The Effect of Arbuscular Mycorrhizal Fungi on Photosystem II of the Host Plant Under Salt Stress: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Data Collection
2.2. Data Acquisition
2.3. Statistical Analysis
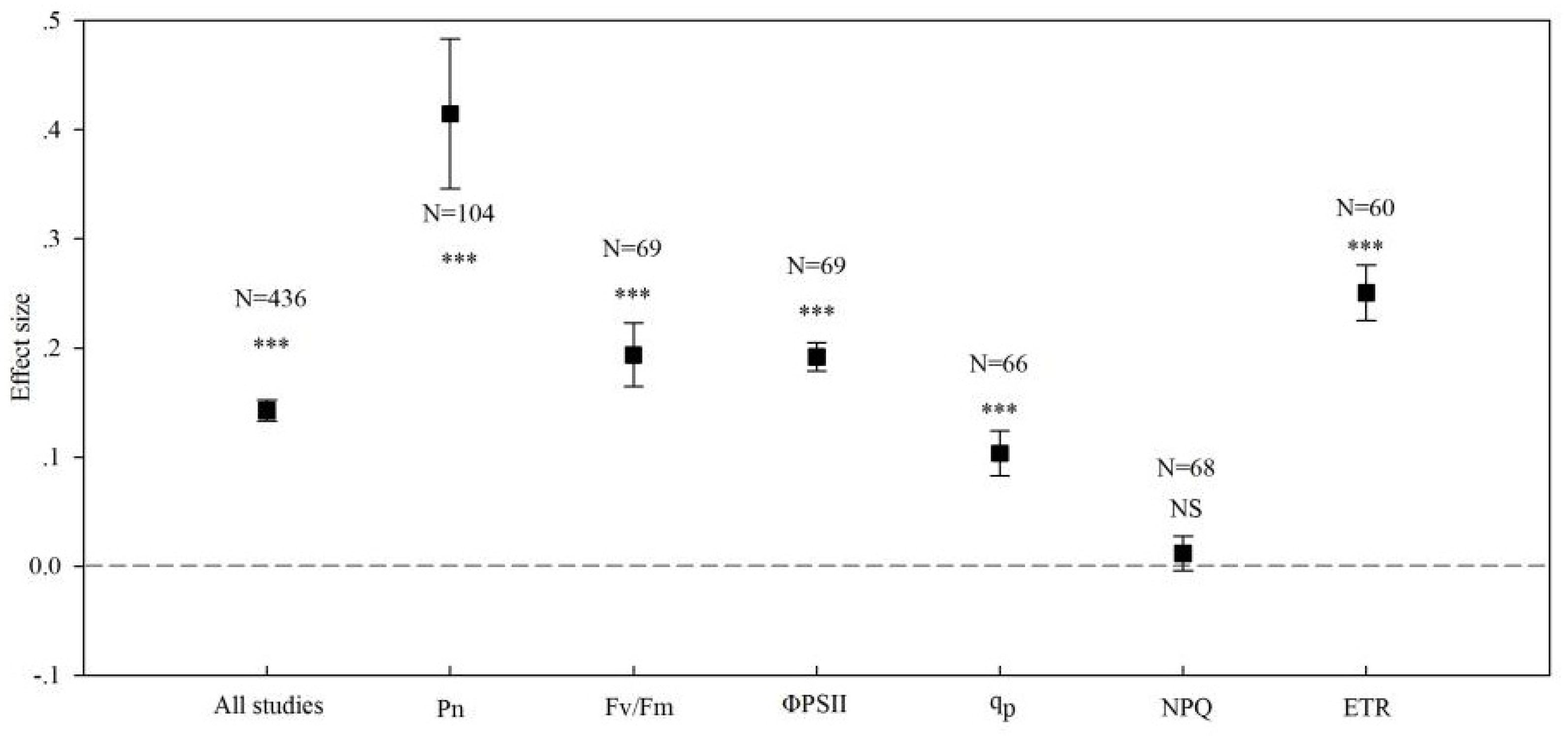
3. Results
3.1. Overview
3.2. Effects of Arbuscular Mycorrhizal Fungi (AMF) Inoculation on the Photosynthetic Rate in Plants
3.3. Effects of AMF Inoculation on the Apparent Quantum Yield of Photosystems II (PS II) in Plants
3.4. Effects of AMF Inoculation on the Actual Quantum Yield of Photochemical Energy Conversion in PS II
3.5. Effects of AMF Inoculation on the Photochemical Quenching Coefficient in Plants
3.6. Effects of AMF Inoculation on the Non-Photochemical Quenching Coefficient in Plants
3.7. Effects of AMF Inoculation on the Electron Transfer Rate in Plants
4. Discussion
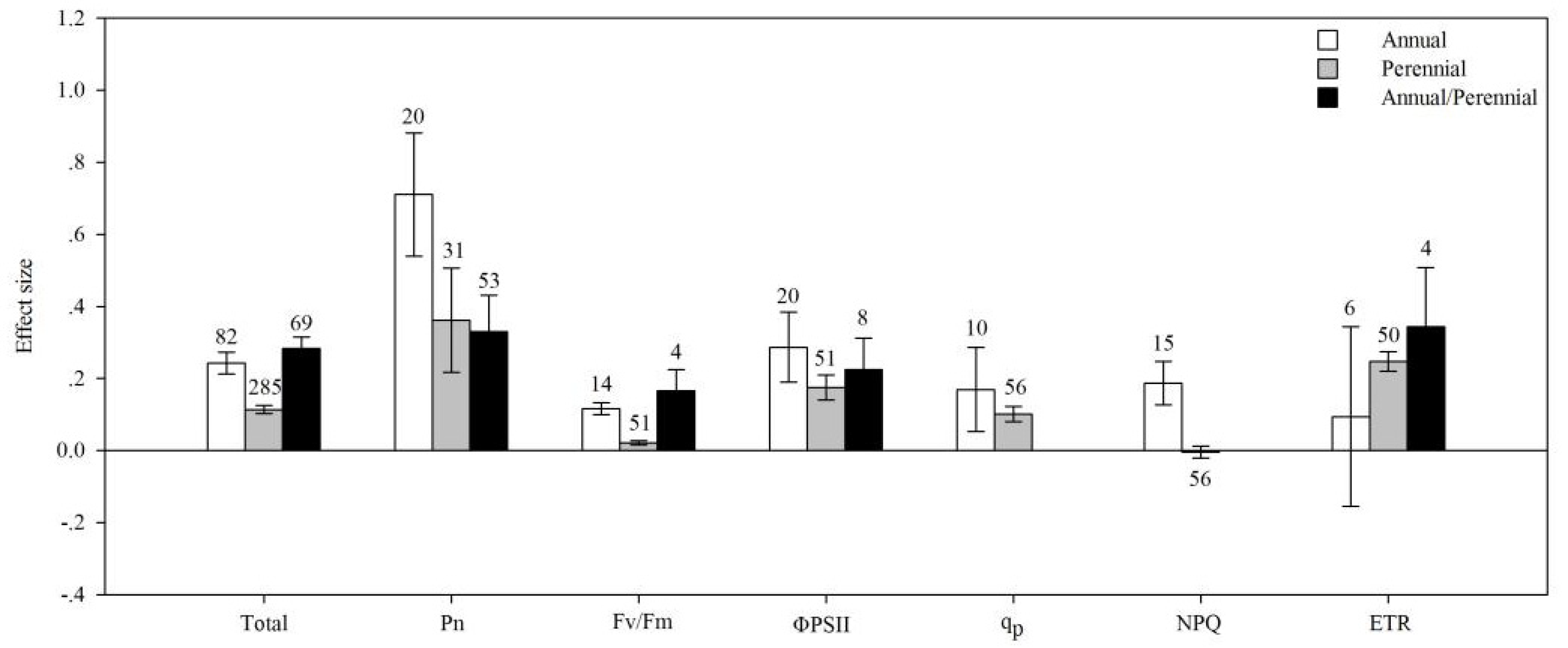
4.1. Total Effects
4.2. Life Cycle
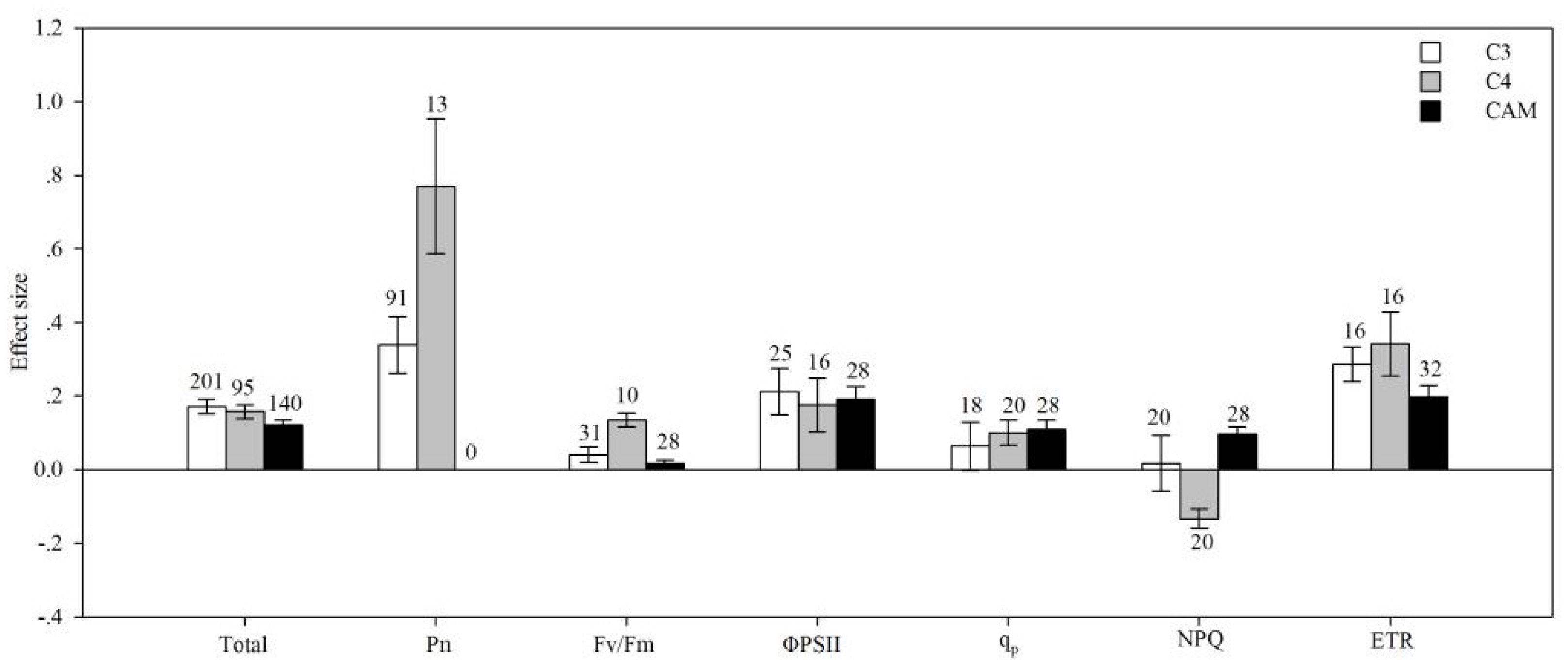
4.3. Photosynthetic Type
4.4. Plant Group
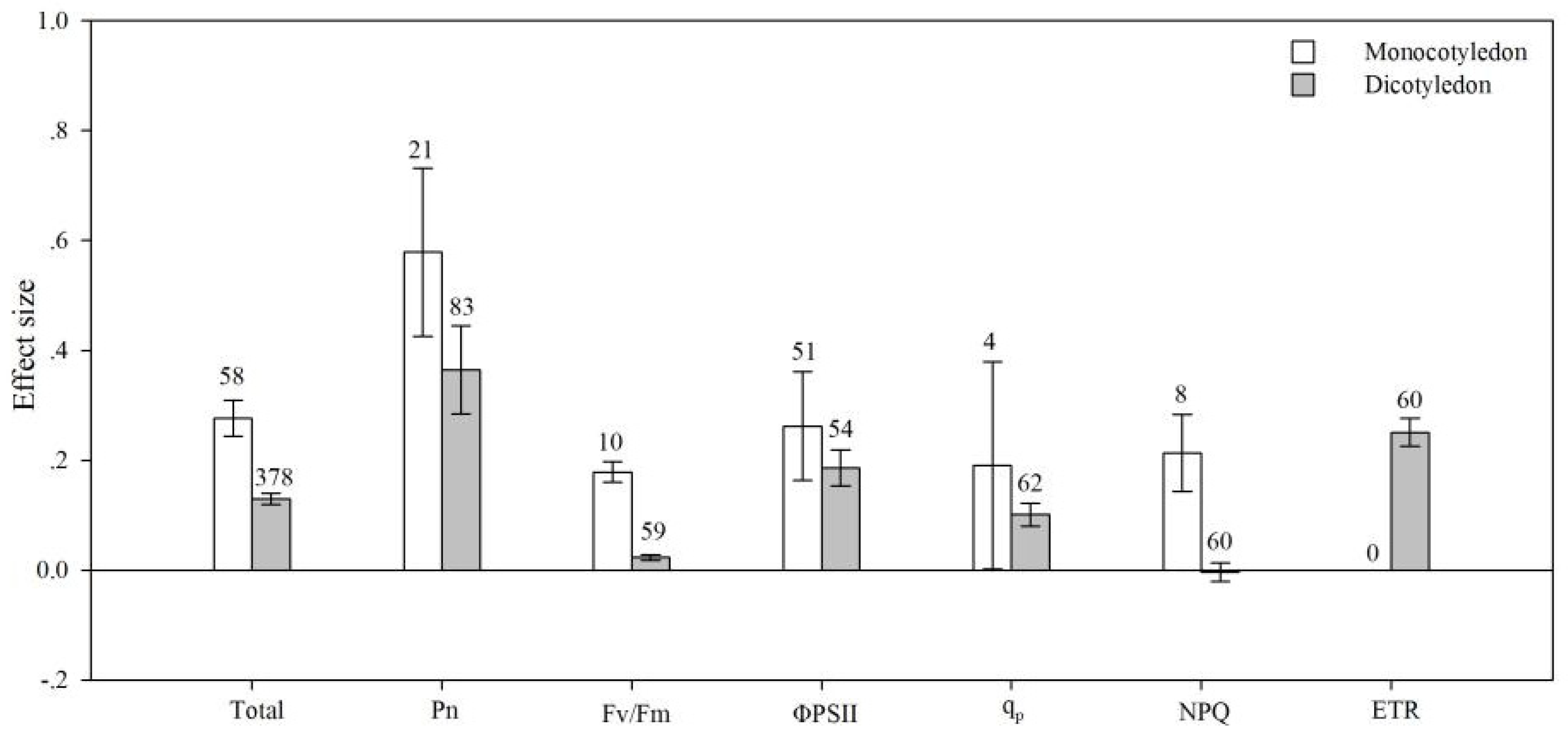
4.5. Plant Type
4.6. Soil Salinity
4.7. AMF Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef] [PubMed]
- Tufail, A.; Li, H.; Naeem, A.; Li, T.X. Leaf cell membrane stability-based mechanisms of zinc nutrition in mitigating salinity stress in rice. Plant Biol. 2018, 20, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Li, Z.; Liu, H.G.; Tang, M. Influence of arbuscular mycorrhiza on photosynthesis and water status of Populus cathayana Rehder males and females under salt stress. Acta Physiol. Plant. 2015, 37, 183. [Google Scholar] [CrossRef]
