apple cultivars (Malus domestica Borkh.) to heading back pruning. Int. J AgriSci. 2013, 3, 628–635. [Google Scholar]
- ‘WA38’ Horticulture: Characteristics and Preliminary Results. Available online: http://treefruit.wsu.edu/web-article/wa38-horticulture/ (accessed on 10 January 2019).
- Celton, J.M.; Kelner, J.J.; Martinez, S.; Bechti, A.; Touhami, A.K.; James, M.J.; Durel, C.E.; Laurens, F.; Costes, E. Fruit self-thinning: A trait to consider for genetic improvement of apple tree. PLoS ONE 2014, 9, e91016. [Google Scholar] [CrossRef] [PubMed]
- Corelli, L.; Sansavini, S. Light interception and photosynthesis related to planting density and canopy management in apple. Acta Horticulturae (Netherlands) 1989, 243, 159–174. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Ferguson, I.B. Crop load interactions in apple. Hort. Rev. 2005, 31, 231–290. [Google Scholar]
- Pallas, B.; Da Silva, D.; Valsesia, P.; Yang, W.; Guillaume, O.; Lauri, P.E.; Vercambre, G.; Génard, M.; Costes, E. Simulation of carbon allocation and organ growth variability in apple tree by connecting architectural and source–sink models. Ann. Bot. 2016, 118, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, J.J. Orchard Microclimate and Its Effect on The Growth Cycle of Apple Trees. Ph.D. Thesis, University of Bristol, Bristol, UK, 1974. [Google Scholar]
- Hansen, P. Source/sink effects in fruit: An evaluation of various elements. In Manipulation of Fruiting; Wright, C.J., Ed.; Butterworth & Co. (publishers), The Anchor Press Ltd.: Tiptree, UK, 1989; pp. 29–37. [Google Scholar]
- Kramer, P.J.; Kozlowski, T.T. Physiology of Woody Plants; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Lakso, A.N.; Wünsche, J.N.; Palmer, J.W.; Corelli Grappadelli, L. Measurement and modeling of carbon balance of the apple tree. HortScience 1999, 34, 1040. [Google Scholar] [CrossRef]
- Coombe, B.G. The development of fleshy fruits. Ann. Rev. Plant Physiol. 1976, 27, 207–228. [Google Scholar] [CrossRef]
- Goffinet, M.C.; Robinson, T.L.; Lakso, A.N. A comparison of ‘Empire’ apple fruit size and anatomy in unthinned and hand-thinned trees. J. Horticul. Sci. 1995, 70, 375–387. [Google Scholar] [CrossRef]
- Lakso, A.N.; Corelli Grappadelli, L. Implications of pruning and training practices to carbon partitioning and fruit development in apple. Acta Hort. 1992, 322, 231–239. [Google Scholar] [CrossRef]
- Williams, M.W. Chemical thinning of apples. Horticul. Rev. 1979, 1, 270–300. [Google Scholar]
- Delong, M.J.; Prange, K.R.; Harrison, A.P.; Embree, G.C.; Nichols, S.D.; Harrison, W.A. The influence of crop-load, delayed cooling and storage atmosphere on post-storage quality of ‘Honeycrisp™’ apples. J. Horticul. Sci. Biotechnol. 2006, 81, 391–396. [Google Scholar] [CrossRef]
- Serra, S.; Leisso, R.; Giordani, L.; Kalcsits, L.; Musacchi, S. Crop Load Influences Fruit Quality, Nutritional Balance, and Return Bloom in ‘Honeycrisp’ Apple. HortScience 2016, 51, 236–244. [Google Scholar] [CrossRef]
- Faust, M. Physiology of Temperate Zone Fruit Trees; John Wiley & Sons, Inc.: New York, NY, USA, 1989. [Google Scholar]
- Embree, C.G.; Myra, M.T.; Nichols, D.S.; Wright, A.H. Effect of blossom density and crop load on growth, fruit quality, and return bloom in ‘Honeycrisp’ apple. HortScience 2007, 42, 1622–1625. [Google Scholar] [CrossRef]
