Simulating Growth and Development Processes of Quinoa (Chenopodium quinoa Willd.): Adaptation and Evaluation of the CSM-CROPGRO Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivar Selection
2.2. Experiments
2.3. Climatic Conditions
2.4. Data Collection
2.4.1. Phenology and Development
2.4.2. Dry Matter Partitioning and Leaf Area
2.4.3. Harvest
2.4.4. Chemical Analysis
2.5. Modeling Approach
- Composition values for plant tissues (seeds, shells, leaves, and stems) were derived from the literature and our own analysis (protein). Moreover, cardinal temperatures for growth or development processes and freezing temperatures were adapted based on literature values and a stepwise parameter estimation.
- Parameters that influence the life cycle (threshold values for the cultivar-specific phase duration of different development phases) were changed to predict the correct dates of the first pod, anthesis, and harvest maturity. Then, to ensure an adequate prediction of the leaf number and canopy height and width, the leaf appearance rate, the internode length per node, and the canopy width per node were adjusted by comparing observed and simulated values.
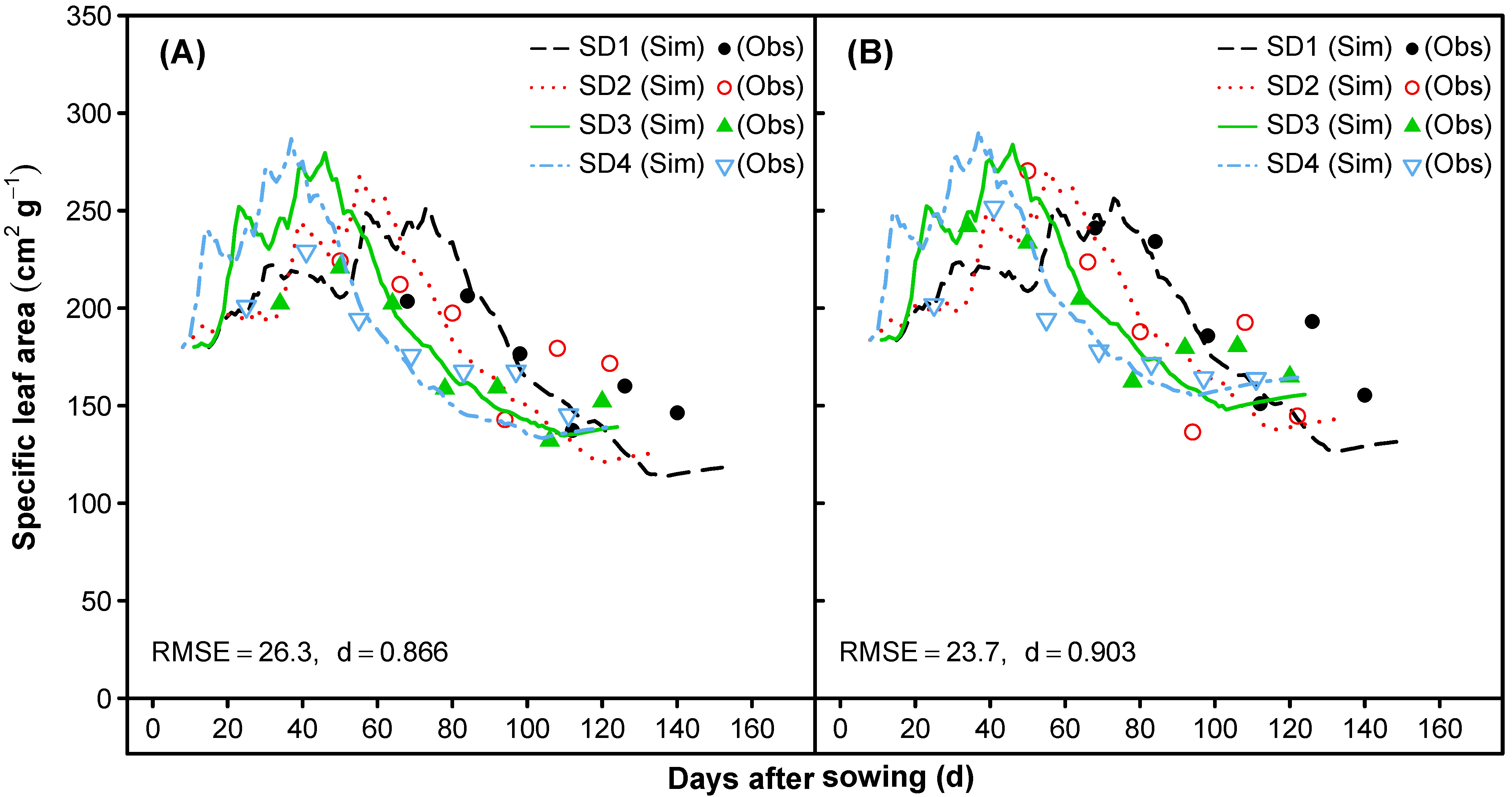
- The next step focused on the parameterization of traits influencing photosynthesis, and consequently, the biomass production during the life cycle. The first target was to match the observed values for the specific leaf area (SLA) over time. Therefore, the default values for the radiation effect on the SLA under saturating irradiance (SLAMIN) and under