- Lin, J.X.; Wang, Y.N.; Sun, S.N.; Mu, C.S.; Yan, X.F. Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci. Total Environ. 2017, 576, 234–241. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31. [Google Scholar] [CrossRef]
- Porcel, R.; Redondo-Gómez, S.; Mateos-Naranjo, E.; Aroca, R.; Garcia, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 2015, 185, 75–83. [Google Scholar] [CrossRef]
- Xu, H.W.; Lu, Y.; Tong, S.Y. Effects of arbuscular mycorrhizal fungi on photosynthesis and chlorophyll fluorescence of maize seedlings under salt stress. Emir. J. Sci. Food Agric. 2018, 30, 199–204. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Tang, M.; Zhang, H.Q. Arbuscular mycorrhizal fungi enhanced the growth, photosynthesis, and calorific value of black locust under salt stress. Photosynthetica 2016, 55, 378–385. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z.; Wu, F.; Tang, M. Comparative photochemistry activity and antioxidant responses in male and female Populus cathayana cuttings inoculated with arbuscular mycorrhizal fungi under salt. Sci. Rep. 2016, 6, 37663. [Google Scholar] [CrossRef]
- Oliveira, D.F.B.D.; Endres, L.; Silva, J.V.; Clemente, P.R.A. Pre-colonized seedlings with arbuscular mycorrhizal fungi: An alternative for the cultivation of Jatropha curcas L. in salinized soils. Theor. Exp. Plant Physiol. 2017, 29, 129–142. [Google Scholar] [CrossRef]
