Biomass and Phosphorus Accumulation and Partitioning of Geranium and Coleus in Response to Phosphorus Availability and Growth Phase
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Culture Methods
2.2. P Treatment
2.3. Growth Measurements
2.4. Root Characteristic Measurements
2.5. Total P Measurements
2.6. Phosphorus Use Efficiency (PUE) Calculations
2.7. Experimental Design and Statistical Analysis
3. Results
3.1. Plant Growth Responses to P Availability
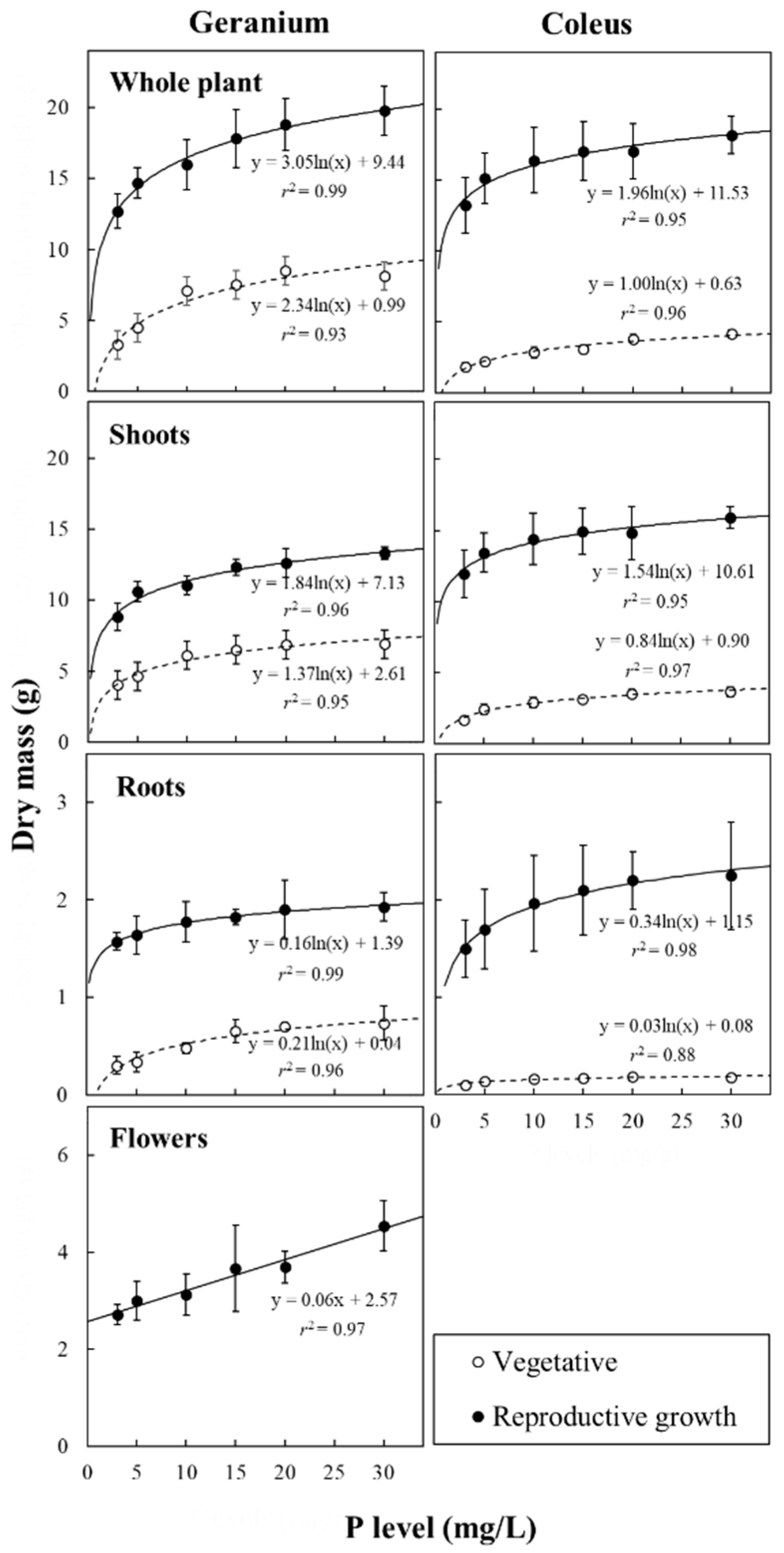
3.2. Plant Biomass Accumulation and Allocation in Response to P Availability
3.3. Plant Root Growth Responses
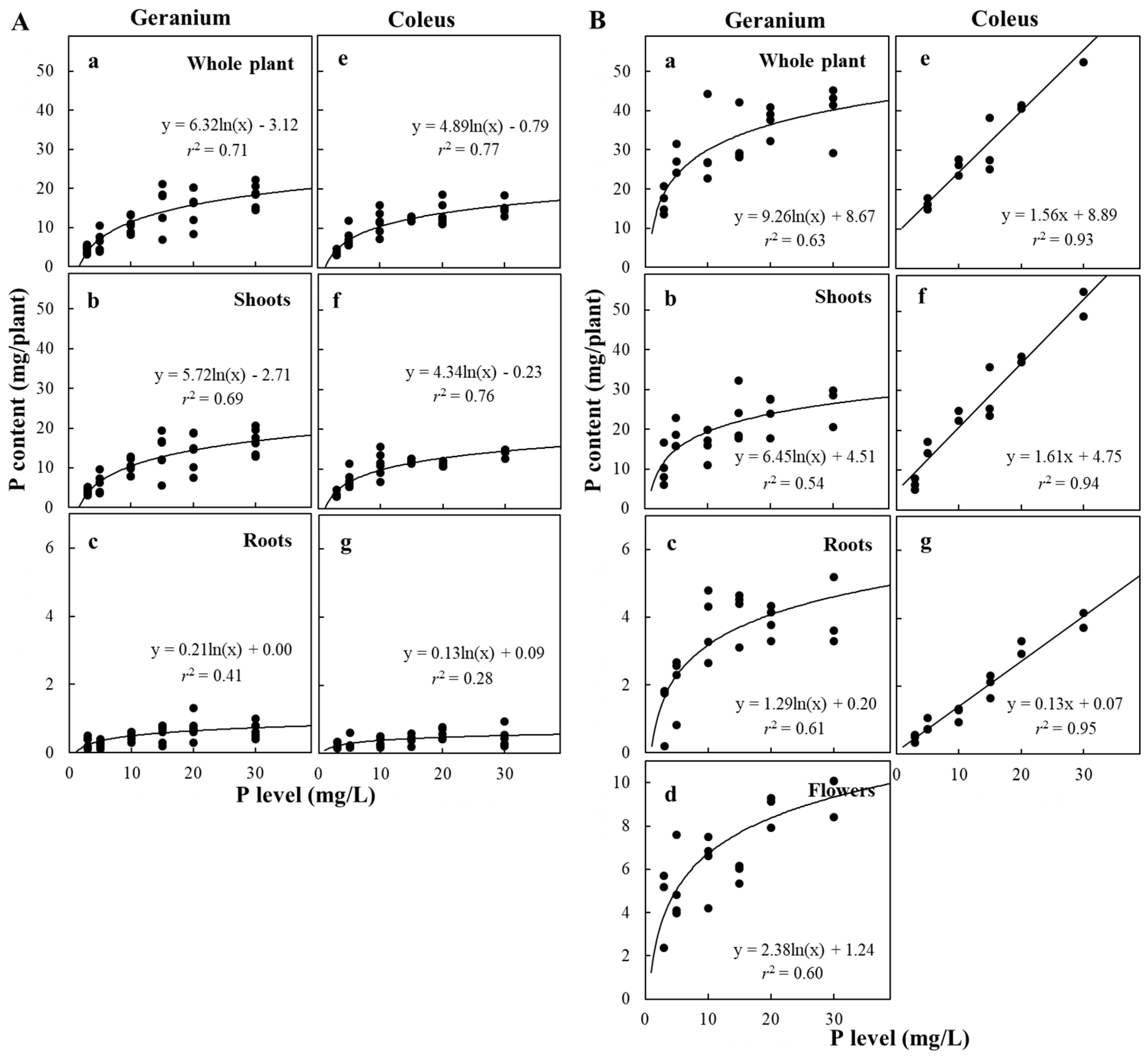
3.4. Plant P Content and Concentration and Allocation in Response to P Availability
4. Discussion
4.1. Biomass Accumulation and Partitioning Are Influenced by P Level, Plant Species, and Growth Phase
4.2. Phosphorus Accumulation and Partitioning Are Influenced by P Level, Plant Species, and Growth Phase
4.3. Optimal P Rate of Geranium and Coleus Plants Is Varied by the Growth Phase
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Data Availability
References
- Correll, D.L. Phosphorus: A rate limiting nutrient in surface waters. Poult. Sci. 1999, 78, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Gburek, W.J.; Sharpley, A.N. Hydrologic controls on phosphorus loss from upland agricultural watersheds. J. Environ. Qual. 1998, 27, 267–277. [Google Scholar] [CrossRef]
- Daniel, T.C.; Sharpley, A.N.; Lemunyon, J.L. Agricultural phosphorus and eutrophication: A symposium overview. J. Environ. Qual. 1998, 27, 251–257. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.; Bowen, J.; McGrath, J.; Nairn, R.; Fox, G.; Brown, G.; Wilson, S.; Gill, C. Evaluation of a universal flow-through model for predicting and designing phosphorus removal structures. Chemosphere 2016, 151, 345–355. [Google Scholar] [CrossRef]
- Steen, I. Phosphorus availability in the 21st Century: Management of a non-renewable resource. Phosphorus Potassium 1998, 217, 25–31. [Google Scholar]
- Smil, V. Phosphorus in the environment: Natural flows and human interferences. Annu. Rev. Energy Environ. 2000, 25, 53–88. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang.-Hum. Policy Dimens. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Driver, J. Phosphates recovery for recycling from sewage and animal waste. Phosphorus Potassium 1998, 216, 17–21. [Google Scholar]
