1. Introduction
In the world, globe artichoke (
Cynara cardunculus L. var.
scolymus (L.) Benth) cultivation covers around 124,941 ha. Its main production sites are in the Mediterranean countries, and they provide around 76% of worldwide artichoke production [
1].
In Tunisia, globe artichoke ranks as the sixth most important vegetable in terms of area, production, and commercialization in local and international markets, especially for the cultivar Violet d’Hyères. In 2015, the total area of globe artichoke cultivation covered 4,400 ha and produced 24,000 tons [
2]. According to the statistics, the export price per ton (2095 dinars per ton = 646 euros per ton) of artichoke heads reached more than 166% of the production costs (1253 dinars per ton = 386 euros per ton) [
2,
3]. Despite its important and valuable financial gains, only 12% from the production were exported in 2015 [
4].
Quality, precocity, and yield of this vegetable are mainly affected by the unsatisfactory quality of cuttings. Most Tunisian farmers adopt the traditional vegetative propagation method for globe artichoke, mainly using stalks and ovoli (underground dormant buds). Despite its economic profitability, this method seems to be a big source of disease spread [
5,
6,
7,
8] due to the absence of phytosanitary and quality control of transplants. The production of vitro plants could be an important solution to decrease the risk of contaminated plants and it could virtually produce an unlimited number of identical plants from one plant [
9,
10,
11]. However, micropropagation is an expensive technique and it is not developed in Tunisia. As a result, the improvement of the vegetative propagation techniques used in the field seems to be a potential strategy to overcome the problem of cutting quality in Tunisia [
8].
According to a previous work, it was demonstrated that ovoli that originate from spring offshoots significantly improve agronomic, marketable, and qualitative traits of production [
8]. Research has targeted the enlargement of cutting production using many techniques such as the application of exogenous cytokinine, the decapitation or the removal of epigeal part of the plant, and mychorhizes [
12].
In fact, the application of exogenous cytokinins such as kinetin and 6-benzylamino purine (BAP) in many crops increases the ratio of cytokinin to auxin in the plant, disrupting the apical dominance that controls branching patterns and plant form, and promotes offshoot outgrowth [
13]. The responses of crops to cytokinine treatments differs from one species to another. For example, in horticulture, BAP increases the amount of branching in many herbaceous plants, and in some cases it increases the number of flowers and spikes, as in
Phalaenopsis Blume and
Dendrobium Sw. orchids [
14,
15]. Regarding vegetables, BAP accelerates flowering and shoot development in tomato plants [
16] and increases the shoot biomass in green leafy crops such as spinach [
17]. Moreover, the use of kinetin was reported by many authors, but mainly in in vitro culture for globe artichoke as an important inducer for bud formation during the proliferation phase [
18,
19,
20,
21,
22].
On the other hand, the removal of the apex by the epigeal part cut-back causes a breakdown of apical dominance, so it promotes a simultaneous development of several offshoots which may originate “ovoli” in globe artichoke mother plants [
12]. The low availability of ovoli, especially in early cultivars such as Violet de Provence and Violet d’Hyères, makes the expansion of this crop limited.
To overcome this problem, a production plan for globe artichoke nursery plantlets was applied by the removal of apex and epigeal parts of field mother plants during active growth. After the plant cut-back, the remaining parts, which were stumps, were removed from field to pots. Within one month, it was possible to collect the emitted offshoots periodically as cuttings for new transplantations [
23]. Another mechanical technique—the decapitation—was also tested on artichoke plants. Under aseptic conditions, artichoke seedlings aged six weeks were decapitated for shoot proliferation in vitro and it was an effective technique, generating many offshoots from each decapitated explant [
24]. According to other authors, the combination of chemical and mechanical treatments in vivo or in vitro to enhance the multiplication of globe artichoke cuttings seems to be more effective than using each technique separately [
6,
25,
26].
