Effect of Long-Term Cropping Systems on the Diversity of the Soil Bacterial Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Sampling
2.2. DNA Extraction and 16 rDNA T-RFLP Analysis
2.3. Statistical Analysis
3. Results and Discussion
3.1. Soil Properties
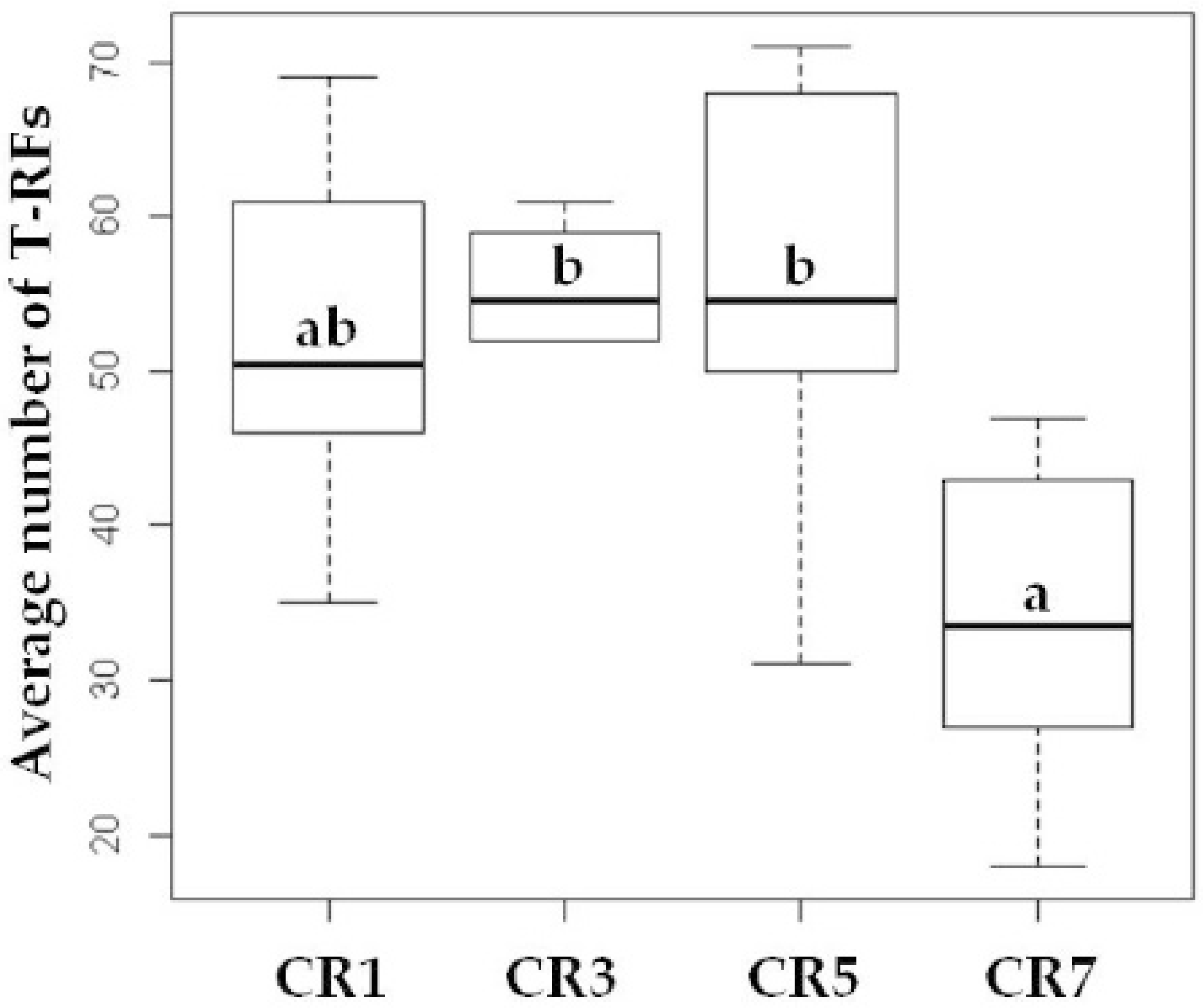
3.2. Richness and Diversity of Bacterial Communities
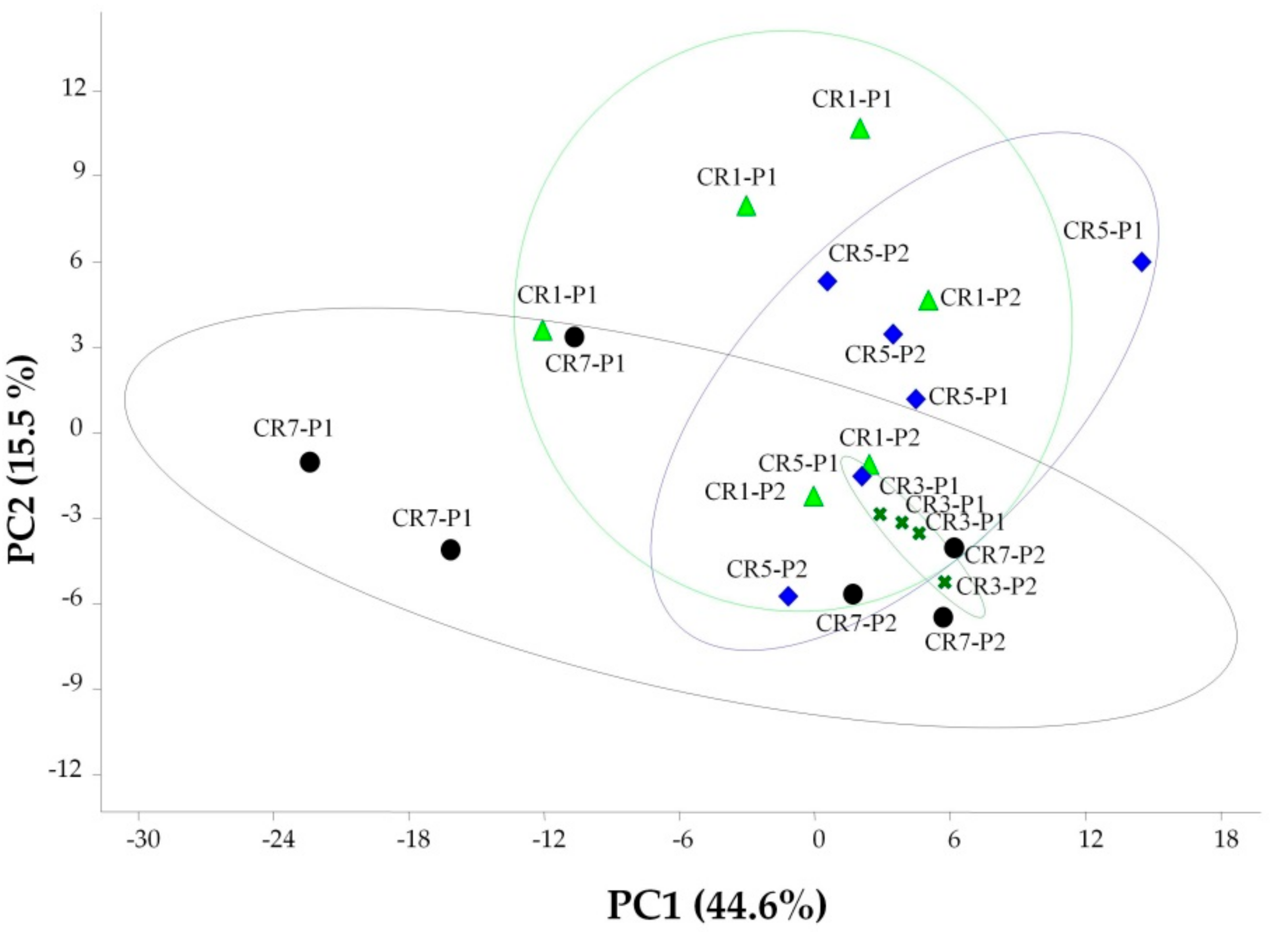
3.3. Differences in Bacterial Communities
3.4. Relationship between Microbial Communities and Environmental Variables
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, P.; Zeng, Z. Dynamics of bacterial communities in a 30-year fertilized paddy field under different organic–inorganic fertilization strategies. Agronomy 2019, 9, 14. [Google Scholar] [CrossRef]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Garbeva, P.; van Veen, J.A.; van Elsas, J.D. Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, S.L. Plant-microbial interactions in agriculture and the use of farming systems to improve diversity and productivity. AIMS Microbiol. 2017, 3, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Sanderlin, J.S.; Reeves, J.H.; Jenkins, M.B.; Endale, D.M.; Coleman, D.C.; Whitman, W.B. Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol. Biochem. 2008, 40, 2843–2853. [Google Scholar] [CrossRef]
- Drechsel, P.; Heffer, P.; Magen, H.; Mikkelsen, R.; Wichelns, D. Managing Water and Fertilizer for Sustainable Agricultural Intensification; IFA: Paris, France; IWMI: Colombo, Sri Lanka; IPNI: Peachtree Corners, GA, USA; IPI: Horgen, Switzerland, 2015; pp. 140–167. [Google Scholar]