- Link, H. Significance of flower and fruit thinning on fruit quality. Plant Growth Regul. 2000, 31, 17–26. [Google Scholar] [CrossRef]
- Tough, H.J.; Park, D.G.; Crutchley, K.J.; Bartholomew, F.B.; Craig, G. August. Effect of crop load on mineral status, maturity and quality of ‘Braeburn’ (Malus domestica Borkh.) apple fruit. In International Postharvest Science Conference Postharvest 96; ISHS: Leuven, Belgium, 1996; pp. 53–58. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Priestley, C.A. Carbohydrate reserves in deciduous fruit trees. Horticul. Rev. 1988, 10, 403–430. [Google Scholar]
- Palmer, J.W.; Giuliani, R.; Adams, H.M. Effect of crop lead on fruiting and leaf photosynthesis of ‘Braeburn’/M. 26 apple trees. Tree Physiol. 1997, 17, 741–746. [Google Scholar] [CrossRef]
- Usenik, V.; Orazem, P.; Stampar, F. Low leaf to fruit ratio delays fruit maturity of “Lapins” sweet cherry on Gisela 5. Sci. Horticul. 2010, 126, 33–36. [Google Scholar] [CrossRef]
- Cittadini, E.D.; de Ridder, N.; Peri, P.L.; van Keulen, H. Relationship Between Fruit Weight and the Fruit-To-Leaf Area Ratio, At the Spur and Whole-Tree Level, for Three Sweet Cherry Varieties. Acta Horticult. 2008, 795, 669–672. [Google Scholar] [CrossRef]
- Whiting, M.D.; Lang, G.A. ‘Bing’ sweet cherry on the dwarfing rootstock Gisela 5′: Thinning affects fruit quality and vegetative growth but not net CO2 exchange. J. Am. Soc. Horticul. Sci. 2004, 129, 407–415. [Google Scholar] [CrossRef]
- Mohamed, M.; Fayed, T.; Hussein, A.; Maged, S.M. Effect of Some Pruning Applications on Leaf to Fruit Ratio, Yield and Fruit Quality of “Florda Prince” Peach Trees. J. Horticul. Sci. Ornamental Plants 2014, 6, 18–26. [Google Scholar]
- Corelli-Grappadelli, L.C.; Lakso, A.N.; Flore, J.A. Early season patterns of carbohydrate partitioning in exposed and shaded apple branches. J. Am. Soc. Horticul. Sci. 1994, 119, 596–603. [Google Scholar] [CrossRef]
- Jackson, J.E. Utilization of light resources by HDP systems. In Symposium on High Density Planting; International Society for Horticultural Science: Leuven, Belgium, 1976; pp. 61–70. [Google Scholar]
- Robinson, T.L.; Watkins, C.B. Cropload of ‘Honeycrisp’ affects not only fruit size but many quality attributes. New York Fruit Quart. 2003, 11, 7–10. [Google Scholar]
- Costes, E.; Lauri, P.E.; Regnard, J.L. Analyzing fruit tree architecture: Implications for tree management and fruit production. Horticul. Rev. 2006, 32, 1–61. [Google Scholar]
- Ziosi, V.; Noferini, M.; Fiori, G.; Tadiello, A.; Trainotti, L.; Casadoro, G.; Costa, G. A new index based on vis spectroscopy to characterize the progression of ripening in peach fruit. Postharvest Biol. Technol. 2008, 49, 319–329. [Google Scholar] [CrossRef]
- DeLong, J.; Prange, R.; Harrison, P.; Nichols, D.; Wright, H. Determination of optimal harvest boundaries for Honeycrisp™ fruit using a new chlorophyll meter. Can. J. Plant Sci. 2014, 94, 361–369. [Google Scholar] [CrossRef]
- Hanrahan, I. Starch Iodine Index Honeycrisp. Washington Tree Fruit Research Commission. 24 April 2015. Available online: https://www.treefruitresearch.com/resources-a-downloads/starch-iodine-index-for-honeycrisp (accessed on 18 February 2019).
