1. Introduction
Increased consumers awareness relating to food safety is one of the drivers that has led to an appreciable expansion of the agricultural land area treated according to organic farming practices in the last two decades. This is clearly reflected in the increase of the total organically cultivated area worldwide, from 11 million hectares in 1999 to 69.8 million ha in 2017 [
1]. Organic farming systems rely on environmentally friendly practices, such as crop rotation, maintenance and enhancement of soil microbial activity, soil fertility and biodiversity, and nourishing plants primarily through the soil ecosystem, while excluding the use of synthetic chemicals [
2]. Calcium (Ca), magnesium (Mg), and sulfur (S) micronutrients are normally available at sufficient levels in arable soils. Furthermore, the supply of phosphorus (P) and potassium (K) does not pose serious difficulties in organic farming systems, since these nutrients are constituents of nonsynthetic organic or inorganic materials, such as bone meal, rock phosphate, potassium magnesium sulfate, and dolomitic lime, which are permitted as fertilizers in organic farming [
3]. However, inorganic nitrogen (N) fertilizers of natural origin that are compatible with certified organic production are rare. Consequently, the availability of N to plants in organic agriculture strongly depends on supply and recycling of organic residues. Animal manure originating from organic or free-range husbandry is a common form of organic residues as a source of N in organic agriculture. However, according to the relevant European Union legislation (EU Directive 889/2008) [
4], the amount of N exogenously supplied to an agricultural ecosystem through animal manure should not exceed 170 kg/ha per year. This amount may be sufficient for open-field crops but is insufficient for greenhouse tomato crops, due to both the length of the cultivation period and the high amounts of fruit removed from the field through harvesting. Indeed, tomato fruit production of 20 kg/m
2, which is a reasonable yield outcome in organic tomato greenhouses [
5], removes about 240 kg N ha
−1 yr
−1 through harvesting, assuming a fruit dry matter content of 6% and a N concentration of 20 mg g
−1 dry weight, as reported by Colla et al. [
6]. Thus, in addition to animal manure, other sources of N compatible with organic agriculture are needed to cover the high N needs of tomato when cultivated organically in greenhouses.
Inadequate levels of available N in soil may result in nutrient deficiency in greenhouse tomato crops, which is manifested as stunted spindly growth and yellowing of the older and intermediate tomato leaves [
7]. Nitrogen deficiency decreases tomato yields considerably due to reduction in both the number of fruit per plant and the mean fruit size, together with a negative effect on fruit quality [
8]. Therefore, N fertilization is of major importance for greenhouse tomato crops and should be carefully managed to optimize both fruit yield and quality. The use of legumes as intercrops or green manure may represent an important source of N in organic agriculture [
9,
10]. The unique ability of legumes to fix N
2 through symbiosis with rhizobia is of paramount importance for crop N supply in organic agriculture [
11]. Nevertheless, the total amount of biologically-fixed N provided to the crop, as well as the timely release of plant-available N originating from biological N
2 fixation (BNF) are crucial factors for the successful application of green manure or intercropping in organic tomato crops grown in greenhouses [
3]. To the best of our knowledge, peer-reviewed reports on the use of legumes in organic tomato greenhouses treated according to the relevant EU legislation are not available so far.
Based on these considerations, the present study was designed to test the hypothesis that legumes can be successfully used as an additional nutrient source in organic greenhouse tomato production. More specifically, the objective of this study was to test whether legumes applied as green manure or intercrops, inoculated or noninoculated with rhizobia and plant growth promoting rhizobacteria (PGPR), can successfully complement farmyard manure (FYM) as an N source.
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Treatments
The research presented in this manuscript includes three individual experiments conducted successively in the same greenhouse. The three experiments (henceforth referred to as E1, E2, and E3, respectively) took place from May 2017 to January 2018 (E1), February 2018 to June 2018 (E2), and June 2018 to January 2019 (E3). The exact dates for each experiment and crop are provided in
Table 1. The experiments were carried out in a standard commercial arch type greenhouse covered by low-density polyethylene films, with vertical sidewalls. The geometrical characteristics of the greenhouse were as follows: eaves height = 2.80 m, ridge height = 3.5 m, span width = 7.5 m, length = 44 m, ground area = 330 m
2. The greenhouse was ventilated by side vents (total opening area of 150 m
2), which were opened whenever the greenhouse air temperature exceeded 26 °C. The greenhouse was NNE–SSW oriented, and located in Preveza, northwestern Greece (38°59′29.2″N; 20°45′36.1″E, 5 m a.s.l.). The plot size was 3.75 × 5.00 m (i.e., 18.75 m
2). The soil type was sandy loam. The greenhouse was not cultivated and had remained uncovered for the 13 years prior to the establishment of the current experiments.