- Wang, Y.N.; Jie, W.G.; Peng, X.Y.; Hua, X.Y.; Yan, X.F.; Zhou, Z.Q.; Lin, J.X. Physiological adaptive strategies of oil seed crop Ricinus communis early seedlings (cotyledon vs. true Leaf) under salt and alkali stresses: From the growth, photosynthesis and chlorophyll fluorescence. Front. Plant Sci. 2019, 9, 1939. [Google Scholar] [CrossRef] [PubMed]
- Hanachi, S.; Van Labeke, M.C.; Mehouachi, T. Application of chlorophyll fluorescence to screen eggplant (Solanum melongena L.) cultivars for salt tolerance. Photosynthetica 2014, 52, 57–62. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Xie, X.H. Effects of Arbuscular Mycorrhizal Fungi on Growth and Photosynthesis in Melon Seedlings under Weak Light with Salt Stress. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2016. [Google Scholar]
- Chen, T.W.; Kahlen, K.; Stützel, H. Disentangling the contributions of osmotic and ionic effects of salinity on stomatal, mesophyll, biochemical and light limitations to photosynthesis. Plant Cell Environ. 2015, 38, 1528–1542. [Google Scholar] [CrossRef]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum Lycopersicum L.) plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, J. Introduction to Meta-Analysis; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Chandrasekaran, M.; Boughattas, S.; Hu, S.J.; Oh, S.H.; Sa, T. A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress. Mycorrhiza 2014, 24, 611–625. [Google Scholar] [CrossRef]
- Auge, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress: A meta-analysis. Front. Plant Sci. 2014, 5, 562. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chanratana, M.; Kim, K.; Seshadri, S.; Sa, T. Impact of Arbuscular mycorrhizal fungi on photosynthesis, water status, and gas exchange of plants under salt stress—A Meta-Analysis. Front. Plant Sci. 2019, 10, 457. [Google Scholar] [CrossRef]