- Knerl, A.; Anthony, B.; Serra, S.; Musacchi, S. Optimization of Leaf Area Estimation in a High-Density Apple Orchard Using Hemispherical Photography. HortScience 2018, 53, 799–804. [Google Scholar] [CrossRef]
- Wilson, P.J.; Thompson, K.E.N.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Hanson, E. Apple Nutrition: Diagnosing and Avoiding Nutrient Deficiencies. Michigan State University Extension. Available online: https://www.canr.msu.edu/uploads/files/Applenutrition-EricHanson.pdf (accessed on 18 February 2019).
- Peck, G.M.; Andrews, P.K.; Reganold, J.P.; Fellman, J.K. Apple orchard productivity and fruit quality under organic, conventional, and integrated management. HortScience 2006, 41, 99–107. [Google Scholar] [CrossRef]
- Dow, A.I. Critical Nutrient Ranges in Northwest Crops; In WREP-Western Region Extension Publication-Cooperative Extension Service (USA); Washington State University: Pullman, WA, USA, 1980. [Google Scholar]
- Radivojevic, D.D.; Milivojevic, J.M.; Oparnica, C.D.; Vulic, T.B.; Djordjevic, B.S.; ERCİŞLİ, S. Impact of early cropping on vegetative development, productivity, and fruit quality of Gala and Braeburn apple trees. Turk. J. Agric. For. 2014, 38, 773–780. [Google Scholar] [CrossRef]
- De Salvador, F.R.; Fisichella, M.; Fontanari, M. Correlations between fruit size and fruit quality in apple trees with high and standard crop load levels. J. Fruit Ornam. Plant Res. 2006, 14, 113–122. [Google Scholar]
- Wünsche, J.N.; Palmer, J.W.; Greer, D.H. Effects of crop load on fruiting and gas-exchange characteristics of “Braeburn”/M.26 apple trees at full canopy. J. Am. Soc. Horticul. Sci. 2000, 125, 93–99. [Google Scholar] [CrossRef]
- Ferguson, I.B.; Watkins, C.B. Crop load affects mineral concentrations and incidence of bitter pit in ‘Cox’s Orange Pippin’ apple fruit. J. Am. Soc. Horticul. Sci. 1992, 117, 373–376. [Google Scholar] [CrossRef]
- Lakso, A.N.; Robinson, T.L. Principles of orchard systems management optimizing supply, demand and partitioning in apple trees. Acta Hortic. 1997, 451, 405–416. [Google Scholar] [CrossRef]
- Lakso, A.N.; Robinson, T.L.; Pool, R.M. Canopy microclimate effects on patterns of fruiting and fruit development in apples and grapes. In Manipulation of Fruiting; 47th Nottingham Easter School: Butterworths, UK, 1989; pp. 263–274. [Google Scholar]
- Doerflinger, F.C.; Miller, W.B.; Nock, J.F.; Watkins, C.B. Relationships between starch pattern indices and starch concentrations in four apple cultivars. Postharvest Biol. Technol. 2015, 110, 86–95. [Google Scholar] [CrossRef]
- Meland, M. Effects of different crop loads and thinning times on yield, fruit quality, and return bloom in Malus × domestica Borkh. ‘Elstar’. J. Horticul. Sci. Biotech. 2009, 84, 117–121. [Google Scholar] [CrossRef]
- Harker, F.R.; Marsh, K.B.; Young, H.; Murray, S.H.; Gunson, F.A.; Walker, S.B. Sensory interpretation of instrumental measurements 2: Sweet and acid taste of apple fruit. Postharvest Biol. Technol. 2002, 24, 241–250. [Google Scholar] [CrossRef]
- Warner, G. Crop Load Affects Flavor of Honeycrisp. Available online: http://www.goodfruit.com/crop-load-affects-flavor-of-honeycrisp/ (accessed on 18 February 2019).