- Gu, Y.; Wang, Y.; Lu, S.; Xiang, Q.; Yu, X.; Zhao, K.; Marco, D.E. Long-term fertilization structures bacterial and archaeal communities along soil depth gradient in a paddy soil. Front. Microbiol. 2017, 8, 1516. [Google Scholar] [CrossRef]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.E.; Hu, C.; Liu, B. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Ding, J.; Jiang, X.; Guan, D.; Zhao, B.; Ma, M.; Zhou, B. Influence of inorganic fertilizer and organic manure application on fungal communities in a long-term field experiment of Chinese Mollisols. Appl. Soil Ecol. 2017, 111, 114–122. [Google Scholar] [CrossRef]
- Sommermann, L.; Geistlinger, J.; Wibberg, D.; Deubel, A.; Zwanzig, J.; Babin, D.; Schlüter, A.; Schellenberg, I. Fungal community profiles in agricultural soils of a long-term field trial under different tillage, fertilization and crop rotation conditions analyzed by high-throughput ITS-amplicon sequencing. PLoS ONE 2018, 13, e0195345. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, J.; Li, L.; Wang, X.; Li, X.; Qu, J. Effect of soybean and maize rotation on soil microbial community structure. Agronomy 2019, 9, 42. [Google Scholar] [CrossRef]
- McDaniel, M.D.; Grandy, A.S.; Tiemann, L.K.; Weintraub, M.N. Crop rotation complexity regulates the decomposition of high and low quality residues. Soil Biol. Biochem. 2014, 78, 243–254. [Google Scholar] [CrossRef]
- Riedell, W.E.; Osborne, S.L.; Pikul, J.L. Soil attributes, soybean mineral nutrition, and yield in diverse crop rotations under no-till conditions. Agron. J. 2013, 105, 1231–1236. [Google Scholar] [CrossRef]
- Ondreičková, K.; Babulicová, M.; Mihálik, D.; Gubišová, M.; Gubiš, J. Screening of bacterial populations in crop rotations with different proportion of cereals. Agriculture 2014, 60, 1–38. [Google Scholar] [CrossRef]
- Pérez-Brandán, C.; Arzeno, J.L.; Huidobro, J.; Conforto, C.; Grümberg, B.; Hilton, S.; Bending, G.D.; Meriles, J.M.; Vargas, G.S. The effect of crop sequences on soil microbial, chemical and physical indicators and its relationship with soybean sudden death syndrome (complex of Fusarium species). Span. J. Agric. 2014, 12, 252–264. [Google Scholar] [CrossRef]