- EcoSanRes. Closing the Loop on Phosphorus; EcoSanRes Factsheet; Stockholm Environment Institute (SEI): Stockholm, Sweden, 2008; Available online: http://www.ecosanres.org/pdf_files/Fact_sheets/ESR4lowres.pdf (accessed on 21 November 2019).
- Cordell, D.; White, S. Peak phosphorus: Clarifying the key issues of a vigorous debate about long-term phosphorus security. Sustainability 2011, 3, 2027–2049. [Google Scholar] [CrossRef]
- Warncke, D.D.; Kraukopf, D.M. Greenhouse Growth Media: Testing & Nutrition Guidelines; MSU Ag Facts Extension Bulletin E-1736; Michigan State University: East Lansing, MI, USA, 1983; Available online: https://archive.lib.msu.edu/DMC/extension_publications/e1736/e1736-1983.pdf (accessed on 21 November 2019).
- Bailey, D.A.; Nelson, P.V. Designing a Greenhouse Crop Fertilization Program; Department of Horticultural Sciences, North Carolina State University: Raleigh, NC, USA, 2004; Available online: http://www.ces.ncsu.edu/depts/hort/floriculture/plugs/fertprog.pdf (accessed on 21 November 2019).
- Kim, H.J.; Li, X.X. Effects of Phosphorus on Shoot and Root Growth, Partitioning, and Phosphorus Utilization Efficiency in Lantana. Hortscience 2016, 51, 1001–1009. [Google Scholar] [CrossRef]
- Broschat, T.K.; Klock-Moore, K.A. Root and shoot growth responses to phosphate fertilization in container-grown plants. HortTechnology 2000, 10, 765–767. [Google Scholar] [CrossRef]
- Lynch, J.P.; Lauchli, A.; Epstein, E. Vegetative growth of the common bean in response to phosphorus-nutrition. Crop Sci. 1991, 31, 380–387. [Google Scholar] [CrossRef]
- Kim, H.J.; Lynch, J.P.; Brown, K.M. Ethylene insensitivity impedes a subset of responses to phosphorus deficiency in tomato and petunia. Plant Cell Environ. 2008, 31, 1744–1755. [Google Scholar] [CrossRef] [PubMed]
- Whitcher, C.L.; Kent, M.W.; Reed, D.W. Phosphorus concentration affects new guinea impatiens and vinca in recirculating subirrigation. Hortscience 2005, 40, 2047–2051. [Google Scholar] [CrossRef]
- Van der Boon, J. A slow-release fertilizer for nursery plants in container. Acta Hortic. 1981, 126, 321–348. [Google Scholar] [CrossRef]