- Opara, L.U.; Studman, C.J.; Banks, N.H. Physico-mechanical Properties of “Gala” apples and Stem-end Splitting as Influenced by Orchard Management Practices and Harvest Date. J. Agric. Eng. Res. 1997, 68, 139–146. [Google Scholar] [CrossRef]
- Gallardo, R.K.; Hanrahan, I.; Hong, Y.A.; Luby, J.J. Crop load management and the market profitability of ‘Honeycrisp’ apples. HortTechnology 2015, 25, 575–584. [Google Scholar] [CrossRef]
- Stopar, M.; Bolcina, U.; Vanzo, A.; Vrhovsek, U. Lower crop load for cv. Jonagold apples (Malus× domestica Borkh.) increases polyphenol content and fruit quality. J. Agric. Food Chem. 2002, 50, 1643–1646. [Google Scholar] [CrossRef]
- Robinson, T.; Lopez, S.; Iungerman, K.; Reginato, G. Crop load management for consistent production of Honeycrisp apples. N. Y. Fruit Q. 2009, 17, 24–28. [Google Scholar]
- Lenz, F. Fruit effects on transpiration and dry matter production in apples. In The Regulation of Photosynthesis in Fruit Trees; Symp. Procee Publ.; New York State Agricultural Experiment Station: Geneva, NY, USA, 1986; pp. 101–104. [Google Scholar]
- Parker, A.K.; Hofmann, R.W.; van Leeuwen, C.; Mclachlan, A.R.G.; Trought, M.C.T. Leaf area to fruit mass ratio determines the time of veraison in Sauvignon Blanc and Pinot Noir grapevines. Aust. J. Grape Wine Res. 2014, 20, 422–431. [Google Scholar] [CrossRef]
- Atay, E. Which Nutrients in The Leaf Decrease Linearly as Fruit Load Increases in Apples? A Preliminary Study. Presented at the International Academic Research Congress, Antalya, Turkey, 24–25 June 2016; pp. 656–659. [Google Scholar]
- Nii, N. Changes of starch and sorbitol in leaves before and after removal of fruits from peach trees. Ann. Bot. 1997, 79, 139–144. [Google Scholar] [CrossRef]
- Smith, P.F. Mineral analysis of plant tissues. Ann. Rev. Plant Physiol. 1962, 13, 81–108. [Google Scholar] [CrossRef]