- Prakash, O.; Pandey, P.K.; Kulkarni, G.J.; Mahale, K.N.; Shouche, Y.S. Technicalities and glitches of terminal restriction fragment length polymorphism (T-RFLP). Indian J. Microbiol. 2014, 54, 255–261. [Google Scholar] [CrossRef]
- Osborn, A.M.; Moore, E.R.B.; Timmis, K.N. An evaluation of terminal restriction fragment length polymorphism (T-RFLP) analysis for the study of microbial community sructure and dynamics. Environ. Microbiol. 2000, 2, 39–50. [Google Scholar] [CrossRef]
- Felföldy, L. Biological Water Qualification, 4th ed.; Vízügyi Hidrológia: Vízdok, Budapest, Hungary, 1987. (In Hungarian) [Google Scholar]
- Egnér, H.; Riehm, H.; Domingo, W.R. Untersuchungen über die chemische Bodenanalyseals Grundlage für die Beurteilung de Nährstoffzustandes der Böden. II. K. LantbrHögsk. Ann. 1960, 26, 199–215. [Google Scholar]
- Berzsenyi, Z.; Győrffy, B.; Lap, D. Effect of crop rotation and fertilization on corn and wheat yields and yield stability in a long-term experiment. Eur. J. Agron. 2000, 13, 225–244. [Google Scholar] [CrossRef]
- Magurno, F.; Sasvári, Z.; Barchi, L.; Posta, K. From monoculture to Norfolk system: How the number of crops in rotation can influence the biodiversity of arbuscular mycorrhiza assemblages in the soil. Open J. Ecol. 2014, 4, 1080–1088. [Google Scholar] [CrossRef][Green Version]
- Culman, S.W.; Bukowski, R.; Gauch, H.G.; Cadillo-Quiroz, H.; Buckley, D.H. T-REX: Software for the processing and analysis of T-RFLP data. BMC Bioinform. 2009, 10. [Google Scholar] [CrossRef] [PubMed]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2016. Available online: https://www.r-project.org/ (accessed on 4 May 2016).