- Yeager, T.H.; Wright, R.D. Phosphorus requirement of Ilex crenata thunb cv Helleri grown in a pine bark medium. J. Am. Soc. Hortic. Sci. 1982, 107, 558–562. [Google Scholar]
- Ristvey, A.G.; Lea-Cox, J.D.; Ross, D.S. Nitrogren and phosphorus uptake efficiency and partitioning of container-grown Azalea during spring growth. J. Am. Soc. Hortic. Sci. 2007, 132, 563–571. [Google Scholar] [CrossRef]
- Shreckhise, J.H.; Owen, J.S.; Niemiera, A.X. Growth response of three containerized woody plant taxa to varying low phosphorus fertilizer concentrations. Hortscience 2018, 53, 628–637. [Google Scholar] [CrossRef]
- Gagnon, V.; Maltais-Landry, G.; Puigagut, J.; Chazarenc, F.; Brisson, J. Treatment of hydroponics wastewater using constructed wetlands in winter conditions. Water Air Soil Pollut. 2010, 212, 483–490. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J.; Sukias, J.P.S. Treatment of hydroponic wastewater by denitrification filters using plant prunings as the organic carbon source. Bioresour. Technol. 2008, 99, 2711–2716. [Google Scholar] [CrossRef] [PubMed]
- Prystay, W.; Lo, K.V. Treatment of greenhouse wastewater using constructed wetlands. J. Environ. Sci. Health Part B-Pestic. Food Contam. Agric. Wastes 2001, 36, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.W.; Lynch, J. Response to phosphorus availability during vegetative and reproductive growth of chrysanthemum: II. Biomass and phosphorus dynamics. J. Am. Soc. Hortic. Sci. 1998, 123, 223–229. [Google Scholar] [CrossRef]
- Kim, H.J.; Yang, T.; Lin, M.Y.; Langenhoven, P. Plant propagation for successful hydroponic production. Acta Hortic. 2018, 109–116. [Google Scholar] [CrossRef]
- Nau, J. Ball Redbook Volume 2 Crop Production, 18th ed.; Ball Publishing: Batavia, IL, USA, 2011. [Google Scholar]
- Epstein, E.; Bloom, A.J. Mineral nutrition of plants: Principles and perspectives. In Mineral Nutrition of Plants Principles and Perspectives, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2005. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347–353. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for determination of phosphate in natural waters. Anal. Chim. Acta 1962, 26, 31–36. [Google Scholar] [CrossRef]