limiting low light (SLAMAX) were adjusted. Furthermore, we set very minor differences in the cultivar-specific SLA under standard growth conditions (SLAVAR), which is similar to the species-standard SLA in saturating irradiance (SLAREF = SLAMIN). Continuing the improvements of the simulated biomass production over time, the specific leaf weight (SLW) giving the maximum photosynthetic rate (LFMAX) was calibrated and LFMAX was defined based on our own field measurements (not shown). The critical leaf N concentrations for photosynthesis were set in accordance to own data collected in a hydroponic experiment that included different N-concentrations (unpublished).
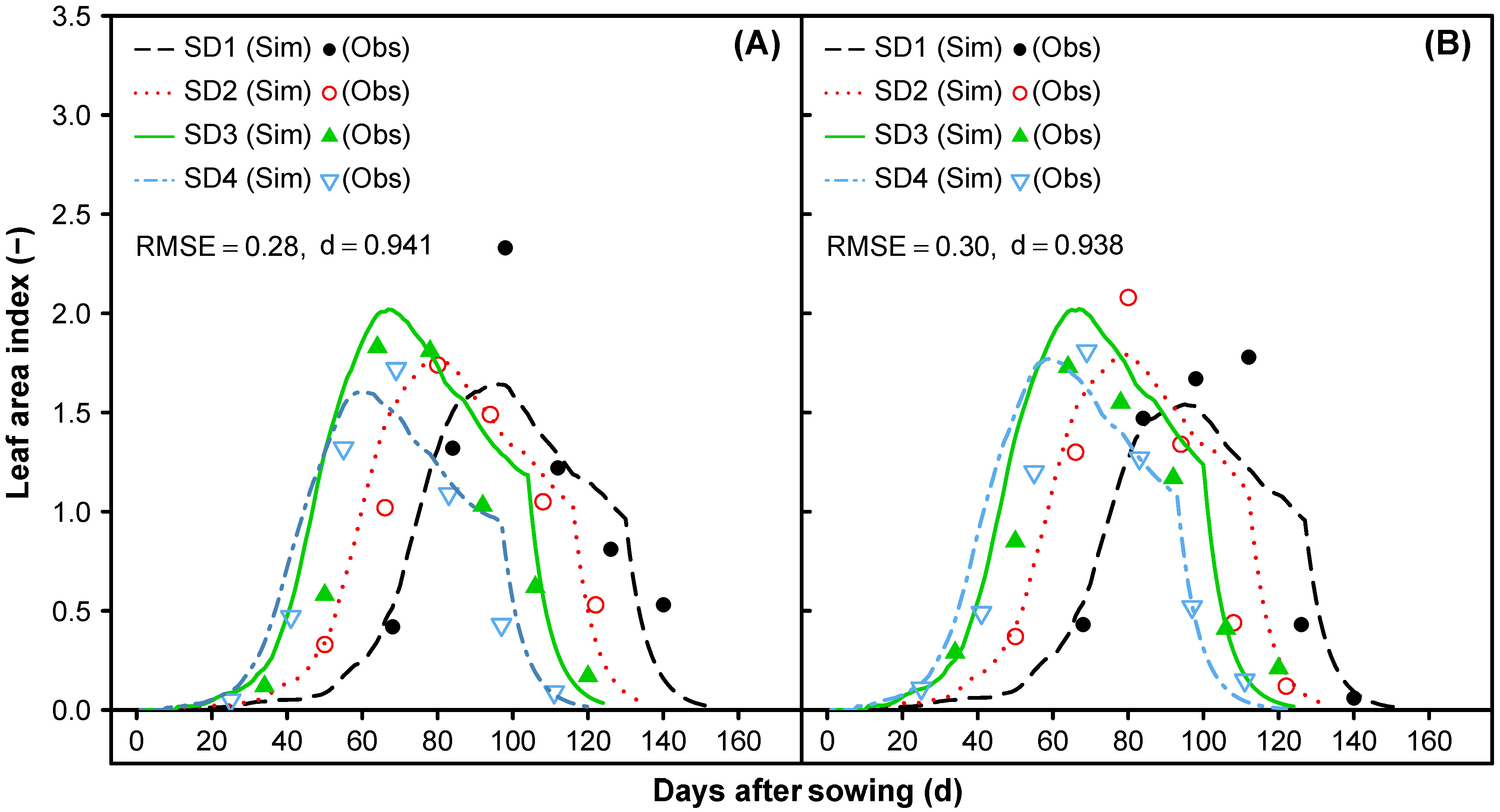
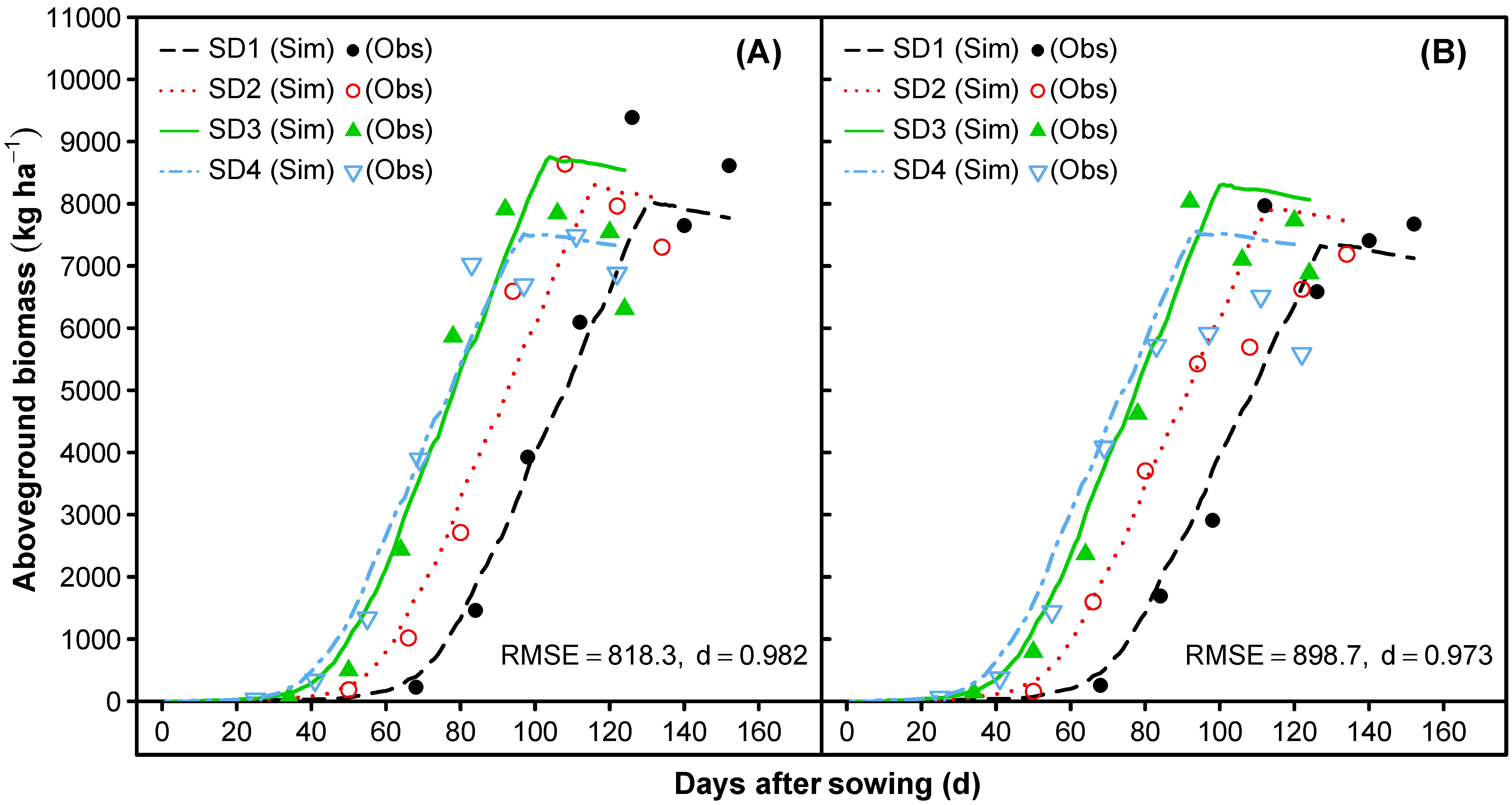
- Time-series comparisons of the predicted and observed dry matter accumulation for leaf, stem, and total aboveground biomass, as well as the leaf area index (LAI), were conducted to parameterize the partitioning functions and to set a final allocation among vegetative tissues. The focus was on the early leaf area development to reach a sufficient leaf area index (LAI), and consequently, a higher photosynthetic performance of the quinoa canopy to adequately predict the dry matter accumulation of the leaf, stem, and aboveground biomass over time.
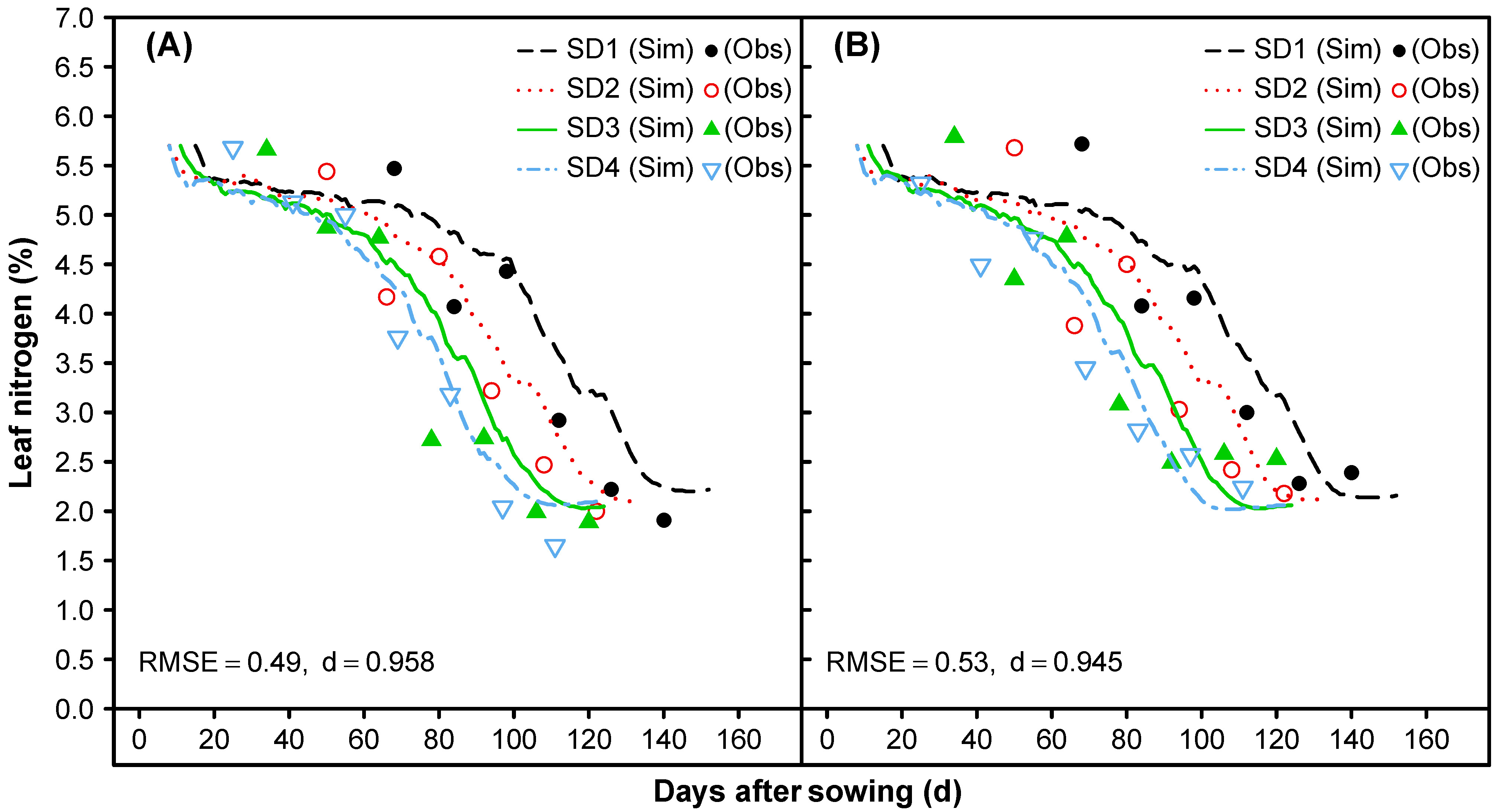
- To match the measured N concentrations of leaves, stems, and shells over time, the maximum mobilization rate (from vegetative tissues) of proteins and carbohydrates during reproductive and vegetative growth was adjusted.