- Van Groenigen, K.J.; Osenberg, C.W.; Hungate, B.A. Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 2011, 475, 214–216. [Google Scholar] [CrossRef]
- Rosenberg, N.J.; Adams, D.C.; Gurevitch, J. Metawin: Statistical Software for Meta-Analysis Version 2.0; Sinauer Associates Inc.: Sunderland, MA, USA, 2000. [Google Scholar]
- Gurevitch, J.; Curtis, P.S.; Jones, M.H. Meta-analysis in ecology. Adv. Ecol. Res. 2001, 32, 199–247. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Gurevitch, J.; Hedges, L.V. Statistical issues in ecological metaanalysis. Ecology 1999, 80, 1142–1149. [Google Scholar] [CrossRef]
- Aroca, R.; Ruiz-Lozano, J.M.; Ángel, M.Z.; José, A.P.; José, M.G.; Mina María, J.P.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.M.; Péreztornero, O.; Morte, A. Alleviation of salt stress in citrus seedlings inoculated with arbuscular mycorrhizal fungi depends on the rootstock salt tolerance. J. Plant Physiol. 2014, 171, 76–85. [Google Scholar] [CrossRef]
- Jiang, Y.; Ding, X.; Zhang, D.; Deng, Q.; Yu, C.L.; Zhou, S. Soil salinity increases the tolerance of excessive sulfur fumigation stress in tomato plants. Environ. Exp. Bot. 2017, 133, 70–77. [Google Scholar] [CrossRef]
- Ding, X.T.; Jiang, Y.P.; Hao, T.; Jin, H.J.; Zhang, H.M.; He, L.Z. Effects of heat shock on photosynthetic properties, antioxidant enzyme activity, and downy mildew of cucumber (Cucumis sativus L.). PLoS ONE 2016, 11, e0152429. [Google Scholar] [CrossRef]