- Rose, T.J.; Wissuwa, M. Rethinking internal phosphorus utilization efficiency: A new approach is needed to improve pue in grain crops. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 116, pp. 185–217. [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Gedroc, J.J.; McConnaughay, K.D.M.; Coleman, J.S. Plasticity in root/shoot partitioning: Optimal, ontogenetic, or both? Funct. Ecol. 1996, 10, 44–50. [Google Scholar] [CrossRef]
- Fredeen, A.L.; Rao, I.M.; Terry, N. Influence of phosphorus-nutrition on growth and carbon partitioning in Glycine max. Plant Physiol. 1989, 89, 225–230. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, H.; Tao, P.; Chen, H. Comparative proteomic analyses provide new insights into low phosphorus stress responses in maize leaves. PLoS ONE 2014, 9, e98215. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol. 2011, 156, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Shipley, B.M.; Meziane, D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct. Ecol. 2002, 16, 326–331. [Google Scholar] [CrossRef]
- Elliott, D.E.; Reuter, D.J.; Reddy, G.D.; Abbott, R.J. Phosphorus nutrition of spring wheat (Triticum aestivum L.). 1. Effects of phosphorus supply on plant symptoms, yield, components of yield, and plant phosphorus uptake. Aust. J. Agric. Res. 1997, 48, 855–867. [Google Scholar] [CrossRef]
- Hood, T.M.; Mills, H.A.; Thomas, P.A. Developmental stage affects nutrient uptake by four snapdragon cultivars. Hortscience 1993, 28, 1008–1010. [Google Scholar] [CrossRef]
- Kallarackal, J.; Milburn, J.A. Respiration and phloem translocation in the roots of chickpea (Cicer arietinum). Ann. Bot. 1985, 56, 211–218. [Google Scholar] [CrossRef]
- Erei, R.; Yermiyahu, U.; Yasuor, H.; Cohen Chamus, D.; Schwartz, A.; Ben-Gal, A.; Dag, A. Phosphorus nutritional level, carbohydrate reserves and flower quality in olives. PLoS ONE 2016, 11, e0167591. [Google Scholar] [CrossRef]
- Williams, R.F. The effects of phosphorus supply on the rates of intake of phosphorus and nitrogen and upon certain aspects of phosphorus metabolism in gramineous plants. Aust. J. Sci. Res. Ser. B-Biol. Sci. 1948, 1, 333–361. [Google Scholar] [CrossRef]