- Kemmitt, S.J.; Wright, D.; Goulding, K.W.T.; Jones, D.L. pH regulation of carbon and nitrogen dynamics in two agricultural soils. Soil Biol. Biochem. 2006, 38, 898–911. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, Y.; Li, C.; Wang, F.; Sui, Y.; Suvannang, N.; Zhou, J.; Sun, B. Crop rotations alter bacterial and fungal diversity in paddy soils across East Asia. Soil Biol. Biochem. 2016, 95, 250–261. [Google Scholar] [CrossRef]
- Zhao, C.; Fu, S.; Mathew, R.P.; Lawrence, K.S.; Feng, Y. Soil microbial community structure and activity in a 100-year-old fertilization and crop rotation experiment. J. Plant Ecol. 2015, 8, 623–632. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Yang, H.J.; Archdeacon, D.; Tippett, O.; Tibi, M.; Whiteley, A.S. Humus-rich compost increases lettuce growth, nutrient uptake, mycorrhizal colonisation, and soil fertility. Pedosphere 2019, 29, 170–179. [Google Scholar] [CrossRef]
- Finn, D.; Kopittke, P.M.; Dennis, P.G.; Dalal, R.C. Microbial energy and matter transformation in agricultural soils. Soil Biol. Biochem. 2017, 111, 176–192. [Google Scholar] [CrossRef]
- Liu, K.; Ma, B.L.; Luan, L.; Li, C. Nitrogen, phosphorus, and potassium nutrient effects on grain filling and yield of high-yielding summer corn. J. Plant Nutr. 2011, 34, 1516–1531. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.M.; Jia, Y. Responses of soil water, nitrogen, and organic matter to the alfalfa crop rotation in semiarid loess area of China. J. Sustain. Agric. 2006, 28, 117–130. [Google Scholar] [CrossRef]
- Zeng, Q.; An, S.; Liu, Y. Soil bacterial community response to vegetation succession after fencing in the grassland of China. Sci. Total Environ. 2017, 609, 2–10. [Google Scholar] [CrossRef]
- Li, R.; Shen, Z.; Sun, L.; Zhang, R.; Fu, L.; Deng, X.; Shen, Q. Novel soil fumigation method for suppressing cucumber Fusarium wilt disease associated with soil microflora alterations. Appl. Soil Ecol. 2016, 101, 28–36. [Google Scholar] [CrossRef]
- Wang, W.; Luo, X.; Chen, Y.; Ye, X.; Wang, H.; Cao, Z.; Ran, W.; Ulrich, A. Succession of composition and function of soil bacterial communities during key rice growth stages. Front. Microbiol. 2019, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, S.L.; Seipel, T.; Yeoman, C.J.; Menalled, F.D. Soil bacterial communities of wheat vary across the growing season and among dryland farming systems. Geoderma 2020, 358, 113989. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Soman, C.; Li, D.; Wander, M.M.; Kent, A.D. Long-term fertilizer and crop-rotation treatments differentially affect soil bacterial community structure. Plant Soil 2017, 413, 145–159. [Google Scholar] [CrossRef]
- Peralta, A.L.; Sun, Y.; McDaniel, M.D.; Lennon, J.T. Crop rotational diversity increases disease suppressive capacity of soil microbiomes. Ecosphere 2018, 9, e02235. [Google Scholar] [CrossRef]
- D’Acunto, L.; Andrade, J.F.; Poggio, S.L.; Semmartin, M. Diversifying crop rotation increased metabolic soil diversity and activity of the microbial community. Agric. Ecosyst. Environ. 2018, 257, 159–164. [Google Scholar] [CrossRef]