- Zribi, L.; Fatma, G.; Fatma, R.; Salwa, R.; Hassan, N.; Néjib, R.M. Application of chlorophyll fluorescence for the diagnosis of salt stress in tomato Solanum lycopersicum (variety riogrande). Sci. Hortic. 2009, 120, 367–372. [Google Scholar] [CrossRef]
- Tang, J. Effects of AMF on Salt Tolerance of Sedum Aizoon L. under NaCl Stress. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2015. [Google Scholar]
- Liu, J.; Shi, D.C. Photosynthesis, chlorophyll fluorescence, inorganic ion and organic acid accumulations of sunflower in responses to salt and salt-alkaline mixed stress. Photosynthetica 2010, 48, 127–134. [Google Scholar] [CrossRef]
- Roumet, C.; Urcelay, C.; Sandra, D. Suites of root traits differ between annual and perennial species growing in the field. New Phytol. 2006, 170, 357–368. [Google Scholar] [CrossRef]
- Lin, G.; Mccormack, M.L.; Guo, D. Arbuscular mycorrhizal fungal effects on plant competition and community structure. J. Ecol. 2015, 103, 1224–1232. [Google Scholar] [CrossRef]
- Chaves, M.M.; Miguel, C.J.; Madeira, S.N.J. Recent advances in photosynthesis under drought and salinity. Adv. Bot. Res. 2011, 57, 49–104. [Google Scholar] [CrossRef]
- Pinto, H.; Sharwood, R.E.; Tissue, D.T.; Ghannoum, O. Photosynthesis of C3, C3-C4, and C4 grasses at glacial CO2. J. Exp. Bot. 2014, 65, 3669–3681. [Google Scholar] [CrossRef] [PubMed]
- Rahim, N.A.; Jais, H.M.; Hassan, H.M. Environment and host affects arbuscular mycorrhiza fungi (AMF) population. Trop. Life Sci. Res. 2016, 27, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.L.; Xing, J.; Hu, J.; Li, X. Effects of AMF on growth of wheat soybean. J. Inn. Mong. Agric. Univ. 2002, 23, 104–106. [Google Scholar] [CrossRef]