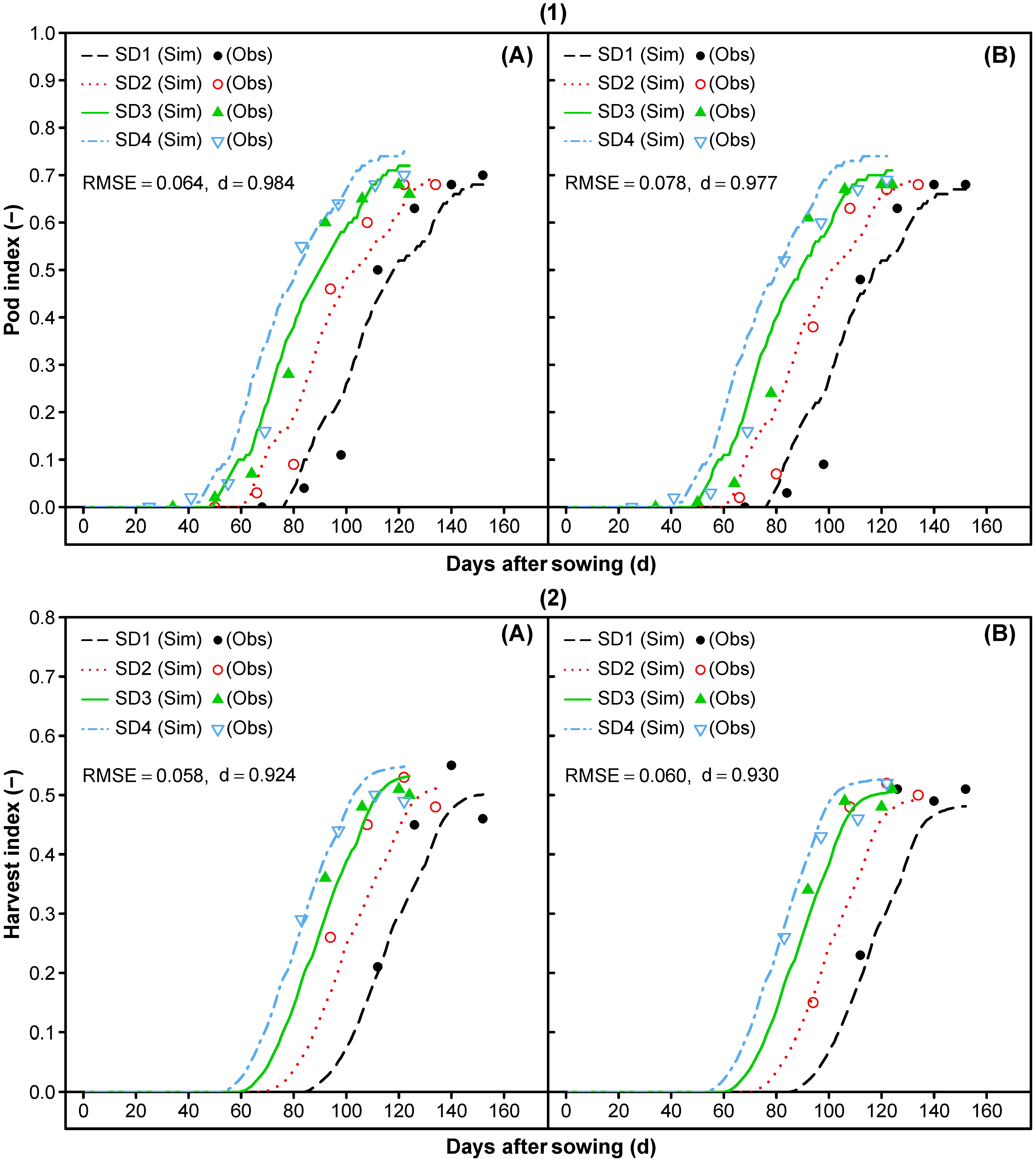
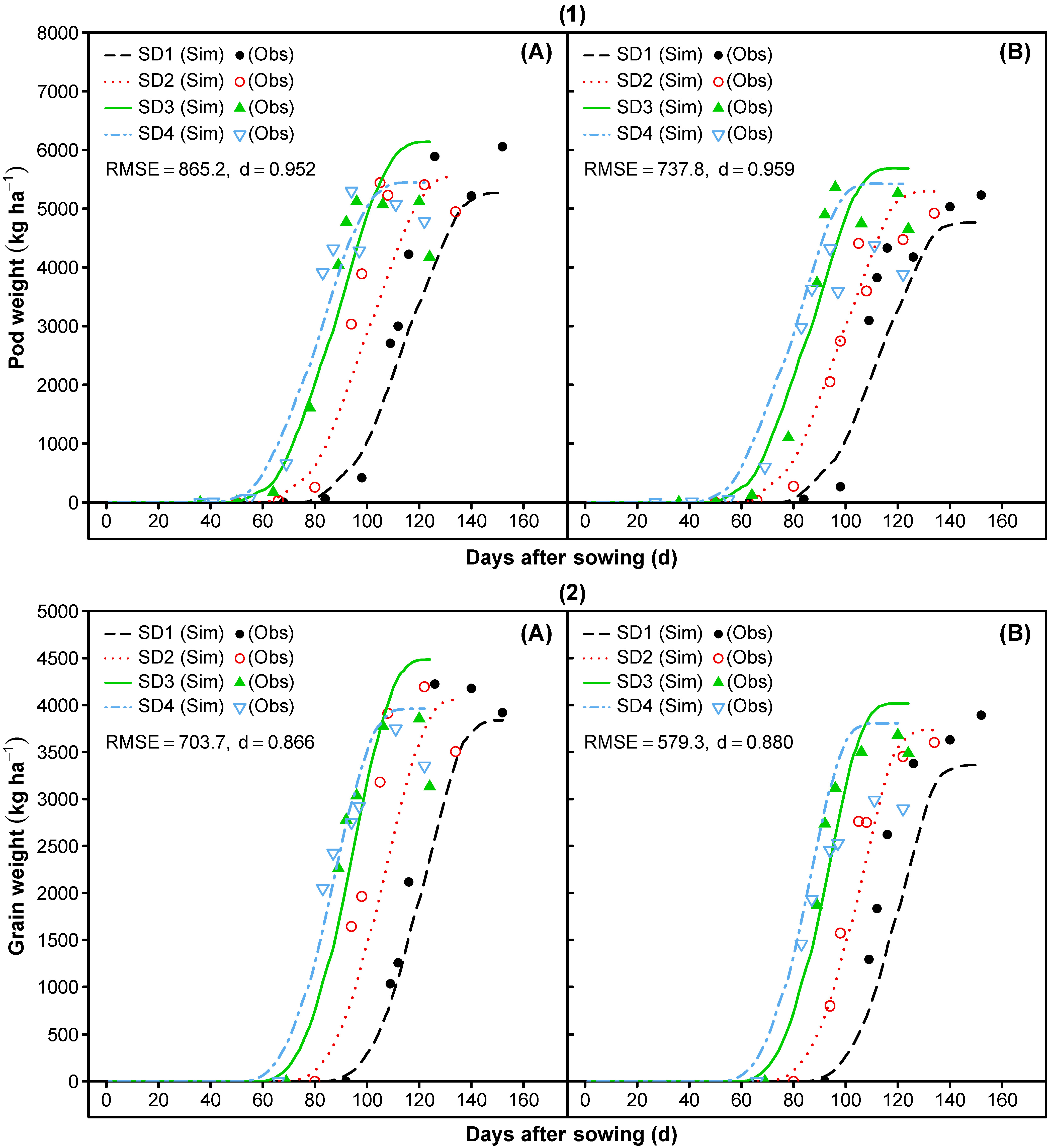
- Re-parameterization of cultivar-specific phase durations was done to match the measured values of the onset and slope of the dry matter accumulation for reproductive tissues. Furthermore, periods for pod addition (PODUR) and seed filling (SFDUR) were shortened and the maximum grain to pod ratio (THRESH) was adjusted to reach an adequate prediction of the pod dry matter, grain dry matter, pod harvest index, grain harvest index, and grain to pod ratio over time.
- Finally, the maximum weight per seed (WTPSD) was set to the lowest value allowed by the model (0.06 g) because the measured values (2.5–3.5 mg) led to source-code-related problems when simulating the reproductive growth (model failed to produce grains). A code change was not attempted because our aim was to be compatible with the default executable of V4.7 DSSAT.