- Aerts, R. Nutrient resorption from senescing leaves of perennials: Are there general patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Santiveri, F.; Royo, C.; Romagosa, I. Growth and yield responses of spring and winter triticale cultivated under Mediterranean conditions. Eur. J. Agron. 2004, 20, 281–292. [Google Scholar] [CrossRef]
- Rose, T.J.; Rengel, Z.; Ma, Q.; Bowden, J.W. Differential accumulation patterns of phosphorus and potassium by canola cuffivars compared to wheat. J. Plant Nutr. Soil Sci.-Z. Pflanzenernahr. Bodenkd. 2007, 170, 404–411. [Google Scholar] [CrossRef]
- Julia, C.; Wissuwa, M.; Kretzshmar, T.; Jeong, K.; Rose, T. Phosphorus uptake, partitioning and redistribution during grain filling in rice. Ann. Bot. 2016, 118, 1151–1162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Brown, K.M. Whole plant adaptations to low phosphorus availability. In Plant-Environment Interactions; Huang, B., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 209–242. [Google Scholar]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of Soil and Fertilizer Phosphorus Use: Reconciling Changing Concepts of Soil Phosphorus Behaviour with Agronomic Information; FAO Fertilizer and Plant Nutrition Bulletin; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2008; Volume 18. [Google Scholar]
- Warren, S.L.; Bilderback, T.E.; Tyler, H.H. Efficacy of three nitrogen and phosphorus sources in container-grown azalea production. J. Environ. Hortic. 1995, 13, 147–151. [Google Scholar]
- Tyler, H.H.; Warren, S.L.; Bilderback, T.E. Reduced leaching fractions improve irrigation use efficiency and nutrient efficacy. J. Environ. Hortic. 1996, 14, 199–204. [Google Scholar]
- Owen, J.S., Jr.; Warren, S.L.; Bilderback, T.E.; Albano, J.P. Phosphorus rate, leaching fraction, and substrate influence on influent quantity, effluent nutrient content, and response of a containerized woody ornamental crop. Hortscience 2008, 43, 906–912. [Google Scholar] [CrossRef]
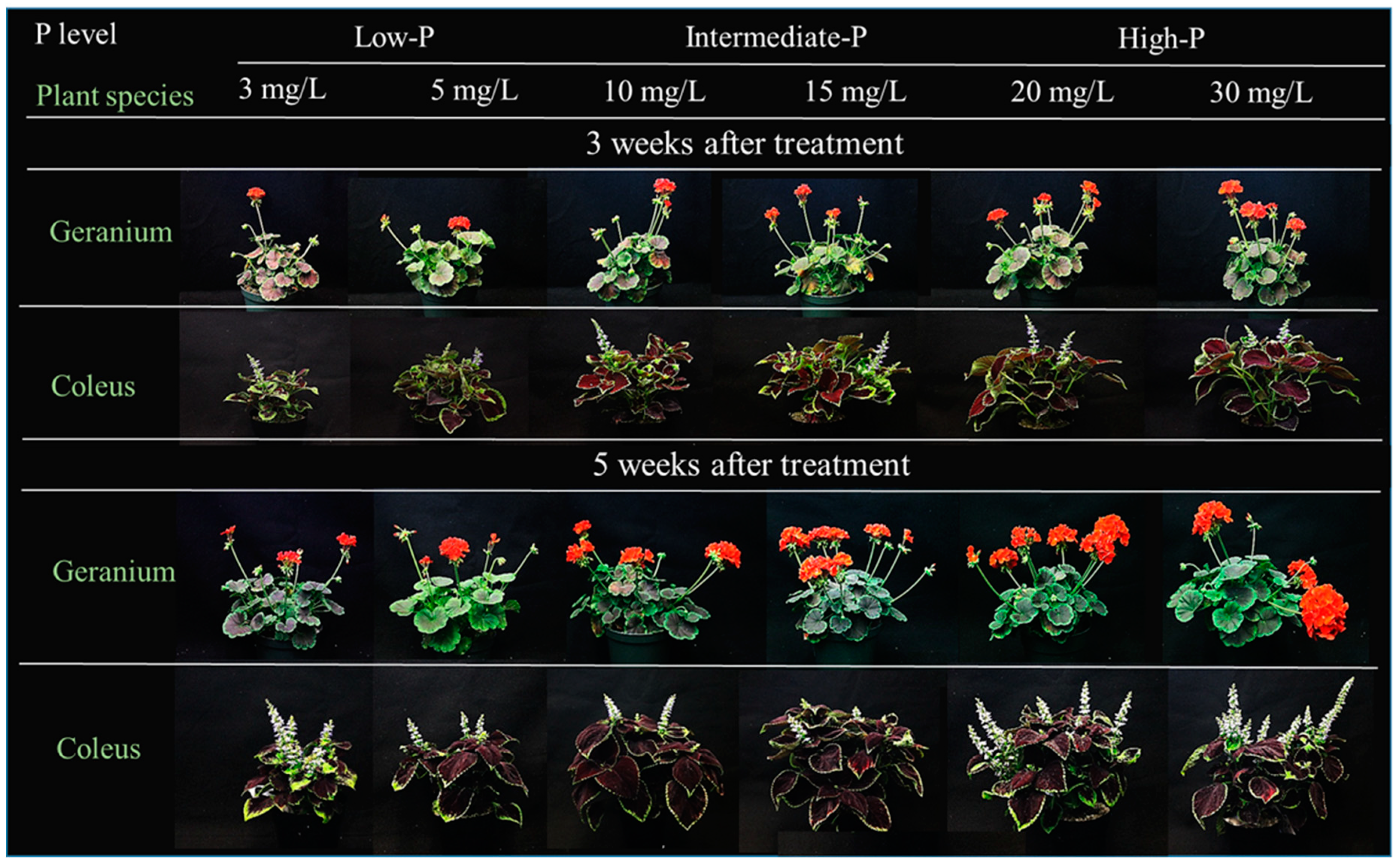


| Plant Species | P Level (mg/L) | Plant Height (cm) | Plant Width (cm) | Branch Number (/Plant) | Leaf Number (/Plant) | Leaf Area (cm2/Plant) | Inflorescence Number (/Plant) | Open floret Number (/Plant) | Inflorescence Diameter (cm/Flower) |
|---|---|---|---|---|---|---|---|---|---|
| Vegetative phase | |||||||||
| Geranium | Low | 11.3b | 15.6b | 10b | 10b | 369b | – | – | – |
| Intermediate | 14.0a | 18.7a | 11ab | 13a | 556ab | – | – | – | |