- Tang, X.L.; Zhou, B.Z.; Zhou, Y.; Ni, X.; Cao, Y.H.; Gu, L.H. Photo-physiological and photo-biochemical characteristics of several herbaceous and woody species based on FvCB model. Chin. J. Appl. Ecol. 2017, 28, 1482–1488. [Google Scholar] [CrossRef]
- Schellenbaum, L.; Berta, G.; Ravolanirina, F.; Tisserant, B.; Gianinazzi, S.; Fitter, A.H. Influence of endomycorrhizal infection on root morphology in a micropropagated woody plant species (Vitis vinifera L.). Ann. Bot. 1991, 68, 135–141. [Google Scholar] [CrossRef]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb. Ecol. 2007, 54, 753–760. [Google Scholar] [CrossRef]
- Andrew, Y.; Nicole, P.; Brian, T.; David, A.C.P.; Julie, E.J.; Gary, D.B. Growth and nutritional responses to arbuscular mycorrhizal fungi are dependent on onion genotype and fungal species. Biol. Fertil. Soils 2015, 51, 801–813. [Google Scholar] [CrossRef]
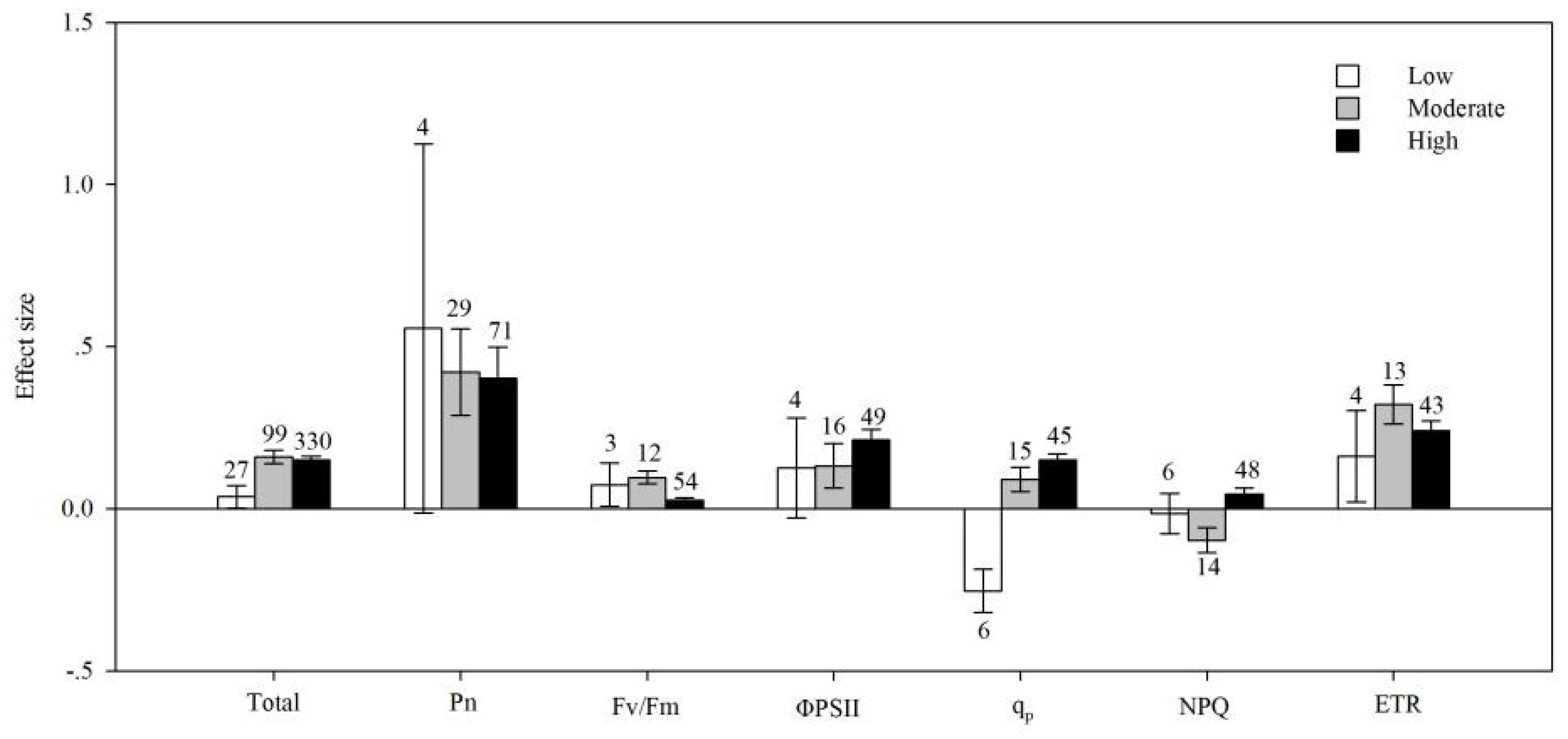
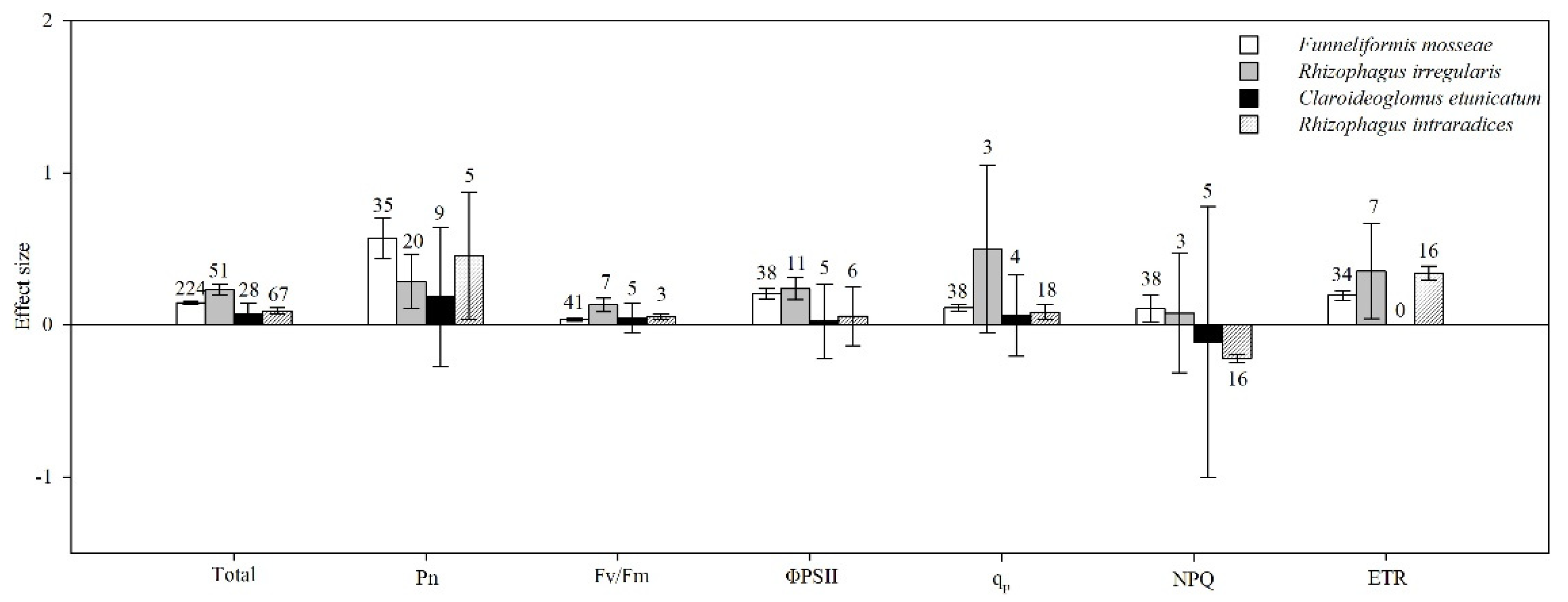
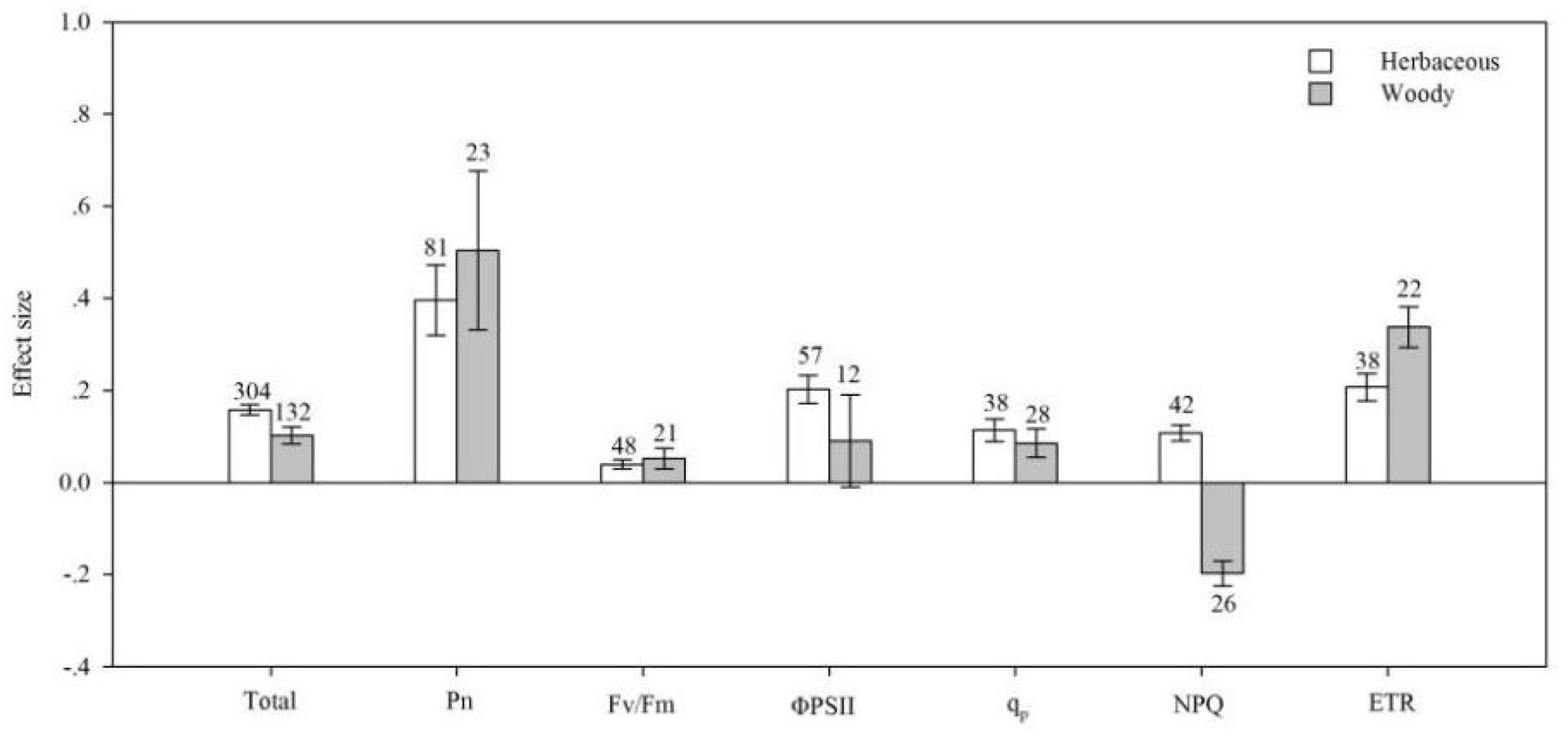
| Noncategorical Predictor Variables | Effect Size | dfa | 95% BS CIs | QTotal | P(chi-square) |
|---|---|---|---|---|---|
| All studies | 0.143 | 435 | 0.133 to 0.152 | 2477.046 | 0 |
| Pn | 0.415 | 103 | 0.346 to 0.483 | 167.351 | 0 |
| Fv/Fm | 0.042 | 68 | 0.033 to 0.050 | 259.403 | 0 |
| ΦPSII | 0.192 | 68 | 0.165 to 0.223 | 182.492 | 0 |
| qp | 0.104 | 65 | 0.083 to 0.124 | 309.468 | 0 |
| NPQ | 0.012 | 67 | −0.004 to 0.027 | 710.539 | 0 |