2.6. Model Evaluation Statistics
3. Results and Discussion
3.1. Model Adaptation
3.1.1. Temperature-Dependent Processes
3.1.2. Plant Tissue Composition
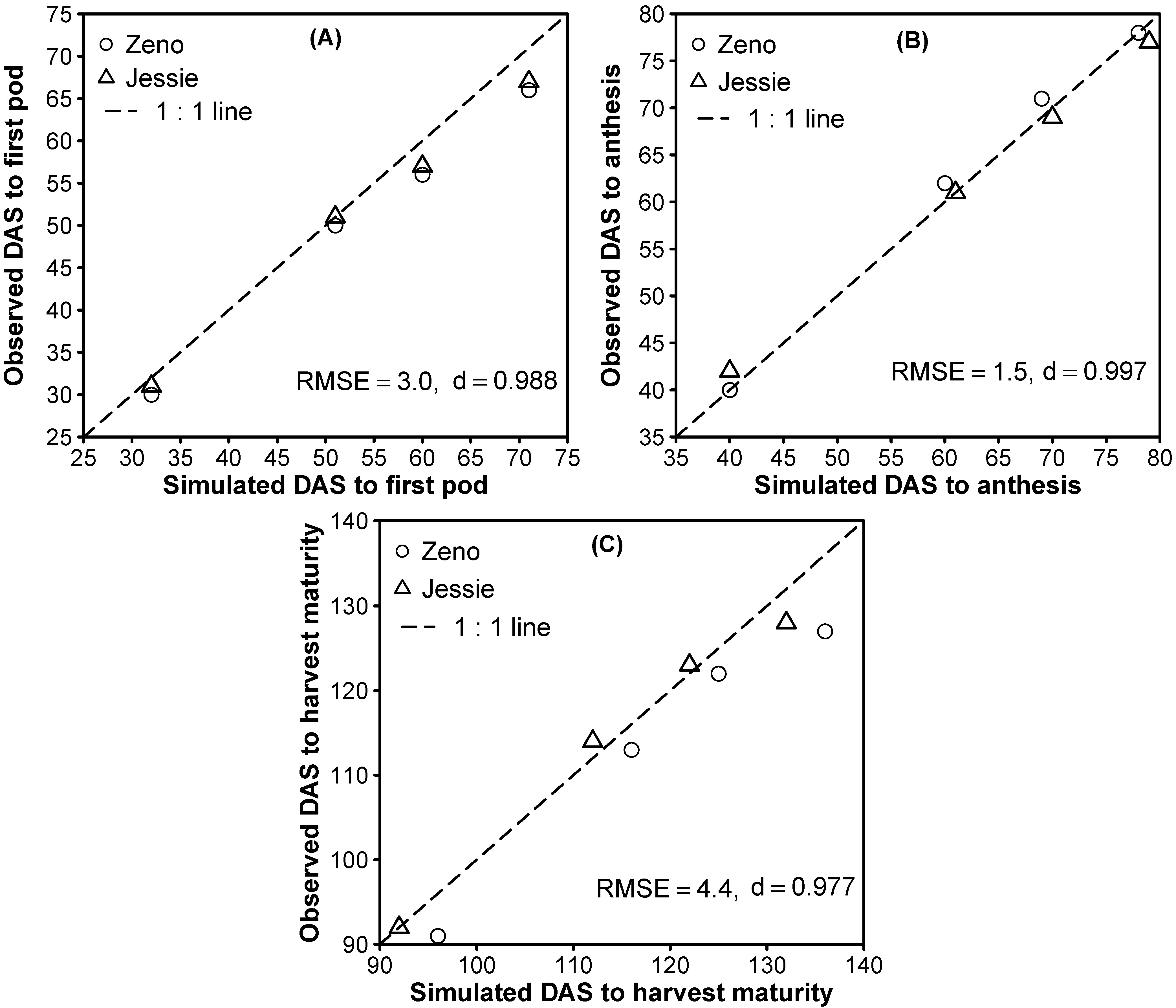
3.1.3. Phase Durations and Phenology
3.1.4. Specific Leaf Area and Photosynthesis
3.1.5. Leaf Area Index and Vegetative Partitioning
3.1.6. Leaf Senescence and Nitrogen Mobilization
3.1.7. Reproductive Partitioning
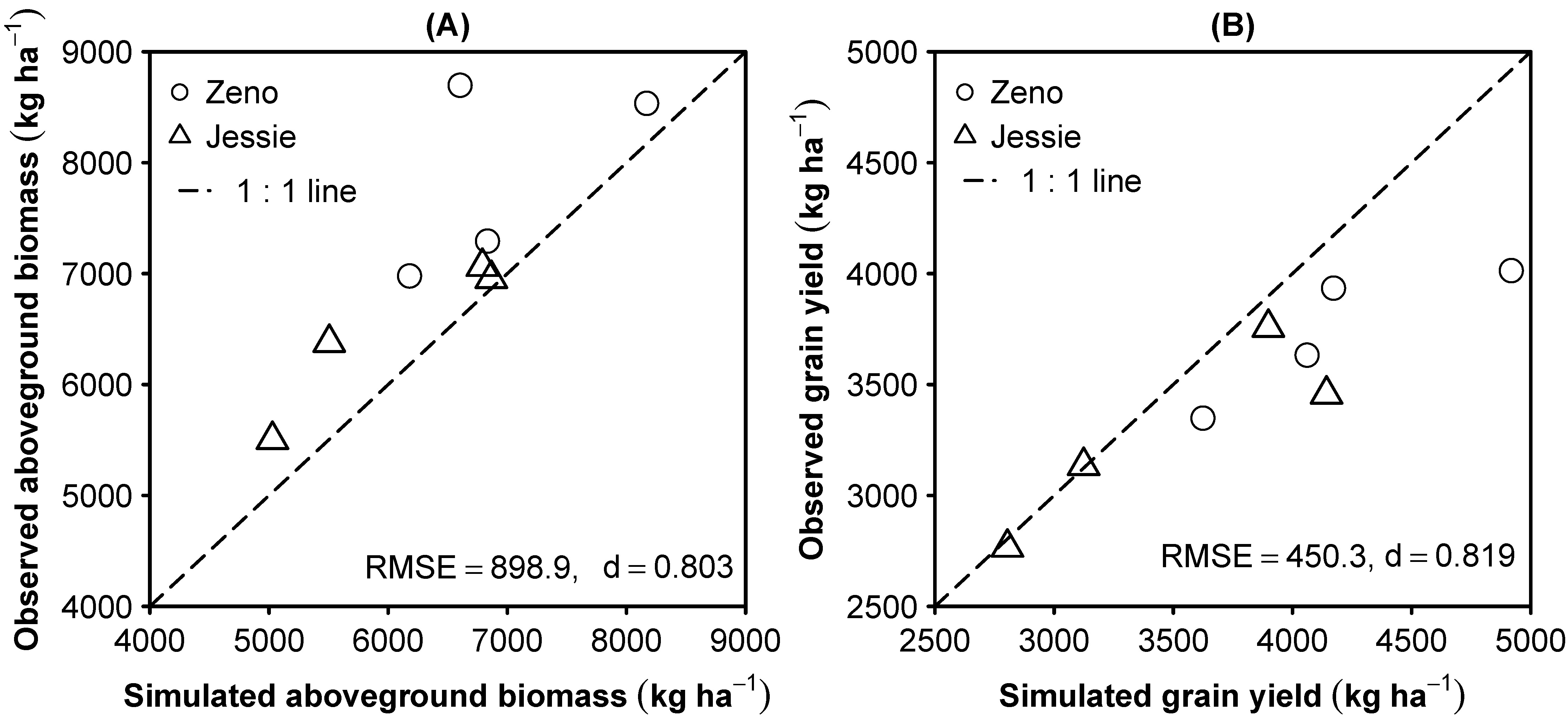
3.2. Model Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olesen, J.E.; Trnka, M.; Kersebaum, K.C.; Skjelvåg, A.O.; Seguin, B.; Peltonen-Sainio, P.; Rossi, F.; Kozyra, J.; Micale, F. Impacts and adaptation of European crop production systems to climate change. Eur. J. Agron. 2011, 34, 96–112. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Iglesias, A.; Yang, X.B.; Epstein, P.R.; Chivian, E. Climate change and extreme weather events; implications for food production, plant diseases, and pests. Glob. Chang. Hum. Health 2001, 2, 90–104. [Google Scholar] [CrossRef]
- Olesen, J.E.; Bindi, M. Consequences of climate change for European agricultural productivity, land use and policy. Eur. J. Agron. 2002, 16, 239–262. [Google Scholar] [CrossRef]
- Rezaei, E.E.; Gaiser, T.; Siebert, S.; Ewert, F. Adaptation of crop production to climate change by crop substitution. Mitig. Adapt. Strateg. Glob. Chang. 2015, 20, 1155–1174. [Google Scholar] [CrossRef]
- Turral, H.; Burke, J.; Faurès, J.M. Climate change, water and food security. In FAO Water Reports No. 36; FAO: Rome, Italy, 2011. [Google Scholar]
- Smit, B.; Skinner, M.W. Adaptation options in agriculture to climate change: A typology. Mitig. Adapt. Strateg. Glob. Chang. 2002, 7, 85–114. [Google Scholar] [CrossRef]
- Bazile, D.; Bertero, D.; Nieto, C. State of the Art Report of Quinoa in the World in 2013, In FAO & CIRAD 2015; FAO: Rome, Italy, 2015; ISBN 978-92-5-108558-5. [Google Scholar]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef]
- Fuentes, F.F.; Bazile, D.; Bhargava, A.; Martínez, E.A. Implications of farmers’ seed exchanges for on-farm conservation of quinoa, as revealed by its genetic diversity in Chile. J. Agric. Sci. 2012, 150, 702–716. [Google Scholar] [CrossRef]
- Bazile, D.; Fuentes, F.; Mujica, A. Historical Perspectives and Domestication. In Quinoa: Botany, Production and Uses; Bhargava, A., Srivastava, S., Eds.; CAB International: Wallingford, Oxfordshire, UK, 2013; pp. 16–36. ISBN 978-1-78064-226-0. [Google Scholar]
- Bazile, D.; Martínez, E.A.; Fuentes, F. Diversity of quinoa in a biogeographical Island: A review of constraints and potential from arid to temperate regions of Chile. Not. Bot. Horti Agrobot. Cluj Napoca 2014, 42, 289–298. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Mujica, A.; Jensen, C.R. The resistance of quinoa (Chenopodium quinoa Willd.) to adverse abiotic factors. Food Rev. Int. 2003, 19, 99–109. [Google Scholar] [CrossRef]
- Martínez, E.A.; Veas, E.; Jorquera, C.; San Martín, R.; Jara, P. Re-introduction of quinoa into Arid Chile: Cultivation of two lowland races under extremely low irrigation. J. Agron. Crop. Sci. 2009, 195, 1–10. [Google Scholar] [CrossRef]
- Sanchez, H.B.; Lemeur, R.; Damme, P.V.; Jacobsen, S.E. Ecophysiological analysis of drought and salinity stress of quinoa (Chenopodium quinoa Willd.). Food Rev. Int. 2003, 19, 111–119. [Google Scholar] [CrossRef]
- Cocozza, C.; Pulvento, C.; Lavini, A.; Riccardi, M.; d’Andria, R.; Tognetti, R. Effects of Increasing Salinity Stress and Decreasing Water Availability on Ecophysiological Traits of Quinoa (Chenopodium quinoa Willd.) Grown in a Mediterranean-Type Agroecosystem. J. Agron. Crop. Sci. 2013, 199, 229–240. [Google Scholar] [CrossRef]
- Bois, J.F.; Winkel, T.; Lhomme, J.P.; Raffaillac, J.P.; Rocheteau, A. Response of some Andean cultivars of quinoa (Chenopodium quinoa Willd.) to temperature: Effects on germination, phenology, growth and freezing. Eur. J. Agron. 2006, 25, 299–308. [Google Scholar] [CrossRef]
- Präger, A.; Munz, S.; Nkebiwe, P.; Mast, B.; Graeff-Hönninger, S. Yield and quality characteristics of different quinoa (Chenopodium quinoa Willd.) Cultivars grown under field conditions in Southwestern Germany. Agronomy 2018, 8, 197. [Google Scholar] [CrossRef]
- Prego, I.; Maldonado, S.; Otegui, M. Seed structure and localization of reserves in Chenopodium quinoa. Ann. Bot. 1998, 82, 481–488. [Google Scholar] [CrossRef]
- McDonell, E. Nutrition Politics in the Quinoa Boom: Connecting Consumer and Producer Nutrition in the Commercialization of Traditional Foods. Int. J. Food. Nutr. Sci. 2016, 3, 1–7. [Google Scholar] [CrossRef][Green Version]
- Furche, C.; Salcedo, S.; Krivonos, E.; Rabczuk, P.; Jara, B.; Fernandez, D.; Correa, F. International Quinoa trade. In State of the Art Report of Quinoa in the World in 2013; Bazile, D., Bertero, D., Nieto, C., Eds.; FAO & CIRAD: Rome, Italy, 2015; pp. 316–329. ISBN 978-92-5-108558-5. [Google Scholar]
- Noulas, C.; Tziouvalekas, M.; Vlachostergios, D.; Baxevanos, D.; Karyotis, T.; Iliadis, C. Adaptation, agronomic potential, and current perspectives of quinoa under mediterranean conditions: Case studies from the lowlands of central Greece. Commun. Soil Sci. Plant Anal. 2017, 48, 2612–2629. [Google Scholar] [CrossRef]
- Szilagyi, L.; Jørnsgǻrd, B. Preliminary agronomic evaluation of Chenopodium quinoa Willd. under climatic conditions of Romania. Sci. Pap. Ser. A Agron. 2014, 57, 339–343. [Google Scholar]