All these methods used to produce globe artichoke cuttings were mainly focused on their impact on quantitative parameters of the produced plants, but not in qualitative ones. In fact, an exogenous application of cytokinine may affect the distribution of minerals in the plant. This can also influence the morphology of the plant by increasing fresh weight and plant height [
27] or leaf shape, leaf size, and leaf number in some ornamental species [
28]. It can also have an impact on the interaction between sink and source organs: Gao et al [
29] showed that the foliar spray of BAP increases maize yield by enhancing source and sink capacity. On the other hand, mineral uptake and biomass accumulation can give information about the quality of cuttings, since many macronutrients such as phosphorus and sulfate are involved in molecular and physiological processes linked to root [
30] and shoot development. Moreover, cytokinins are involved in the cell division process [
31], photosynthesis [
32], and nutrient uptake [
33] in plants so it may influence the chemical aspects of cuttings.
In view of this, the objective of this study was the evaluation of the foliar spray of 6-benzylamino purine effects with different concentrations combined with the leaf cut-back at the collar level of the plant on offshoot production and their quality in terms of biomass and mineral composition.
2. Materials and Methods
2.1. Experimental Site
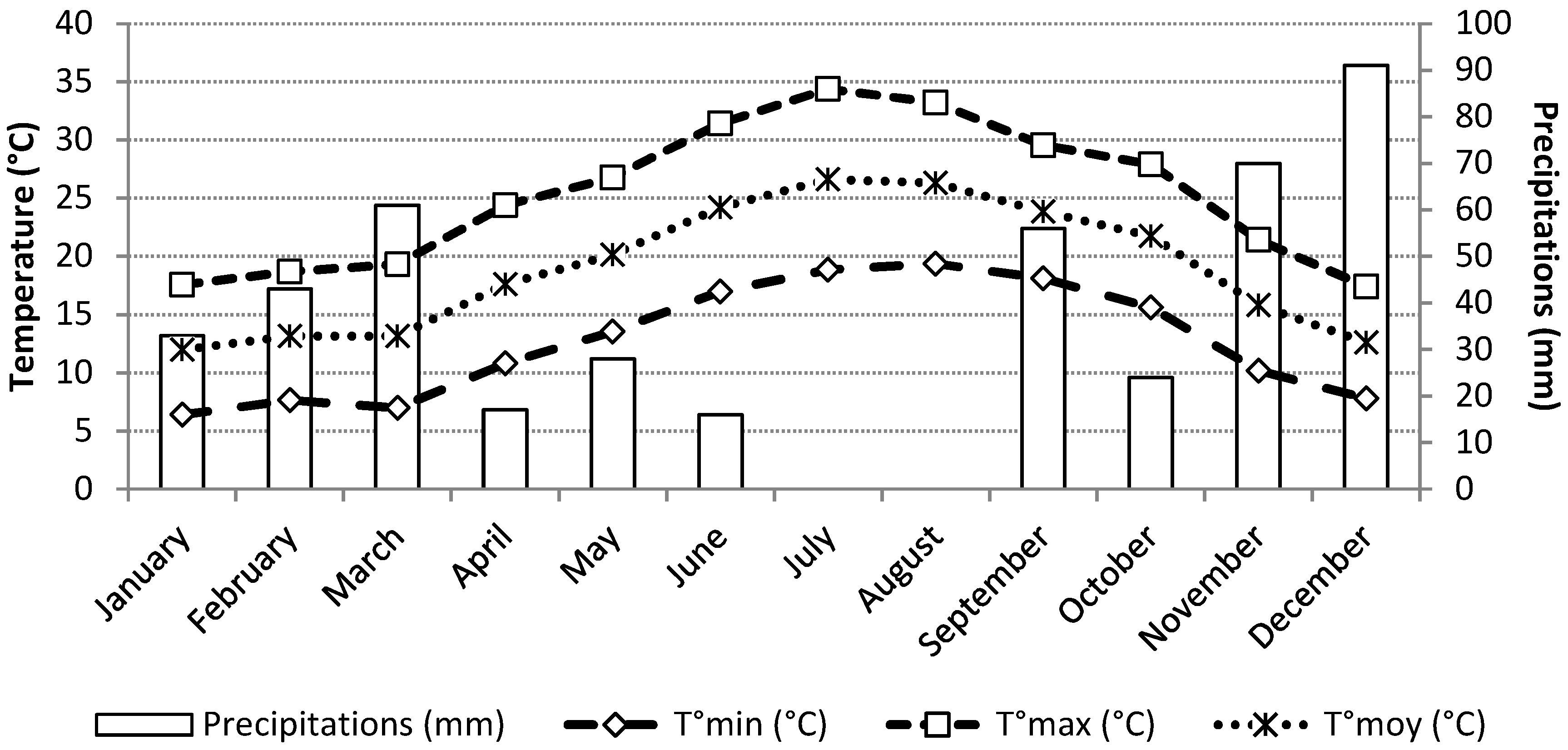
The trials were conducted in the experimental area of the Support Station of Manouba (SAM), which is under the supervision of the Inter-professional Group of Vegetables (GIL). It is situated in the north of Tunisia, more precisely in the region of Manouba (36°48′ N; 10°03′ E, altitude 469 m). The local climate of this area is characterized by an upper semi-arid stage (Technical Center of Potato and Artichoke meteorological station, Manouba, Tunisia). The lowest temperature recorded was around 6 °C in January and the highest one was in July about 34 °C. The rainfall is irregular with an average of 439 mm per year (
Figure 1).
2.2. Plant Material
To optimize the cutting production for globe artichoke cultivation, two trials were set up in the agronomic experimental station of Manouba using two different vegetative materials from the Violet d’Hyères variety: offshoots and field mother plants.