- Benitez, M.S.; Osborne, S.L.; Lehman, R.M. Previous crop and rotation history effects on maize seedling health and associated rhizosphere microbiome. Sci. Rep. 2017, 7, 15709. [Google Scholar] [CrossRef]
- Higo, M.; Isobe, K.; Yamaguchi, M.; Ishii, R.; Drijber, R.A.; Jeske, E.S. Diversity and vertical distribution of indigenous arbuscular mycorrhizal fungi under two soybean rotational systems. Biol. Fertil. Soils 2013, 49, 1085–1096. [Google Scholar] [CrossRef]
- Naylor, D.; Coleman-Derr, D. Drought atress and root-associated bacterial communities. Front. Plant Sci. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Culman, S.W.; Gauch, H.G.; Blackwood, C.B.; Thies, J.E. Analysis of T-RFLP data using analysis of variance and ordination methods: A comparative study. J. Microbiol. Methods 2008, 75, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Noya, Y.E.; Gómez-Acata, S.; Montoya-Ciriaco, N.; RojasValdez, A.; Suárez-Arriaga, M.C.; Valenzuela-Encinas, C.; Jiménez-Bueno, N.; Verhulst, N.; Govaerts, B.; Dendooven, L. Relative impacts of tillage, residue management and crop-rotation on soil bacterial communities in a semi-arid agroecosystem. Soil Biol. Biochem. 2013, 65, 86–95. [Google Scholar] [CrossRef]
- Xuan, D.T.; Guong, V.T.; Rosling, A.; Alström, S.; Chai, B.; Högberg, N. Different crop rotation systems as drivers of change in soil bacterial community structure and yield of rice, Oryza sativa. Biol. Fertil. Soils 2012, 48, 217–225. [Google Scholar] [CrossRef]
- Yin, C.; Jones, K.L.; Peterson, D.E.; Garrett, K.A.; Hulbert, S.H.; Paulitz, T.C. Members of soil bacterial communities sensitive to tillage and crop rotation. Soil Biol. Biochem. 2010, 42, 2111–2118. [Google Scholar] [CrossRef]
- Li, C.G.; Li, X.M.; Kong, W.D.; Wu, Y.; Wang, J.G. Effect of monoculture soybean on soil microbial community in the Northeast China. Plant Soil 2010, 330, 423–433. [Google Scholar] [CrossRef]
- Silva, A.P.; Babujia, L.; Matsumoto, M.F.; Guimarães, M.F.; Hungria, M. Bacterial diversity under different tillage and crop rotation systems in an oxisol of Southern Brazil. Open Agric. J. 2013, 7, 40–47. [Google Scholar] [CrossRef]
- Buckley, D.H.; Schmidt, T.M. The structure of microbial communities in soil and the lasting impact of cultivation. Microb. Ecol. 2011, 42, 11–21. [Google Scholar]
- Xin-ya, W.E.N.; Dubinsky, E.; Yao, W.U.; Rong, Y.; Fu, C. Wheat, maize and sunflower cropping systems selectively influence bacteria community structure and diversity in their and succeeding crop’s rhizosphere. J. Integr. Agric. 2016, 15, 1892–1902. [Google Scholar]
- Hou, P.F.; Chien, C.H.; Chiang-Hsieh, Y.F.; Tseng, K.C.; Chow, C.N.; Huang, H.J.; Chang, W.C. Paddy-upland rotation for sustainable agriculture with regards to diverse soil microbial community. Sci. Rep. 2018, 8, 7966. [Google Scholar] [CrossRef]
- Hilton, S.; Bennett, A.J.; Keane, G.; Bending, G.D.; Chandler, D.; Stobart, R.; Mills, P. Impact of shortened crop rotation of oilseed rape on soil and rhizosphere microbial diversity in relation to yield decline. PLoS ONE 2013, 8, e59859. [Google Scholar] [CrossRef]