- Palmer, J.W. Effects of varying crop load on photosynthesis, dry matter production and partitioning of Crispin/M. 27 apple trees. Tree Physiol. 1992, 11, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Viškelienė, A.; Sirtautas, R.; Kviklys, D. Relationships between apple tree rootstock, crop-load, plant nutritional status and yield. Sci. Horticul. 2016, 211, 167–173. [Google Scholar] [CrossRef]
- Havis, L.; Gilkeson, A.L. Interrelationships of nitrogen and potassium fertilization and pruning practice in mature peach trees. In Proceedings of the American Society for Horticultural Science, Minneapolis, MN, USA, 10–12 September 1951; Amer Soc Horticultural Science: Alexandria, VA, USA, 1951; pp. 24–30. [Google Scholar]
- Telias, A.; Hoover, E.; Rosen, C.; Bedford, D.; Cook, D. The effect of calcium sprays and fruit thinning on bitter pit incidence and calcium content in ‘Honeycrisp’apple. J. Plant Nutr. 2006, 29, 1941–1957. [Google Scholar] [CrossRef]
- Koutinas, N.; Pepelyankov, G.; Lichev, V. Flower induction and flower bud development in apple and sweet cherry. Biotechnol. Biotec. Eq. 2010, 24, 1549–1558. [Google Scholar] [CrossRef]
- Monselise, S.P.; Goldschmidt, E.E. Alternate bearing in fruit trees. Horticul. Rev. 1982, 4, 128–173. [Google Scholar]
- Treder, W. Crop loading studies with ‘Jonagold’apple tree. J. Fruit Ornamental Plant Res. 2010, 18, 59–69. [Google Scholar]
- Hoblyn, T.N.; Grubb, N.H.; Painter, A.C.; Wates, B.L. Studies in Biennial Bearing—I. J. Pomol. Horticul. Sci. 1937, 14, 39–76. [Google Scholar] [CrossRef]
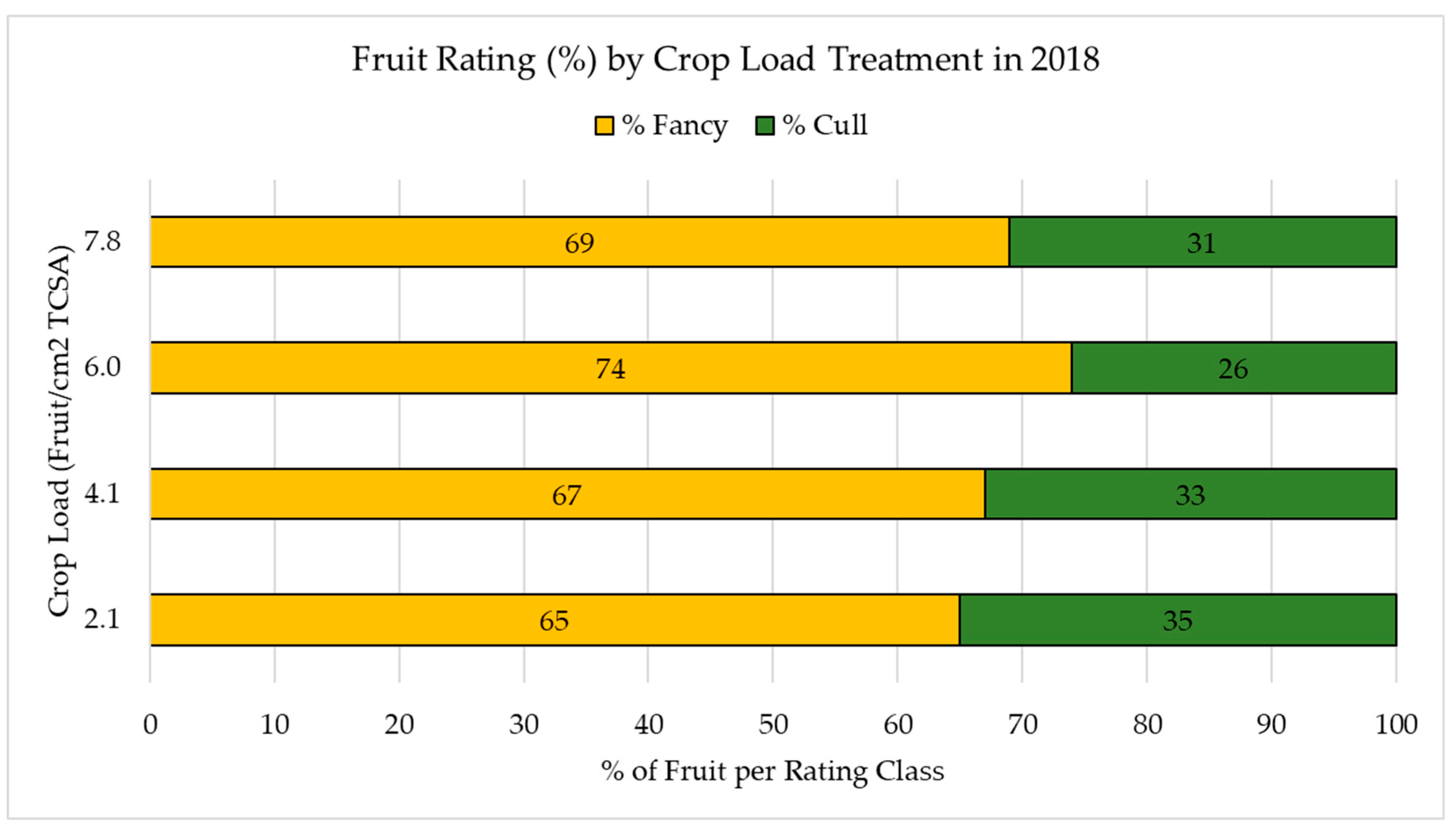
| Crop Load (no. fruits/cm2 TCSA) | Fruit (no.)/tree 2017 | Yield (kg/tree) 2017 | Average Fruit Weight a (g) 2017 | Yield (MT/ha) 2017 |
|---|---|---|---|---|
| 2.1 | 26 D | 7.8 D | 303 A | 28.2 D |
| 4.1 | 50 C | 13.3 C | 265 B | 47.8 C |
| 6.0 | 72 B | 18.8 B | 262 B | 67.8 B |
| 7.8 | 95 A | 23.0 A | 242 B | 82.8 A |
| Significance | *** | *** | *** | *** |
| Crop Load (no. fruits/cm2 TCSA) in 2017 | Fruit (no.)/tree 2018 | Yield (kg/tree) 2018 | Average Fruit Weight a (g) 2018 | Yield (MT/ha) 2018 |