- Stikic, R.; Glamoclija, D.; Demin, M.; Vucelic-Radovic, B.; Jovanovic, Z.; Milojkovic-Opsenica, D.; Jacobsen, S.E.; Milovanovic, M. Agronomical and nutritional evaluation of quinoa seeds (Chenopodium quinoa Willd.) as an ingredient in bread formulations. J. Cereal Sci. 2012, 55, 132–138. [Google Scholar] [CrossRef]
- Pulvento, C.; Riccardi, M.; Lavini, A.; d’Andria, R.; Iafelice, G.; Marconi, E. Field trial evaluation of two chenopodium quinoa genotypes grown under rain-fed conditions in a typical Mediterranean environment in South Italy. J. Agron. Crop. Sci. 2010, 196, 407–411. [Google Scholar] [CrossRef]
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusari, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Sepahvand, N.A.; et al. Worldwide Evaluations of Quinoa: Preliminary Results from Post International Year of Quinoa FAO Projects in Nine Countries. Front. Plant Sci. 2016, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Boote, K.J.; Angadi, S.V.; Grover, K.; Begna, S.; Auld, D. Adapting the CROPGRO model to simulate growth and yield of spring safflower in semiarid conditions. Agron. J. 2016, 108, 64–72. [Google Scholar] [CrossRef]
- Blade, S.F.; Slinkard, A.E. New crop development: The Canadian experience. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002; pp. 62–75. ISBN 0-970756-5-5. [Google Scholar]
- Fodor, N.; Challinor, A.; Droutsas, I.; Ramirez-Villegas, J.; Zabel, F.; Koehler, A.K.; Foyer, C.H. Integrating plant science and crop modeling: Assessment of the impact of climate change on soybean and maize production. Plant Cell Physiol. 2017, 58, 1833–1847. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ahuja, L.R.; Islam, A.; Trout, T.J.; Saseendran, S.A.; Malone, R.W. Modeling yield and biomass responses of maize cultivars to climate change under full and deficit irrigation. Agric. Water Manag. 2017, 180, 88–98. [Google Scholar] [CrossRef]
- Attia, A.; Rajan, N.; Xue, Q.; Nair, S.; Ibrahim, A.; Hays, D. Application of DSSAT-CERES-Wheat model to simulate winter wheat response to irrigation management in the Texas High Plains. Agric. Water Manag. 2016, 165, 50–60. [Google Scholar] [CrossRef]
- Ewert, F.; Rötter, R.P.; Bindi, M.; Webber, H.; Trnka, M.; Kersebaum, K.C.; Olesen, J.E.; van Ittersum, M.K.; Janssen, S.; Rivington, M.; et al. Crop modelling for integrated assessment of risk to food production from climate change. Environ. Model. Softw. 2015, 72, 287–303. [Google Scholar] [CrossRef]
- Ngwira, A.R.; Aune, J.B.; Thierfelder, C. DSSAT modelling of conservation agriculture maize response to climate change in Malawi. Soil Till. Res. 2014, 143, 85–94. [Google Scholar] [CrossRef]
- Zamora, D.S.; Jose, S.; Jones, J.W.; Cropper, W.P. Modeling cotton production response to shading in a pecan alleycropping system using CROPGRO. Agrofor. Syst. 2009, 76, 423–435. [Google Scholar] [CrossRef]
- Boote, K.J.; Jones, J.W.; Pickering, N.B. Potential uses and limitations of crop models. Agron. J. 1996, 88, 704–716. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D.; Garcia, M.; Miranda, R.; Cusicanqui, J.A.; Taboada, C.; Mendoza, J.; Huanca, R.; Mamani, A.; Condori, O.; et al. Simulating yield response of quinoa to water availability with AquaCrop. Agron. J. 2009, 101, 499–508. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D.; Garcia, M.; Taboada, C.; Miranda, R.; Cusicanqui, J.; Mhizha, T.; Vacher, J. Modeling the potential for closing quinoa yield gaps under varying water availability in the Bolivian Altiplano. Agric. Water Manag. 2009, 96, 1652–1658. [Google Scholar] [CrossRef]
- Hirich, A.; Choukr-Allah, R.; Ragab, R.; Jacobsen, S.E.; El Youssfi, L.; El Omari, H. The SALTMED model calibration and validation using field data from Morocco. J. Mater. Environ. Sci. 2012, 3, 342–359. [Google Scholar]
- Pulvento, C.; Riccardi, M.; Lavini, A.; D’andria, R.; Ragab, R. SALTMED model to simulate yield and dry matter for quinoa crop and soil moisture content under different irrigation strategies in south Italy. Irrig. Drain. 2013, 62, 229–238. [Google Scholar] [CrossRef]
- Fghire, R.; Wahbi, S.; Anaya, F.; Issa Ali, O.; Benlhabib, O.; Ragab, R. Response of quinoa to different water management strategies: Field experiments and SALTMED model application results. Irrig. Drain. 2015, 64, 29–40. [Google Scholar] [CrossRef]
- Kaya, Ç.I.; Yazar, A.; Sezen, S.M. SALTMED model performance on simulation of soil moisture and crop yield for quinoa irrigated using different irrigation systems, irrigation strategies and water qualities in Turkey. Agric. Agricu. Sci. Procedia 2015, 4, 108–118. [Google Scholar] [CrossRef]
- Kaya, Ç.I.; Yazar, A. SALTMED model performance for quinoa irrigated with fresh and saline water in a Mediterranean environment. Irrig. Drain. 2016, 65, 29–37. [Google Scholar] [CrossRef]
- Steduto, P.; Raes, D.; Hsiao, T.C.; Fereres, E.; Heng, L.K.; Howell, T.A.; Evett, S.R.; Rojas-Lara, B.A.; Farahani, H.J.; Izzi, G.; et al. Concepts and applications of AquaCrop: The FAO crop water productivity model. In Crop Modeling and Decision Support; Cao, W., White, J.W., Wang, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 175–191. ISBN 978-3-642-01132-0. [Google Scholar]
- Ragab, R. Integrated management tool for water, crop, soil and N-fertilizers: The SALTMED model. Irrig. Drain. 2015, 64, 1–12. [Google Scholar] [CrossRef]
- Ragab, R. A holistic generic integrated approach for irrigation, crop and field management: The SALTMED model. Environ. Model. Softw. 2002, 17, 345–361. [Google Scholar] [CrossRef]