| High | 14.4a | 19.6a | 13a | 14a | 685a | – | – | – | |
| P | *** | ** | ** | ** | ** | – | – | – | |
| Coleus | Low | 11.6b | 16.1b | – | 26b | 332b | – | – | – |
| Intermediate | 13.4ab | 20.3a | – | 33a | 515a | – | – | – | |
| High | 14.9a | 22.0a | – | 35a | 646a | – | – | – | |
| P | * | ** | *** | *** | *** | – | – | – | |
| Reproductive phase | |||||||||
| Geranium | Low | 15.5b | 22.0c | 35b | 38c | 872b | 6.6a | 35.6b | 4.1b |
| Intermediate | 18.8a | 24.1b | 41ab | 45b | 1102ab | 6.0a | 30.3b | 4.6ab | |
| High | 20.1a | 25.6a | 43a | 50a | 1207a | 7.0a | 57.5a | 5.1a | |
| P | *** | *** | * | *** | ** | ns | ** | *** | |
| Coleus | Low | 19.7b | 30.3a | – | 54b | 1671b | – | – | – |
| Intermediate | 22.8ab | 34.1a | – | 76a | 2209a | – | – | – | |
| High | 24.0a | 35.9a | – | 87a | 2216a | – | – | – | |
| P | ** | ns | *** | * | |||||
| ANOVA | |||||||||
| P level (P) | *** | *** | * | *** | *** | ns | ** | ** | |
| Plant species (PS) | *** | *** | – | *** | *** | – | – | – | |
| Growth phase (G) | *** | *** | *** | *** | *** | – | – | – | |
| P × PS | ns | ns | – | *** | ns | – | – | – | |
| P × G | ns | ns | ns | *** | ns | – | – | – | |
| PS × G | *** | *** | – | *** | *** | – | – | – | |
| P × PS × G | ns | ns | – | ** | ns | – | – | – | |
| P Level (mg/L) | Dry Mass (g DW/Plant) | Root-to-Shoot | ||||
|---|---|---|---|---|---|---|
| Whole Plant | Shoots | Roots | Inflorescences | |||
| Vegetative phase | ||||||
| Geranium | Low | 4.7b | 4.3b | 0.32b | 0.08 | |
| Intermediate | 7.2a | 6.7ab | 0.56a | – | 0.09 | |
| High | 7.8a | 7.1a | 0.71a | – | 0.11 | |
| P | * | * | ** | ns | ||
| Coleus | Low | 2.0b | 1.9b | 0.11b | – | 0.06 |
| Intermediate | 2.8b | 2.7b | 0.16ab | – | 0.06 | |
| High | 3.9a | 3.7a | 0.20a | – | 0.06 | |
| P | *** | *** | ns | ns | ||
| Reproductive phase | ||||||
| Geranium | Low | 14.0b | 9.8b | 1.54a | 2.69b | 0.17 |
| Intermediate | 15.8b | 11.3ab | 1.72a | 2.73b | 0.17 | |
| High | 21.5a | 14.6a | 2.03a | 4.85a | 0.14 | |
| P | ** | * | * | ** | ns | |
| Coleus | Low | 12.6b | 11.4b | 1.19b | – | 0.10 |
| Intermediate | 17.9ab | 15.8a | 2.10a | – | 0.13 | |
| High | 18.6a | 16.4a | 2.20a | – | 0.13 | |
| P | * | * | * | ns | ||
| ANOVA | ||||||
| P level (P) | *** | *** | *** | ** | ns | |