|---|---|---|---|---|
| 2.1 | 114 | 24.45 | 213 | 88.0 |
| 4.1 | 90 | 21.78 | 235 | 78.3 |
| 6.0 | 102 | 25.51 | 245 | 91.8 |
| 7.8 | 73 | 19.21 | 280 | 69.1 |
| Significance | ns | ns | ns | ns |
| Crop load (no. fruits/cm2 TCSA) | Average Fruit weight a | IAD b | Red Overcolor (%) | Average Red Intensity (1–5) | Firmness (kg/cm2) | SSC (°Brix) | Dry Matter (%) | Starch c (1–6) | Titratable Acidity (malic acid %) |
|---|---|---|---|---|---|---|---|---|---|
| 2.1 | 302 A | 0.45 D | 96.2 | 4.03 A | 9.07 A | 14.59 A | 16.02 A | 1.77 | 0.80 A |
| 4.1 | 282 B | 0.60 C | 96.5 | 4.02 A | 8.84 B | 13.40 B | 15.33 B | 1.56 | 0.54 B |
| 6.0 | 282 B | 0.78 B | 94.6 | 3.81 B | 8.23 C | 13.29 B | 14.50 C | 1.66 | 0.54 B |
| 7.8 | 277 B | 0.93 A | 95.6 | 3.95 A | 8.25 C | 13.18 B | 14.39 C | 1.55 | 0.56 B |
| Significance | *** | *** | ns | ** | *** | *** | * | ns | ** |
| Crop Load (no. fruits/cm2 TCSA) | Total Leaf Area (TLA) (cm2) per tree | Total Leaf no. per Tree | Leaf-Area-to-Fruit (LA:F) ratio | Leaf-to-Fruit (L:F) Ratio | LAI (m2/m2) | SLA (cm2/g) | Leaf Area (cm2) per Individual Leaf | Individual Leaf Width (cm) | TCSA Annual Growth 2017–2018 (cm2) |
|---|---|---|---|---|---|---|---|---|---|
| 2.1 | 36506 | 1268 | 1375 A | 48 A | 1.31 | 43.3 | 28.9 | 5.01 | 1.78 |
| 4.1 | 36272 | 1367 | 707 B | 27 B | 1.30 | 47.8 | 26.7 | 4.89 | 1.62 |
| 6.0 | 42492 | 1408 | 585 B | 19 BC | 1.52 | 43.8 | 30.5 | 5.09 | 1.12 |
| 7.8 | 40410 | 1470 | 401 B | 15 C | 1.45 | 44.0 | 26.6 | 4.79 | 1.02 |
| Significance | ns | ns | *** | *** | ns | Ns | ns | ns | ns |
| Macro-Nutrients | Micro-Nutrients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop Load (no. fruits/cm2 TCSA) | Total N (%) | P (%) | K (%) | S (%) | Ca (%) | Mg (%) | Zn (ppm) | Mn (ppm) | Cu (ppm) | Fe (ppm) | B (ppm) |
| 2.1 | 2.06 B | 0.33 | 2.49 A | 0.14 B | 1.90 | 0.28 | 15.2 | 31.7 | 8.77 B | 183.8 | 32.7 A |