The nursery of early spring offshoots was established in 4 February 2016. It covered an area of 79 m2 and contained 675 cuttings. At the beginning, offshoots were preselected and removed from controlled and disease-free mother plants located in the SAM globe artichoke field. After that, they were trimmed by 0.15 m from the base and immersed in a copper hydroxide solution (50%) for 2 min to disinfect them. Then, they were placed on a simple line ridge with a density of 11 plants m−2, respecting gaps of 0.15 m between the plants and 0.6 m between ridges.
The field was composed of 2-year-old artichoke mother plants produced from vitro plants by the SAM laboratory. Since its establishment in 2014, the mother plants field was regularly controlled and cleaned of abnormal plants. The plantation density was 6666 plants ha−1. Plants were spaced 1 m apart per row and 1.5 m between the plantation lines. The trial was realized on a surface of 1057 m². The soil of the field was a clay type composed of 55% of clay, 22% of silt, and 23% of sand. The water used for irrigation was an alkaline drilling water (pH = 8) with 1.2 g L−1 of dry residue, electric conductivity 1924 μS cm−1, a quite high amount of chlorides (521 mg L−1), and total dissolved salts (TDS) of 966 mg L−1. The crop water consumption was about 740 mm, and its requirements in terms of fertilizers was 316 kg ha−1 of N, 160 kg ha−1 P2O5, and 210 kg ha−1 K2O fractionated depending on crop phases needs.
2.3. BAP Application
Different concentrations of exogenous BAP (Sigma Aldrich, Germany) ranging from 0 (T1, T2) to 300 mg L−1 (100, 200, and 300 mg L−1) were sprayed in the same day on the foliage of nursery plants and field mother plants. The powder of BAP was first dissolved in 100 mL of distilled water containing some drops of NaOH, then fulfilled with pure water until getting the volume of 1 L. Each plant from nursery has received a volume of 10 mL of BAP solution except those of treatments T1 and T2, which were sprayed with the same volume but of distilled water. Field mother plants were treated the same as nursery plants but with a volume of 50 mL each.