- Hassanli, M.; Ebrahimian, H.; Mohammadi, E.; Rahimi, A.; Shokouhi, A. Simulating maize yields when irrigating with saline water, using the AquaCrop, SALTMED, and SWAP models. Agric. Water Manag. 2016, 176, 91–99. [Google Scholar] [CrossRef]
- Jones, J.W.; Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Batchelor, W.D.; Hunt, L.A.; Wilkens, P.W.; Singh, U.; Gijsman, A.J.; Ritchie, J.T. The DSSAT cropping system model. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Boote, K.J.; Jones, J.W.; Hoogenboom, G. Simulation of crop growth: CROPGRO model. In Agricultural Systems Modeling and Simulation; Peart, R.M., Curry, R.B., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1998; pp. 651–692. ISBN 0824700414. [Google Scholar]
- Boote, K.J.; Jones, J.W.; Hoogenboom, G.; Pickering, N.B. The CROPGRO model for grain legumes. In Understanding Options for Agricultural Production; Tsuji, G.Y., Hoogenboom, G., Thornton, P.K., Eds.; Springer Science+Business Media: Berlin/Heidelberg, Germany, 1998; pp. 99–128. ISBN 978-94-017-3624-4. [Google Scholar]
- Hoogenboom, G.; Porter, C.H.; Shelia, V.; Boote, K.J.; Singh, U.; White, J.W.; Hunt, L.A.; Ogoshi, R.; Lizaso, J.I.; Koo, J.; et al. Decision Support System for Agrotechnology Transfer (DSSAT) Version 4.7 (www.DSSAT.net); DSSAT Foundation: Gainesville, FL, USA, 2017. [Google Scholar]
- Boote, K.J.; Mínguez, M.I.; Sau, F. Adapting the CROPGRO legume model to simulate growth of faba bean. Agron. J. 2002, 94, 743–756. [Google Scholar] [CrossRef]
- Alderman, P.D.; Boote, K.J.; Jones, J.W.; Bhatia, V.S. Adapting the CSM-CROPGRO model for pigeonpea using sequential parameter estimation. Field Crop. Res. 2015, 181, 1–15. [Google Scholar] [CrossRef]
- Deligios, P.A.; Farci, R.; Sulas, L.; Hoogenboom, G.; Ledda, L. Predicting growth and yield of winter rapeseed in a Mediterranean environment: Model adaptation at a field scale. Field Crop. Res. 2013, 144, 100–112. [Google Scholar] [CrossRef]
- Pathak, T.B.; Jones, J.W.; Fraisse, C.W.; Wright, D.; Hoogenboom, G. Uncertainty analysis and parameter estimation for the CSM-CROPGRO-Cotton model. Agron. J. 2012, 104, 1363–1373. [Google Scholar] [CrossRef]
- Pedreira, B.C.; Pedreira, C.G.; Boote, K.J.; Lara, M.A.; Alderman, P.D. Adapting the CROPGRO perennial forage model to predict growth of Brachiaria brizantha. Field Crop. Res. 2011, 120, 370–379. [Google Scholar] [CrossRef]
- Hartkamp, A.D.; Hoogenboom, G.; White, J.W. Adaptation of the CROPGRO growth model to velvet bean (Mucuna pruriens): I. Model development. Field Crop. Res. 2002, 78, 9–25. [Google Scholar] [CrossRef]
- Jacobsen, S.E. The scope for adaptation of quinoa in Northern Latitudes of Europe. J. Agron. Crop. Sci. 2017, 203, 603–613. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006, First Update 2007. In World Soil Resources Reports No. 103; FAO: Rome, Italy, 2007. [Google Scholar]
- Jacobsen, S.E.; Stølen, O. Quinoa—Morphology, phenology and prospects for its production as a new crop in Europe. Eur. J. Agron. 1993, 2, 19–29. [Google Scholar] [CrossRef]
- Sosa-Zuniga, V.; Brito, V.; Fuentes, F.; Steinfort, U. Phenological growth stages of quinoa (Chenopodium quinoa) based on the BBCH scale. Ann. Appl. Biol. 2017, 171, 117–124. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists, Inc.: Arlington, TX, USA, 1990; p. 70. ISBN 0-935584-42-0. [Google Scholar]
- Fensterseifer, C.A.; Streck, N.A.; Baigorria, G.A.; Timilsina, A.P.; Zanon, A.J.; Cera, J.C.; Rocha, T.S. On the number of experiments required to calibrate a cultivar in a crop model: The case of CROPGRO-soybean. Field Crop. Res. 2017, 204, 146–152. [Google Scholar] [CrossRef]
- Willmott, C.J. Some comments on the evaluation of model performance. Bull. Am. Meteorol. Soc. 1982, 63, 1309–1313. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Bach, A.P. The influence of temperature on seed germination rate in quinoa (Chenopodium quinoa Willd.). Seed Sci. Technol. 1998, 26, 515–523. [Google Scholar]
- Bertero, H.D.; King, R.W.; Hall, A.J. Photoperiod and temperature effects on the rate of leaf appearance in quinoa (Chenopodium quinoa). Funct. Plant Biol. 2000, 27, 349–356. [Google Scholar] [CrossRef]
- Bertero, H.D.; King, R.W.; Hall, A.J. Modelling photoperiod and temperature responses of flowering in quinoa (Chenopodium quinoa Willd.). Field Crop. Res. 1999, 63, 19–34. [Google Scholar] [CrossRef]
- Bertero, H.D. Environmental Control of Development. In State of the Art Report of Quinoa in the World in 2013; Bazile, D., Bertero, D., Nieto, C., Eds.; FAO & CIRAD: Rome, Italy, 2015; pp. 120–130. ISBN 978-92-5-108558-5. [Google Scholar]
- Bertero, H.D.; King, R.W.; Hall, A.J. Photoperiod-sensitive development phases in quinoa (Chenopodium quinoa Willd.). Field Crop. Res. 1999, 60, 231–243. [Google Scholar] [CrossRef]
- Walters, H.; Carpenter-Boggs, L.; Desta, K.; Yan, L.; Matanguihan, J.; Murphy, K. Effect of irrigation, intercrop, and cultivar on agronomic and nutritional characteristics of quinoa. Agroecol. Sustain. Food Syst. 2016, 40, 783–803. [Google Scholar] [CrossRef]
- Hafid, R.E.; Imaalem, H.A.; Driedger, D.; Bandara, M.; Stevenson, J. Quinoa…The next Cinderella crop for Alberta? Alberta Agric. Food Rural Dev. 2005, 1–28. Available online: https://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/afu9961/$FILE/quinoa_final_report_june_05.pdf (accessed on 1 December 2019).