| 4.1 | 2.09 B | 0.31 | 2.23 B | 0.14 B | 1.87 | 0.29 | 13.9 | 32.5 | 8.56 B | 211.7 | 31.2 A |
| 6.0 | 2.32 A | 0.29 | 2.04 B | 0.14 B | 2.02 | 0.28 | 13.6 | 30.4 | 8.98 B | 189.8 | 26.8 B |
| 7.8 | 2.30 A | 0.29 | 2.07 B | 0.15 A | 2.07 | 0.29 | 14.6 | 30.3 | 9.63 A | 201.7 | 30.6 A |
| Significance | ** | ns | *** | ** | ns | ns | ns | ns | * | ns | ** |
| CNR a | 1.7–2.0 | <0.1 | 0.8–1.5 | 0.01–0.025 | <1.2 | 0.2–0.3 | 15–20 | 25–30 | 5–6 | <100 | 20–25 |
| Deficiency threshold | 2.0 | 0.1 | 1.0 | - | 0.5 | 0.2 | 15.0 | 20.0 | 4.0 | 25.0 | 25.0 |
| Macro-Nutrients | Micro-Nutrients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop load (no. fruits/cm2 TCSA) | Total N (%) | P (%) | K (%) | S (%) | Ca (%) | Mg (%) | Zn (ppm) | Mn (ppm) | Cu (ppm) | Fe (ppm) | B (ppm) |
| 2.1 | 0.24 | 0.08 A | 0.82 A | 0.02 | 0.03 | 0.04 | 0.80 | 2.06 B | 2.78 | 27.4 | 20.2 A |
| 4.1 | 0.22 | 0.07 B | 0.78 B | 0.02 | 0.03 | 0.04 | 0.69 | 2.18 B | 3.06 | 14.9 | 16.1 AB |
| 6.0 | 0.23 | 0.06 C | 0.72 C | 0.02 | 0.03 | 0.04 | 1.26 | 2.45 B | 2.84 | 15.3 | 12.3 C |
| 7.8 | 0.25 | 0.06 C | 0.67 D | 0.02 | 0.04 | 0.04 | 0.86 | 2.95 A | 2.85 | 20.4 | 14.1 BC |
| Significance | ns | *** | *** | ns | ns | ns | ns | ** | ns | ns | ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anthony, B.; Serra, S.; Musacchi, S. Optimizing Crop Load for New Apple Cultivar: “WA38”. Agronomy 2019, 9, 107. https://doi.org/10.3390/agronomy9020107
Anthony B, Serra S, Musacchi S. Optimizing Crop Load for New Apple Cultivar: “WA38”. Agronomy. 2019; 9(2):107. https://doi.org/10.3390/agronomy9020107
Chicago/Turabian StyleAnthony, Brendon, Sara Serra, and Stefano Musacchi. 2019. "Optimizing Crop Load for New Apple Cultivar: “WA38”" Agronomy 9, no. 2: 107. https://doi.org/10.3390/agronomy9020107
APA StyleAnthony, B., Serra, S., & Musacchi, S. (2019). Optimizing Crop Load for New Apple Cultivar: “WA38”. Agronomy, 9(2), 107. https://doi.org/10.3390/agronomy9020107