| Plant species (PS) | ** | ns | * | – | ** | |
| Growth phase (G) | *** | *** | *** | – | *** | |
| P × PS | ns | ns | ns | – | ns | |
| P × G | * | ns | ** | – | ns | |
| PS × G | * | *** | ** | – | ns | |
| P × PS × G | ns | ns | * | – | ns | |
| Plant Species | P Level (mg/L) | Total Root Length (m) | Total Root Surface Area (cm2) | Total Root Volume (cm3) | Average Root Diameter (cm) | Root-to-Leaf Area (g/m2) |
|---|---|---|---|---|---|---|
| Vegetative phase | ||||||
| Geranium | Low | 8.0a | 158a | 2.0b | 0.53a | 3.78 |
| Intermediate | 9.4a | 188a | 2.5ab | 0.57a | 3.94 | |
| High | 11.4a | 204a | 3.2a | 0.59a | 2.90 | |
| P | * | ns | * | ns | ns | |
| Coleus | Low | 8.4b | 142a | 1.7b | 0.53a | 10.60 |
| Intermediate | 9.0ab | 148a | 2.1a | 0.53a | 9.55 | |
| High | 10.6a | 161a | 2.6a | 0.53a | 10.74 | |
| P | * | ns | *** | ns | ns | |
| Reproductive phase | ||||||
| Geranium | Low | 13.2b | 280b | 5.1b | 0.66b | 8.80 |
| Intermediate | 18.4a | 357b | 6.8ab | 0.70ab | 9.59 | |
| High | 22.0a | 450a | 7.6a | 0.73a | 10.02 | |
| P | *** | *** | ** | ** | ns | |
| Coleus | Low | 19.7b | 464b | 8.6b | 0.68b | 17.53 |
| Intermediate | 28.7ab | 644ab | 13.1ab | 0.75ab | 18.24 | |
| High | 44.3a | 846a | 17.7a | 0.85a | 15.31 | |
| P | * | ** | ** | ** | ns | |
| ANOVA | ||||||
| P level (P) | *** | *** | *** | *** | ns | |
| Plant species (PS) | *** | *** | *** | ns | *** | |
| Growth phase (G) | *** | *** | *** | *** | *** | |
| P × PS | ** | *** | ** | * | ns | |
| P × G | * | *** | *** | *** | ns | |
| PS × G | *** | *** | *** | *** | ns | |
| P × PS × G | * | * | ** | * | ns | |
| P Level (mg/L) | P Content (mg/Plant) | P concentration (mg P/g DW) | PUE (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Whole Plant | Shoots | Roots | Flowers | Whole Plant | Shoots | Roots | Flowers | |||
| Vegetative phase | ||||||||||
| Geranium | Low | 5.3c | 4.9b | 0.4c | – | 1.3b | 1.2b | 1.3b | – | 11.9a |
| Intermediate | 12.9b | 11.9a | 1.0b | – | 2.1a | 2.1a | 2.0a | – | 9.1b | |
| High | 17.0a | 15.4a | 1.6a | – | 2.3a | 2.3a | 2.3a | – | 6.1c | |
| P | *** | *** | *** | *** | *** | *** | *** | |||
| Coleus | Low | 5.6b | 5.3b | 0.3b | – | 2.1b | 2.6b | 2.0b | – | 12.0a |
| Intermediate | 11.7a | 11.4a | 0.4ab | – | 2.7ab | 3.6a | 2.3ab | – | 8.5b | |