2.4. Plant Cut-Back
One week after the application of BAP treatments (3 April 2016), the foliage of plants, except those of T1, were all cut back at the collar level using a sharp saw knife to enhance more outgrowth of offshoots. The treatments were, respectively, T1 (plants sprayed with pure water and not cut back), T2 (plants sprayed with pure water and cut back), T3 (plants sprayed with 100 ppm BAP and cut back), T4 (plants sprayed with 200 ppm BAP and cut back), and T5 (plants sprayed with 300 ppm BAP and cut back).
2.5. Experimental Design
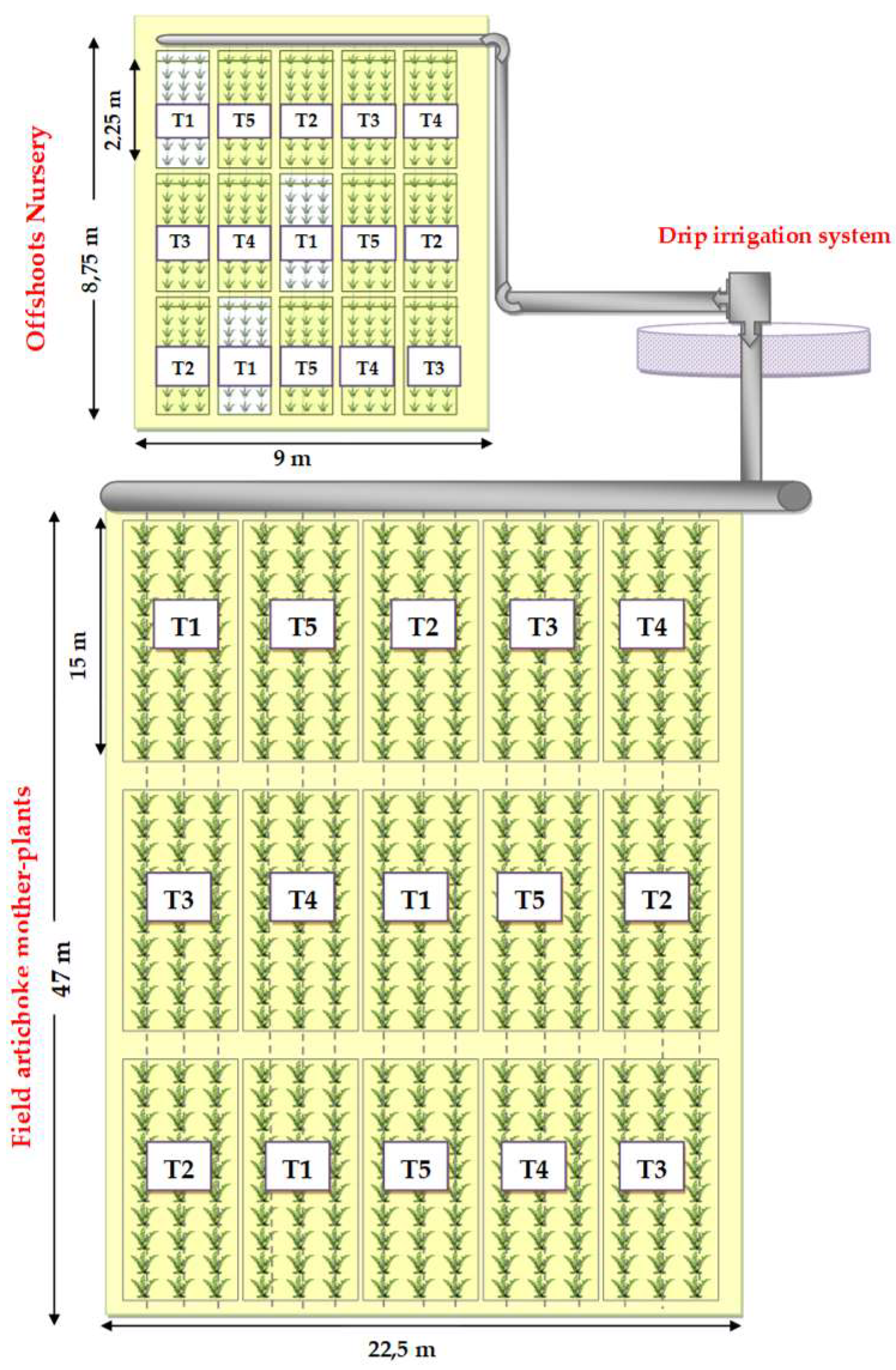
For the two trials, the same treatments were applied, and the same experimental design was adopted for the nursery and for the field of artichoke mother plants (
Figure 2). The treatments were arranged in a randomized block design with three replications. Each block was constituted by five plots. Each plot was composed of 45 plants for both nursery and field trial irrigated by a drip irrigation system.