- Li, Q.H.; Wu, F.Z.; Yang, Y.; Wang, X.Z. Effects of rotation and interplanting on soil bacterial communities and cucumber yield. Acta Agric. Scand. B-S. P. 2009, 59, 431–439. [Google Scholar] [CrossRef]
- Rousk, J.; Baath, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. Microb. Ecol. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Knight, R. Global patterns in bacterial diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 11436–11440. [Google Scholar] [CrossRef] [PubMed]

| Cropping System | Humus (%) 1 | pH (KCl) 1 | CaCO3 (%) 1 | P2O5 (mg kg−1) 1 | K2O (mg kg−1) 1 | (NO3–+NO2–)–N (mg kg−1) 1 |
|---|---|---|---|---|---|---|
| CR1 (mono) | 2.31 ± 0.05 | 5.65 ± 0.14 | 0.29 ± 0.01 | 85.85 ± 9.22 | 259.83 ± 19.96 | 4.22 ± 1.63 |
| CR3 (rotation) | 2.72 ± 0.05 *** | 5.13 ± 0.10 | 0.29 ± 0.03 | 61.27 ± 6.37 * | 244.50 ± 13.09 | 9.57 ± 2.01 * |
| CR5 (rotation) | 2.74 ± 0.05 *** | 5.68 ± 0.06 | 0.16 ± 0.02 | 59.82 ± 3.92 * | 275.33 ± 14.27 | 5.50 ± 0.71 |
| CR7 (rotation) | 2.75 ± 0.08 *** | 5.95 ± 0.14 | 0.30 ± 0.09 | 73.10 ± 5.81 | 287.17 ± 8.48 | 4.50 ± 0.52 |
| One-way ANOVA 2 | F = 11.99, p = 0.0001 *** | F = 2.974, n.s. | F = 2.067, n.s. | F = 3.366, p = 0.039 * | F = 1.630, n.s. | F = 3.295, p = 0.0416 * |
| Dunnett’s post-hoc test | CR3: t = 4.74, p = 0.000365 *** CR5: t = 4.89, p = 0.000247 *** CR7: t = 5.04, p = 0.000174 *** | CR3: t = −2.63, p = 0.0409 * CR5: t = −2.78, p = 0.0300 * | CR3: t = 2.77, p = 0.0308 * |
| Cropping System | Estimated Number of T-RFs (Chao1) 1 | Evenness eH/S 1 | Shannon Index H’ 1 |
|---|---|---|---|
| CR1 | 91.88 ± 4.11 b | 0.70 ± 0.01 a | 3.57 ± 0.03 b |
| CR3 | 85.37 ± 1.98 ab | 0.70 ± 0.10 a | 3.65 ± 0.02 b |
| CR5 | 94.55 ± 5.72 b | 0.71 ± 0.01 a | 3.62 ± 0.03 b |
| CR7 | 47.67 ± 3.58 a | 0.79 ± 0.01 a | 3.22 ± 0.05 a |
| One-way ANOVA 2 | F = 4.462, p = 0.0164 * | F = 2.602, n.s. | F = 4.979, p = 0.0109 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer, Z.; Sasvári, Z.; Szentpéteri, V.; Pethőné Rétháti, B.; Vajna, B.; Posta, K. Effect of Long-Term Cropping Systems on the Diversity of the Soil Bacterial Communities. Agronomy 2019, 9, 878. https://doi.org/10.3390/agronomy9120878
Mayer Z, Sasvári Z, Szentpéteri V, Pethőné Rétháti B, Vajna B, Posta K. Effect of Long-Term Cropping Systems on the Diversity of the Soil Bacterial Communities. Agronomy. 2019; 9(12):878. https://doi.org/10.3390/agronomy9120878
Chicago/Turabian StyleMayer, Zoltán, Zita Sasvári, Viktor Szentpéteri, Beatrix Pethőné Rétháti, Balázs Vajna, and Katalin Posta. 2019. "Effect of Long-Term Cropping Systems on the Diversity of the Soil Bacterial Communities" Agronomy 9, no. 12: 878. https://doi.org/10.3390/agronomy9120878
APA StyleMayer, Z., Sasvári, Z., Szentpéteri, V., Pethőné Rétháti, B., Vajna, B., & Posta, K. (2019). Effect of Long-Term Cropping Systems on the Diversity of the Soil Bacterial Communities. Agronomy, 9(12), 878. https://doi.org/10.3390/agronomy9120878