| High | 13.8a | 13.3a | 0.5a | – | 2.9a | 3.7a | 2.6a | – | 5.0c | |
| P | *** | *** | ** | * | *** | ** | *** | |||
| Reproductive phase | ||||||||||
| Geranium | Low | 21.7b | 14.2b | 2.4b | 5.1b | 1.6b | 1.3b | 1.5b | 2.1b | 21.4a |
| Intermediate | 30.1ab | 20.7ab | 4.0a | 5.5b | 2.6a | 2.2a | 2.5a | 3.0a | 9.8b | |
| High | 38.6a | 25.4a | 4.1a | 9.1a | 2.7a | 2.3a | 2.6a | 3.0a | 6.3b | |
| P | *** | ** | * | ** | *** | ** | *** | *** | *** | |
| Coleus | Low | 11.6b | 11.0b | 0.7b | – | 0.8b | 1.0b | 0.7c | – | 24.2a |
| Intermediate | 28.1a | 26.5a | 1.6b | – | 1.7b | 1.8ab | 1.7b | – | 20.1ab | |
| High | 42.5a | 38.1a | 4.4a | – | 2.8a | 2.7a | 3.7a | – | 14.7b | |
| P | *** | ** | *** | *** | ** | *** | * | |||
| ANOVA | ||||||||||
| P level (P) | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Plant species (PS) | ns | * | *** | - | ns | *** | ns | - | *** | |
| Growth phase (G) | *** | *** | *** | - | ns | *** | ns | - | *** | |
| P × PS | ns | * | * | - | ns | ns | *** | - | ns | |
| P × G | *** | ** | *** | - | ns | ns | *** | - | *** | |
| PS × G | ns | ** | * | - | *** | *** | *** | - | *** | |
| P × PS × G | ** | ** | *** | - | ns | ns | *** | - | * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Choi, S.; Fan, J.; Kim, H.-J. Biomass and Phosphorus Accumulation and Partitioning of Geranium and Coleus in Response to Phosphorus Availability and Growth Phase. Agronomy 2019, 9, 813. https://doi.org/10.3390/agronomy9120813
Zhang J, Choi S, Fan J, Kim H-J. Biomass and Phosphorus Accumulation and Partitioning of Geranium and Coleus in Response to Phosphorus Availability and Growth Phase. Agronomy. 2019; 9(12):813. https://doi.org/10.3390/agronomy9120813
Chicago/Turabian StyleZhang, Jiayin, Seunghyun Choi, Jingping Fan, and Hye-Ji Kim. 2019. "Biomass and Phosphorus Accumulation and Partitioning of Geranium and Coleus in Response to Phosphorus Availability and Growth Phase" Agronomy 9, no. 12: 813. https://doi.org/10.3390/agronomy9120813
APA StyleZhang, J., Choi, S., Fan, J., & Kim, H.-J. (2019). Biomass and Phosphorus Accumulation and Partitioning of Geranium and Coleus in Response to Phosphorus Availability and Growth Phase. Agronomy, 9(12), 813. https://doi.org/10.3390/agronomy9120813