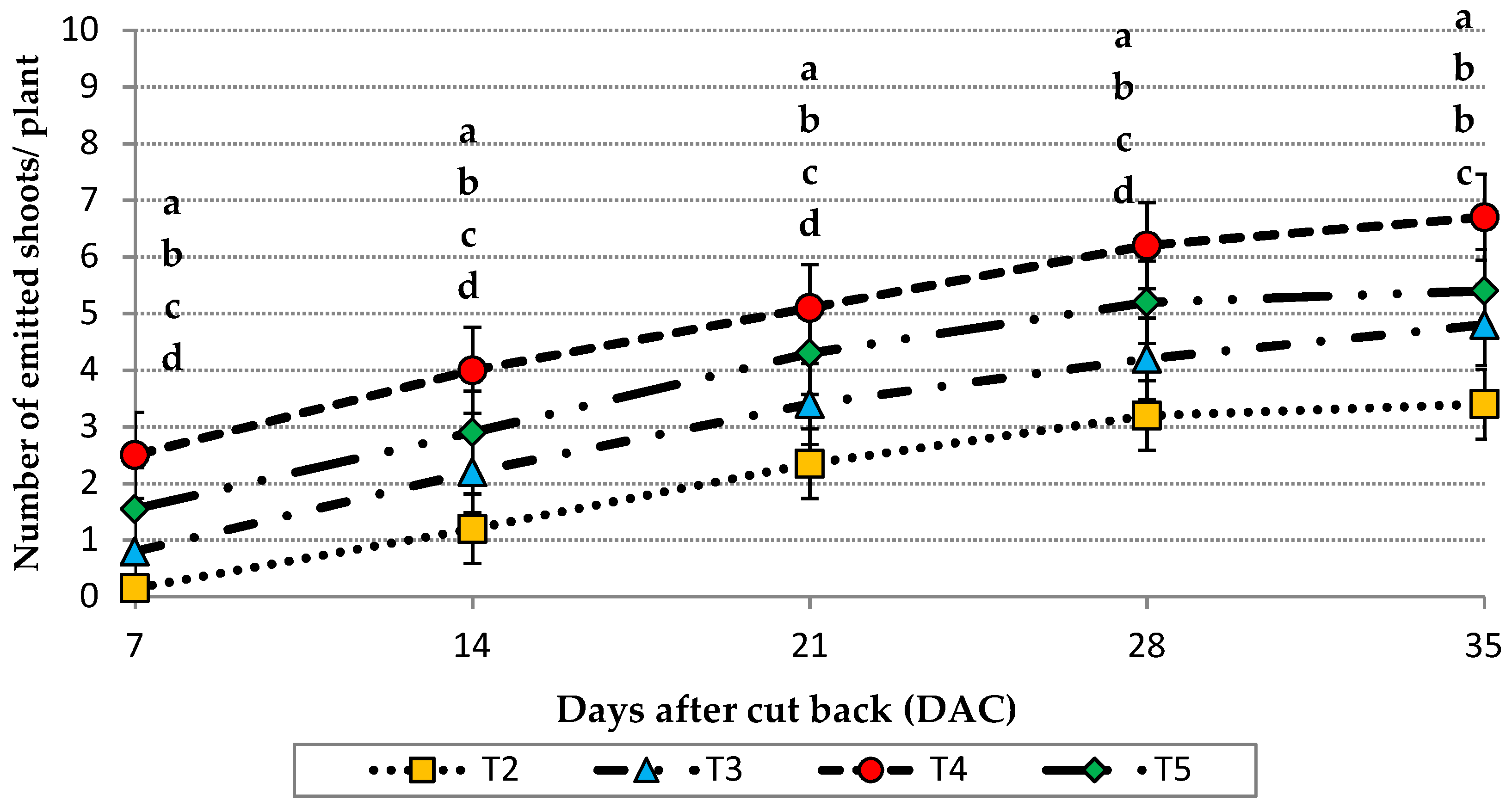
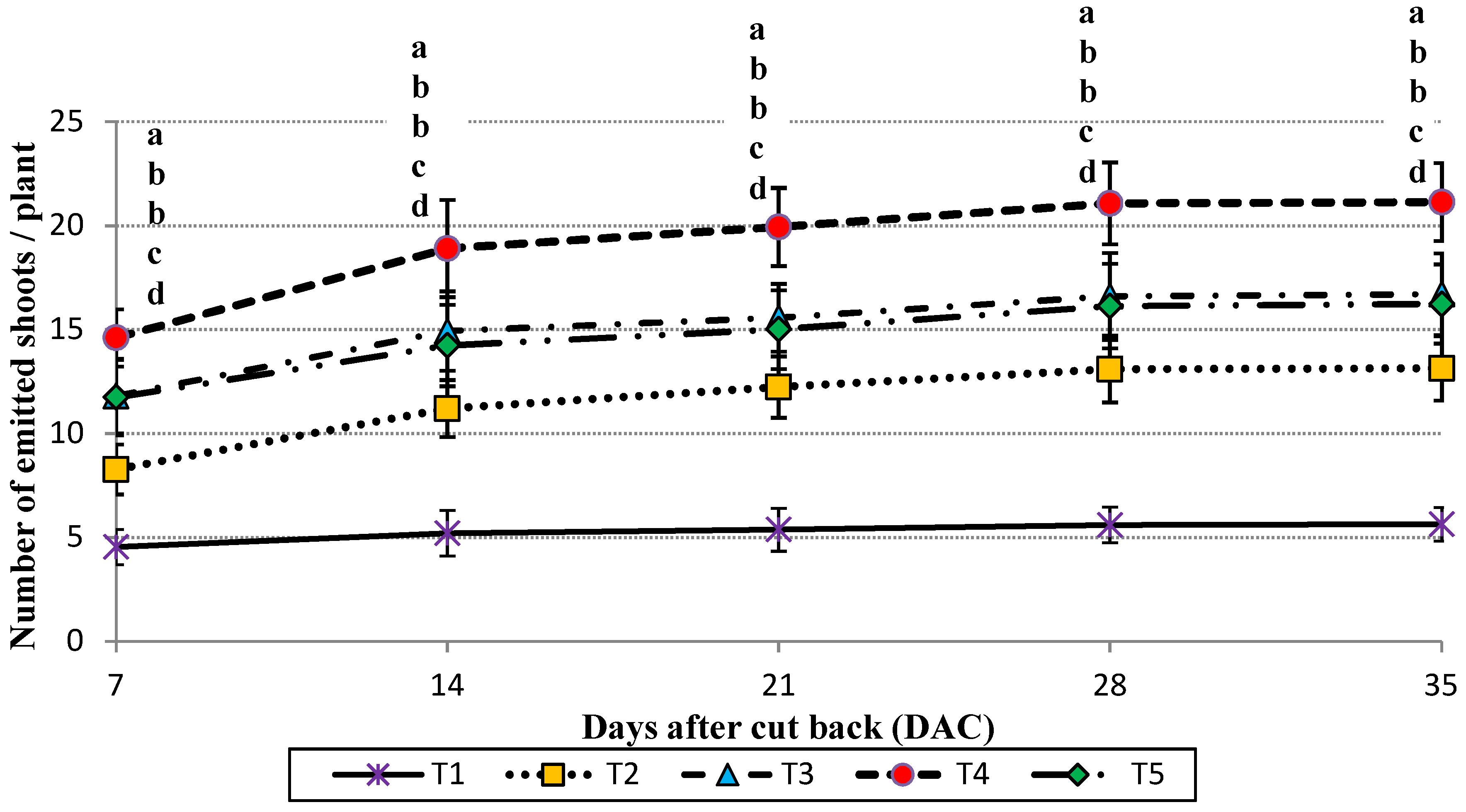
2.6. Vegetative Growth Parameters for Emitted Offshoots
Concerning vegetative growth parameters, 15 plants from each plot and each trial were monitored from the 7th day after the cut-back of plants until the 35th day. During this period, the number of emitted offshoots per plant was counted and the offshoot emission rhythm was calculated. On the 36th day after cutting, the emitted offshoots were harvested from the considered plants from each plot. After that, they were weighed and, according their weights, classified into 3 size groups. The adopted offshoot size classification is reported in
Table 1.
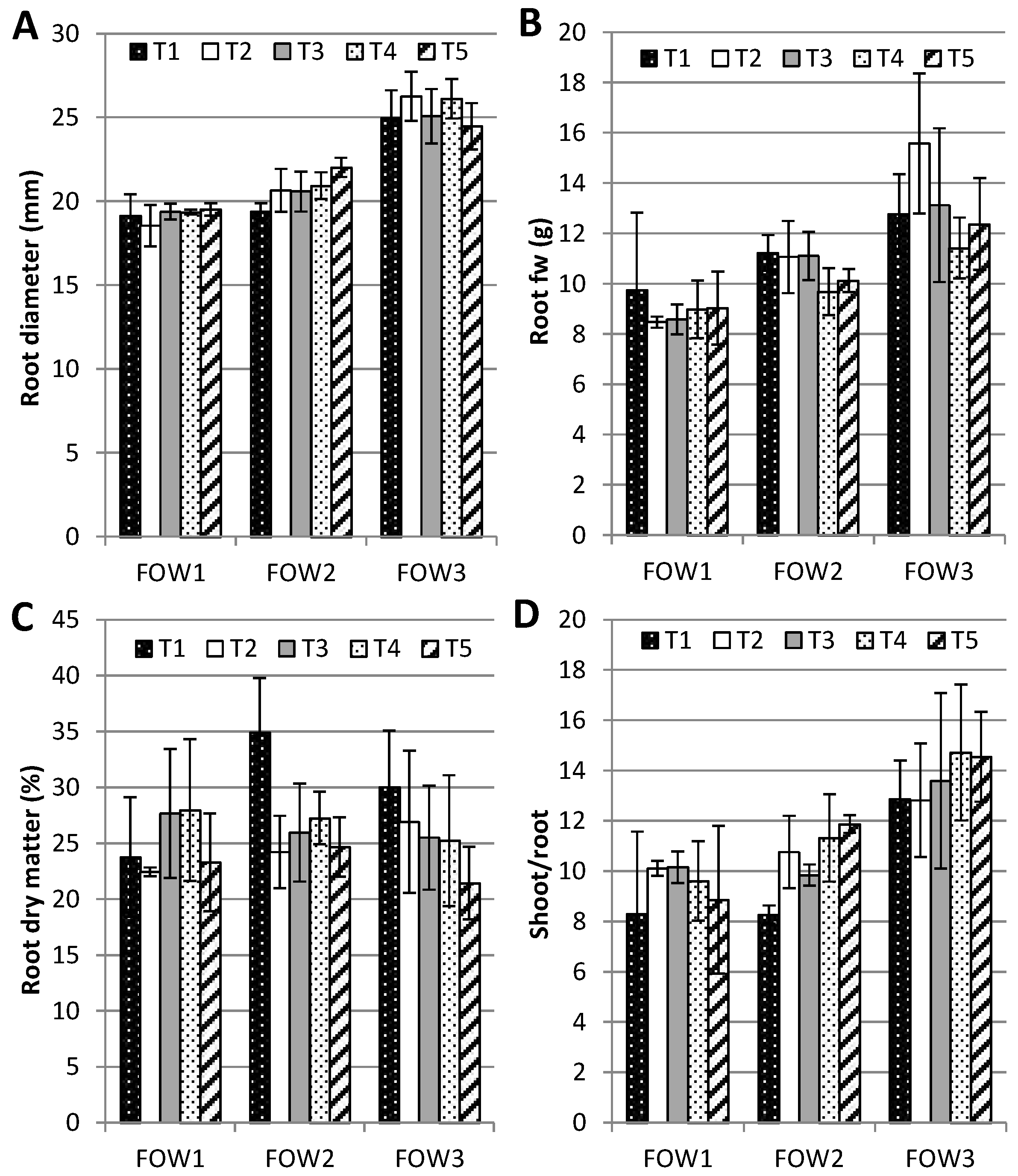