| ETR | 0.251 | 59 | 0.225 to 0.276 | 152.112 | 0 |
| Non-Categorical Predictor Variable | Categorical Predictor Variable | QB | QT | QB/QT | P(random) |
|---|---|---|---|---|---|
| Pn | AMF species | 14.692 | 126.905 | 0.116 | 0.429 |
| plant group | 6.558 | 160.387 | 0.041 | 0.068 | |
| life cycle | 16.623 | 149.681 | 0.111 | 0.011 | |
| plant type | 1.391 | 163.991 | 0.008 | 0.398 | |
| photosynthetic type | 21.774 | 163.697 | 0.133 | 0.009 | |
| soil salinity | 0.702 | 141.474 | 0.005 | 0.828 | |
| Fv/Fm | AMF species | 33.038 | 257.847 | 0.128 | 0.232 |
| plant group | 320.848 | 647.156 | 0.496 | 0.001 | |
| life cycle | 187.509 | 535.195 | 0.350 | 0.003 | |
| plant type | 1.213 | 257.056 | 0.005 | 0.589 | |
| photosynthetic type | 166.424 | 422.353 | 0.394 | 0.001 | |
| soil salinity | 54.171 | 320.944 | 0.169 | 0.023 | |
| ΦPS II | AMF species | 16.530 | 209.559 | 0.079 | 0.406 |
| plant group | 2.432 | 182.479 | 0.013 | 0.369 | |
| life cycle | 6.032 | 182.427 | 0.033 | 0.339 | |
| plant type | 5.485 | 209.639 | 0.026 | 0.158 | |
| photosynthetic type | 0.658 | 201.819 | 0.003 | 0.908 | |
| soil salinity | 7.365 | 209.721 | 0.035 | 0.267 | |
| qp | AMF species | 12.761 | 368.440 | 0.035 | 0.625 |
| plant group | 2.246 | 309.377 | 0.007 | 0.429 | |
| life cycle | 1.731 | 309.382 | 0.006 | 0.578 | |
| pPlant type | 2.058 | 368.368 | 0.006 | 0.573 | |
| photosynthetic type | 1.967 | 373.050 | 0.005 | 0.852 | |
| soil salinity | 213.493 | 503.059 | 0.424 | 0.001 | |
| NPQ | AMF species | 417.222 | 840.666 | 0.496 | 0.001 |