- Hinojosa, L.; Matanguihan, J.B.; Murphy, K.M. Effect of high temperature on pollen morphology, plant growth and seed yield in quinoa (Chenopodium quinoa Willd). J. Agron. Crop. Sci. 2019, 205, 33–45. [Google Scholar] [CrossRef]
- Bunce, J.A. Variation in yield responses to elevated CO2 and a brief high temperature treatment in quinoa. Plants 2017, 6, 26. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Amjad, M.; Iqbal, S.; Jacobsen, S.E. Growth and physiological responses of quinoa to drought and temperature stress. J. Agron. Crop. Sci. 2016, 202, 445–453. [Google Scholar] [CrossRef]
- Bunce, J.A. Thermal acclimation of the temperature dependence of the VCmax of Rubisco in quinoa. Photosynthetica 2018, 56, 1171–1176. [Google Scholar] [CrossRef]
- Pearcy, R.W.; Tumosa, N.; Williams, K. Relationships between growth, photosynthesis and competitive interactions for a C 3 and C 4 plant. Oecologia 1981, 48, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Penning de Vries, F.W.T.; van Laar, H.H. Simulation of growth processes and the model BACROS. In Simulation of Plant Growth and Crop Production; Penning de Vries, F.W.T., van Laar, H.H., Eds.; PUDOC: Wageningen, The Netherlands, 1982; pp. 114–136. ISBN 9789022008096. [Google Scholar]
- Ahamed, N.T.; Singhal, R.S.; Kulkarni, P.R.; Pal, M. A lesser-known grain, Chenopodium quinoa: Review of the chemical composition of its edible parts. Food Nutr. Bull. 1998, 19, 61–70. [Google Scholar] [CrossRef]
- Kozioł, M.J. Chemical composition and nutritional evaluation of quinoa (Chenopodium quinoa Willd.). J. Food Compos. Anal. 1992, 5, 35–68. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Srivastava, J.; Singh, N.; Ohri, D. Genetic diversity for mineral accumulation in the foliage of Chenopodium spp. Sci. Hortic. 2008, 118, 338–346. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Mineral composition in foliage of some cultivated and wild species of Chenopodium. Span. J. Agric. Res. 2010, 8, 371–376. [Google Scholar] [CrossRef]
- Schlick, G. Nutritional Characteristics and Biomass Production of Chenopodium Quinoa Grown in Controlled Environments. Master’s Thesis, San Jose State University, San Jose, CA, USA, 2000. [Google Scholar]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Marcone, M.F.; Liu, R.; Tsao, R. Lipids, tocopherols, and carotenoids in leaves of amaranth and quinoa cultivars and a new approach to overall evaluation of nutritional quality traits. J. Agric. Food Chem. 2014, 62, 12610–12619. [Google Scholar] [CrossRef]
- Carrasco, C.; Cuno, D.; Carlqvist, K.; Galbe, M.; Lidén, G. SO2-catalysed steam pretreatment of quinoa stalks. J. Chem. Technol. Biotechnol. 2015, 90, 64–71. [Google Scholar] [CrossRef]
- Boote, K.J.; Pickering, N.B. Modeling photosynthesis of row crop canopies. HortScience 1994, 29, 1423–1434. [Google Scholar] [CrossRef]
- Bascuñán-Godoy, L.; Sanhueza, C.; Pinto, K.; Cifuentes, L.; Reguera, M.; Briones, V.; Zurita-Silva, A.; Álvarez, R.; Morales, A.; Silva, H. Nitrogen physiology of contrasting genotypes of Chenopodium quinoa Willd.(Amaranthaceae). Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Monteros, C.; Corcuera, L.J.; Bravo, L.A.; Christiansen, J.L.; Mujica, A. Frost resistance mechanisms in quinoa (Chenopodium quinoa Willd.). Eur. J. Agron. 2007, 26, 471–475. [Google Scholar] [CrossRef]




| Cultivar | Origin | Breeder | Note |
|---|---|---|---|
| Jessie | France | Abbott Agra 1 | Sweet 3 |
| Zeno | Austria | Zeno Projekte 2 | Bitter 3 |
| Year | Trial | Depth (cm) | Clay (%) | Sand (%) | Silt (%) |
|---|---|---|---|---|---|
| 2016 | 1 | 0–30 | 25.6 | 14.5 | 59.9 |
| 2016 | 1 | 30–60 | 16.6 | 28.7 | 54.7 |
| 2016 | 1 | 60–90 | - | - | - |
| 2017 | 2 | 0–30 | 27.1 | 2.7 | 70.2 |
| 2017 | 2 | 30–60 | 27.2 | 2.4 | 70.4 |
| 2017 | 2 | 60–90 | 33.1 | 3.3 | 63.6 |
| Year | Trial | Depth (cm) | Nmin 1 (kg ha−1) | Nt 2 (%) | Corg 3 (%) | pH |
|---|---|---|---|---|---|---|
| 2016 | 1 | 0–30 | 6.5 | 0.11 | 1.94 | 7.45 |
| 2016 | 1 | 30–60 | 5.1 | 0.02 | 0.83 | - |
| 2016 | 1 | 60–90 | 11.3 | 0.01 | 0.03 | - |
| 2017 | 2 | 0–30 | 26.67 | 0.19 | 1.33 | 6.63 |
| 2017 | 2 | 30–60 | 23.38 | 0.11 | 0.61 | - |
| 2017 | 2 | 60–90 | 3.46 | 0.08 | 0.41 | - |
| Trial | Cultivar | Sowing Date | Date of Sowing (DD.MM.YYYY) | Emergence 1 (DAS 2) | Harvest (DAS 2) | Final Plant Density (plants m−2) |
|---|---|---|---|---|---|---|
| 1 | Zeno | 1 | 17.03.2016 | 13 | 152 | 40 |
| 1 | Zeno | 2 | 04.04.2016 | 9 | 134 | 48 |
| 1 | Zeno | 3 | 20.04.2016 | 11 | 124 | 71 |
| 1 | Zeno | 4 | 29.04.2016 | 7 | 115 | 51 |
| 1 | Jessie | 1 | 17.03.2016 | 15 | 152 | 48 |
| 1 | Jessie | 2 | 04.04.2016 | 10 | 134 | 74 |
| 1 | Jessie | 3 | 20.04.2016 | 11 | 124 | 83 |
| 1 | Jessie | 4 | 29.04.2016 | 8 | 115 | 76 |
| 2 | Zeno | 1 | 15.03.2017 | 14 | 147 | 24 |
| 2 | Zeno | 2 | 30.03.2017 | 10 | 132 | 31 |
| 2 | Zeno | 3 | 12.04.2017 | 11 | 127 | 42 |
| 2 | Zeno | 4 | 10.05.2017 | 6 | 98 | 42 |
| 2 | Jessie | 1 | 15.03.2017 | 13 | 147 | 31 |
| 2 | Jessie | 2 | 30.03.2017 | 11 | 132 | 32 |
| 2 | Jessie | 3 | 12.04.2017 | 13 | 127 | 36 |
| 2 | Jessie | 4 | 10.05.2017 | 5 | 98 | 58 |
| Month | Tmax (°C) | Tmin (°C) | SRAD (MJ m−2 day−1) | Rainfall (mm) | ||||
|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| March | 7.1 | 12.2 | 0.2 | 2.3 | 9.6 | 11.7 | 29.3 | 63.2 |
| April | 11.7 | 12.1 | 2.8 | 1.8 | 13.8 | 15.6 | 47.4 | 29.0 |
| May | 16.8 | 19.1 | 7.2 | 7.8 | 17.5 | 18.9 | 88.0 | 47.0 |
| June | 20.6 | 24.0 | 11.4 | 11.9 | 17.7 | 23.5 | 108.3 | 72.2 |
| July | 23.5 | 23.3 | 12.7 | 13.0 | 19.4 | 18.1 | 64.8 | 109.9 |
| August | 24.0 | 23.6 | 11.5 | 12.6 | 18.3 | 16.2 | 29.3 | 69.3 |
| September | 22.1 | 17.0 | 13.2 | 7.2 | 14.4 | 11.3 | 50.6 | 52.2 |
| Development or Growth Processes | Description | Cardinal Temperatures (°C) | |||
|---|---|---|---|---|---|
| Tb 1 | Topt1 2 | Topt2 3 | Tmax 4 | ||
| Vegetative development | Node expression/germination rate | 1.0 | 22.0 | 30.0 | 45.0 |
| Early reproductive development | Flowering to first seed | 2.0 | 24.0 | 30.0 | 45.0 |
| Late reproductive development | First seed to maturity | 4.0 | 22.0 | 30.0 | 45.0 |
| Light-saturated leaf photosynthesis | LFmax 5 vs. current temperature | 2.0 | 32.0 | 36.0 8 | 44.0 |
| Light-saturated leaf photosynthesis | LFmax 5 vs. night temperature | −3.0 | 15.0 | ‒ 9 | ‒ 9 |
| Relative leaf area expansion | Effect on specific leaf area | 6.0 6 | 22.0 | ‒ 9 | ‒ 9 |
| Internode expansion | Effect on height and width | 9.0 7 | 19.0 | ‒ 9 | ‒ 9 |
| Pod set | Effect on Pod and seed addition | 10.0 | 22.0 | 26.5 | 41.0 |
| Compound | Plant Tissue | CROPGRO PARAMETER | Quinoa Value | Source of Data 1 |
|---|---|---|---|---|
| Protein | Leaf | PROLFI | 0.356 | Estimated from own nitrogen data |
| Stem | PROSTI | 0.200 | Estimated from own nitrogen data | |