After size classification, offshoots were cut into two parts at the collar level to separate the leaves from roots. The number of leaves was counted whereas root length and root diameter was measured for offshoots derived from field plants using a caliper. Therefore, each part from the offshoot was weighed to determine its fresh weight (g), oven-dried (4 days, 65 °C) and reweighed to determine the dry weight (g) and the dry matter percentage.
2.7. Determination of Mineral Components of the Harvested Offshoots by Ion Chromatography (IC)
A total number of 72 offshoots collected from nursery (4 treatments × 3 classes × 2 sampling areas × 3 replications) were used to determine the mineral contents of leaves. For field mother plants, 90 offshoots in total (5 treatments × 3 classes × 2 sampling areas × 3 replications) were used for the determination of mineral composition in leaves and in roots. For each analysis, 200 mg of dry matter from each sample were used. Analyses were performed by IC using an ICS-900 Ion Chromatography system (Dionex Corporation, Sunnyvale, CA, USA, which is equipped with a dual piston pump, a model AS-DV auto sampler, an isocratic column at room temperature, a DS5 conductivity detector and an AMMS 300 suppressor (4 mm) for anions and CMMS 300 suppressor (4 mm) for cations. Chromeleon 6.5 Chromatography Management Software (ThermoFisher, Sunnyvale, CA, USA) was used for system control and data processing. A Dionex Ion-Pac AS23 analytical column (4 mm × 250 mm) and a guard column (4 mm × 50 mm) were used for anion separations, whereas a Dionex IonPac CS12A analytical column (4 mm × 250 mm) (Dionex Corporation, Sunnyvale, CA, USA) and a guard column (4 mm × 50 mm) (Dionex Corporation, Sunnyvale, CA, USA) were used for cation separations. The eluent consisted of 4.5 mM sodium carbonate and 0.8 mM sodium bicarbonate at a flow rate of 1 mL min−1 for anions and of 20 mM methanesulfonic acid for cations at the same flow rate. Anions and cations were quantified following a calibration method. Dionex solutions containing seven anions at different concentrations and five cations were taken as standards and the calibration curves were generated with concentrations ranging from 0.4 mg L−1 to 20 mg L−1 and from 0.5 mg L−1 to 50 mg L−1 of standards, respectively.
2.8. Statistical Analysis
Data were analyzed by analysis of variance (ANOVA) and treatments were compared with Tukey’s Honestly Significant Difference test at the significance level of p < 0.05. Letters were reported only in the case of significant differences.