| plant group | 49.912 | 716.181 | 0.070 | 0.051 | |
| life cycle | 44.551 | 715.785 | 0.062 | 0.068 | |
| plant type | 379.257 | 837.278 | 0.453 | 0.001 | |
| photosynthetic type | 210.668 | 789.315 | 0.267 | 0.001 | |
| soil salinity | 51.491 | 741.433 | 0.069 | 0.116 | |
| ETR | AMF species | 35.317 | 158.558 | 0.223 | 0.003 |
| plant group | - | 152.112 | 0.000 | ||
| life cycle | 5.879 | 152.183 | 0.039 | 0.320 | |
| plant type | 25.296 | 162.155 | 0.156 | 0.003 | |
| photosynthetic type | 29.543 | 162.254 | 0.182 | 0.006 | |
| soil salinity | 11.037 | 158.732 | 0.070 | 0.118 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, J.; Yan, X.; Sun, S.; Lin, J. The Effect of Arbuscular Mycorrhizal Fungi on Photosystem II of the Host Plant Under Salt Stress: A Meta-Analysis. Agronomy 2019, 9, 806. https://doi.org/10.3390/agronomy9120806
Wang Y, Wang J, Yan X, Sun S, Lin J. The Effect of Arbuscular Mycorrhizal Fungi on Photosystem II of the Host Plant Under Salt Stress: A Meta-Analysis. Agronomy. 2019; 9(12):806. https://doi.org/10.3390/agronomy9120806
Chicago/Turabian StyleWang, Yingnan, Jinghong Wang, Xiufeng Yan, Shengnan Sun, and Jixiang Lin. 2019. "The Effect of Arbuscular Mycorrhizal Fungi on Photosystem II of the Host Plant Under Salt Stress: A Meta-Analysis" Agronomy 9, no. 12: 806. https://doi.org/10.3390/agronomy9120806
APA StyleWang, Y., Wang, J., Yan, X., Sun, S., & Lin, J. (2019). The Effect of Arbuscular Mycorrhizal Fungi on Photosystem II of the Host Plant Under Salt Stress: A Meta-Analysis. Agronomy, 9(12), 806. https://doi.org/10.3390/agronomy9120806