| Root | PRORTI | 0.092 | Soybean model | |
| Shell (hull) | PROSHI | 0.380 | Estimated from own nitrogen data | |
| Seed | SDPROS | 0.135 | Präger et al. [17] | |
| Carbohydrate | Leaf | PCARLF | 0.360 | Koziol [77] |
| Stem | PCARST | 0.511 | Carrasco et al. [82] | |
| Root | PCARRT | 0.711 | Soybean model | |
| Shell (hull) | PCARSH | 0.330 | Calculated/Assumed | |
| Seed | PCARSD | 0.705 | Ahamed et al. [76] | |
| Lipid | Leaf | PLIPLF | 0.040 | Tang et al. [81] |
| Stem | PLIPST | 0.020 | Soybean model | |
| Root | PLIPRT | 0.020 | Soybean model | |
| Shell (hull) | PLIPSH | 0.020 | Soybean model | |
| Seed | PLIPSD | 0.070 | Präger et al. [17] | |
| Lignin | Leaf | PLIGLF | 0.080 | Schlick [80] |
| Stem | PLIGST | 0.219 | Carrasco et al. [82] | |
| Root | PLIGRT | 0.070 | Soybean model | |
| Shell (hull) | PLIGSH | 0.200 | Calculated/Assumed | |
| Seed | PLIGSD | 0.025 | Ahamed et al. [76] | |
| Organic acid | Leaf | POALF | 0.050 | Calculated/Assumed |
| Stem | POAST | 0.025 | Calculated/Assumed | |
| Root | POART | 0.050 | Soybean model | |
| Shell (hull) | POASH | 0.040 | Soybean model | |
| Seed | POASD | 0.040 | Calculated/Assumed | |
| Minerals | Leaf | PMINLF | 0.114 | Bhargava et al. [79] |
| Stem | PMINST | 0.025 | Calculated/Assumed | |
| Root | PMINRT | 0.057 | Soybean model | |
| Shell (hull) | PMINSH | 0.030 | Soybean model | |
| Seed | PMINSD | 0.025 | Ahmed et al. [76] |
| CROPGRO Parameter | Description | Quinoa Cultivar | |
|---|---|---|---|
| Zeno | Jessie | ||
| PP-SEN | Slope of the relative response of development vs. photoperiod (1 h−1) | 0.008 | 0.008 |
| EM-FL | Time between plant emergence and flower appearance (PD 1) | 11.200 | 11.200 |
| FL-SH | Time between first flower and first pod (PD) | 2.900 | 3.000 |
| FL-SD | Time between first flower and first seed (PD) | 8.600 | 9.100 |
| SD-PM | Time between first seed and physiological maturity (PD) | 34.400 | 30.900 |
| LFMAX | Maximum leaf photosynthetic rate under 30 °C, 350 µL L−1 CO2, and high light (mg CO2 m−2 s−1) | 1.730 | 1.700 |
| SLAVR | Specific leaf area of a cultivar under standard growth conditions (cm2 g−1) | 250.000 | 255.000 |
| SIZLF | Maximum size of full leaf (cm2) | 7.000 | 6.500 |
| XFRT | Maximum fraction of daily growth that is partitioned to seeds + shells | 1.000 | 0.980 |
| WTPSD | Maximum weight per seed (g) | 0.060 | 0.060 |
| SFDUR | Seed filling duration for pod cohort under standard growth conditions (PD) | 22.000 | 22.500 |
| PODUR | Duration of pod addition under optimal conditions (PD) | 16.000 | 15.000 |
| THRSH | Maximum grain to pod ratio at maturity (%) | 75.500 | 74.500 |
| SDPRO | Fraction protein in seeds (g(protein) g(seed)−1) | 0.125 | 0.150 |
| SDLIP | Fraction oil in seeds (g(oil) g(seed)−1) | 0.055 | 0.070 |
| CROPGRO Parameter | Description | Quinoa Ecotype | |
|---|---|---|---|
| 1 (cv. Zeno) | 2 (cv. Jessie) | ||
| PL-EM | Time between planting and emergence (thermal days) | 3.2 | 3.2 |
| EM-V1 | Time required from emergence to first true leaf (thermal days) | 2.0 | 2.0 |
| FL-VS | Time from first flower to last leaf on main stem (PD 1) | 20.0 | 20.0 |
| TRIFL | Rate of leaf appearance on the main stem (leaves per thermal day) | 0.9 | 1.2 |
| RWDTH | Relative width of this ecotype in comparison to the standard width per node defined in the species file | 1.0 | 0.8 |
| RHGHT | Relative height of this ecotype in comparison to the standard height per node defined in the species file | 1.0 | 0.8 |
| Height or Width (m Internode−1) | Node Number (Leaf Number) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 6 | 8 | 10 | 14 | 18 | 25 | 50 | |
| Internode length | 0.010 | 0.013 | 0.013 | 0.016 | 0.019 | 0.022 | 0.029 | 0.036 | 0.042 | 0.028 |
| Canopy width | 0.010 | 0.010 | 0.011 | 0.012 | 0.013 | 0.013 | 0.012 | 0.011 | 0.011 | 0.010 |
| Development or Growth Processes | CROPGRO Parameter | Description | Quinoa Value |
|---|---|---|---|
| Photosynthesis | SLWREF | Specific leaf weight at which LFMAX is defined (g cm−2) | 0.0034 |
| LNREF | Leaf N concentration (%) at which LFMAX is defined | 4.5 | |
| Nitrogen or carbon mining | NMOBMX | Maximum fraction of protein pool (from vegetative tissues) mobilized per day during reproductive growth | 0.125 |
| NVSMOB | Relative protein mobilization during vegetative growth (ratio on NMOBMX) | 0.65 | |
| Vegetative partitioning | PORPT | Gram (g) of stem mass abscised per g of leaf | 0.06 |
| FRSTMF | Fraction of vegetative dry matter growth allocated to the stem at the final stage | 0.65 | |
| FRLFF | Fraction of vegetative dry matter growth allocated to leaves at the final stage | 0.20 | |
| Leaf growth | SLAREF | The specific leaf area (cm2 g−1) of the reference cultivar at peak vegetative phase | 250.0 |
| SIZREF | Leaf area (cm2) for the leaf at the fifth node position | 30.0 | |
| SLAMAX | Maximum specific leaf area (cm2 g−1) under low light | 760.0 | |
| SLAMIN | Minimum specific leaf area (cm2 g−1) under saturating light | 250.0 | |
| Leaf senescence | SENRT2 | Rate of leaf abscision after physiological maturity (fraction day−1) | 0.16 |
| FREEZ1 | Temperature (°C) that kills leaves, but stems, pods, and seeds remain alive and seeds can grow on mobilized reserves | −5.50 | |
| FREEZ2 | Temperature (°C) below which development and crop growth simulation stops | −8.50 |
| Relative Dry Matter Partitioning | Node Number (XLEAF) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 11 | 16 | 25 | 37 | 40 | |
| Leaf (YLEAF) | 0.41 | 0.41 | 0.41 | 0.41 | 0.39 | 0.33 | 0.30 | 0.30 |
| Stem (YSTEM) | 0.09 | 0.13 | 0.20 | 0.28 | 0.35 | 0.48 | 0.49 | 0.49 |
| Root | 0.50 | 0.46 | 0.39 | 0.31 | 0.26 | 0.19 | 0.21 | 0.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Präger, A.; Boote, K.J.; Munz, S.; Graeff-Hönninger, S. Simulating Growth and Development Processes of Quinoa (Chenopodium quinoa Willd.): Adaptation and Evaluation of the CSM-CROPGRO Model. Agronomy 2019, 9, 832. https://doi.org/10.3390/agronomy9120832
Präger A, Boote KJ, Munz S, Graeff-Hönninger S. Simulating Growth and Development Processes of Quinoa (Chenopodium quinoa Willd.): Adaptation and Evaluation of the CSM-CROPGRO Model. Agronomy. 2019; 9(12):832. https://doi.org/10.3390/agronomy9120832
Chicago/Turabian StylePräger, Achim, Kenneth J. Boote, Sebastian Munz, and Simone Graeff-Hönninger. 2019. "Simulating Growth and Development Processes of Quinoa (Chenopodium quinoa Willd.): Adaptation and Evaluation of the CSM-CROPGRO Model" Agronomy 9, no. 12: 832. https://doi.org/10.3390/agronomy9120832
APA StylePräger, A., Boote, K. J., Munz, S., & Graeff-Hönninger, S. (2019). Simulating Growth and Development Processes of Quinoa (Chenopodium quinoa Willd.): Adaptation and Evaluation of the CSM-CROPGRO Model. Agronomy, 9(12), 832. https://doi.org/10.3390/agronomy9120832