During the experimental period, climatic data, particularly air temperature and relative humidity, were collected on an hourly basis. Monthly means of temperature (minimum, maximum, and average) and relative humidity (%) for all experiments are presented in
Table 2.
To test the impact of legumes applied as green manure on N nutrition and yield of organic greenhouse tomato, cowpea was cultivated before the tomato cultivation in summer 2017 (E1) and summer 2018 (E3). Furthermore, on October 2017, faba bean was sown between the tomato rows in E2 and incorporated into the soil together with the tomato residues at crop termination in January 2018, to test whether the legume intercrop could substantially enhance the N availability to the next tomato crop in Spring 2018 (E2). The treatments applied in the three experiments are listed in
Table 3. All treatments were applied in the same plots in the three successive experiments.
In E1, farmyard manure (FYM) originating from free-range cattle farming was applied on July 30, 2017, at a rate of 50 t/ha in all treatments. The FYM contained 0.34% N, 0.15% P, and 0.48% K. This amount of FYM is equivalent to N supply of 170 kg/ha in order to comply with European Union Directive 889/2008. In treatment 1 (FYM), which was considered the control, no other source of N was applied except for FYM. In treatments 2 (FYM + legume noninoculated: L-NI), 3 (FYM + legume inoculated with rhizobia: L-I-Rh), and 4 (FYM + legume inoculated with rhizobia and PGPR: L-I-Rh-PGPR), additional N was provided through green manure by sowing cowpea (
Vigna unguiculata (L) Walp.) on May 23 2017, and incorporating it into the soil on 27 July 2017 (i.e., 6 days before planting tomato, which took place on 2 August 2017). In FYM + L-NI, the seeds of cowpea were not inoculated with rhizobia. In FYM + L-I-Rh, the seeds of cowpea were inoculated with
Bradyrhizobium sp. VULI11 (BV) [
12], while in FYM + L-I-Rh-PGPR, the seeds of cowpea were inoculated with a mix of BV and putative plant growth promoting rhizobacteria (PGPR), isolated from cowpea nodules (
Enterobacter sp. C1.2,
Enterobacter sp. C1.5,
Enterobacter sp. C3.1., and
Lelliottia sp. D2.4. Strains have been characterized by multi-locus sequence analysis (unpublished data). Strains’ designations “C” and “D” represent the geographical regions of field-collected cowpea root nodules in Greece, that is Epirus and Crete, respectively, and followed by a lab code number.
In treatments FYM + L-NI, FYM + L-I-Rh, and FYM + L-I-Rh-PGPR, faba bean was sown as an intercrop between the tomato rows at a density of 10.67 plants/m2 on 26 October 2017. The faba bean plants were intended to be incorporated into the soil as green manure for the next tomato crop (E2) after termination of E1. In the FYM + L-NI treatment, the seeds of faba bean were not inoculated with any rhizobia. In the FYM + L-I-Rh treatment, the seeds of faba bean were inoculated with Rhizobium sp. svmbiovar (sv.) viciae VFBL1, isolated from field-grown faba bean nodules in Greece, while in the FYM + L-I-Rh-PGPR treatment, the seeds of faba bean were inoculated with a mix of VFBL1 and PGPR (Enterobacter sp. C1.2, Enterobacter sp. C1.5, Enterobacter sp. C3.1, and Lelliottia sp. D2.4). Upon termination of the tomato crop on January 25, 2018, the faba bean plants and the aboveground parts of the tomato residues were incorporated into the soil. The root residues of tomato were removed and disposed out of the greenhouse because they had been infected by root-knot nematodes (Meloidogyne sp.) during E1. Subsequently, on 29 January 2018, FYM was applied again in all four treatments at a rate of 50 t/ha. Finally, on 8 February 2018, new tomato seedlings were planted to establish a spring–summer tomato crop (E2). This crop was terminated on 11 June 2018, and the residues were incorporated again into the soil, including the roots, because the incidence of nematode infection was very low during E2.
On 12 June 2018, cowpea was sown again in the plots of FYM + L-NI, FYM + L-I-Rh, and FYM + L-I-Rh-PGPR, which was intended to be used as green manure for the next tomato crop (E3). Similarly to E1, the seeds of cowpea in E3 were either not inoculated with any rhizobia (FYM + L-NI), inoculated with
Bradyrhizobium sp. VULI11 only (FYM + L-I-Rh), or inoculated with both
Bradyrhizobium sp. VULI11 and the same PGPR bacteria as in E1 and E2 (FYM + L-I-Rh-PGPR). In E3, the commercial cowpea cultivar Iron and Clay (Seed Ranch Company, Odessa, TX, USA) was used, which is considered nematode-resistant [
13]. The cowpea plants were incorporated into the soil on 7 August 2018 (i.e., 56 days after sowing). On 10 August 2018, FYM was applied again in all treatments at a rate of 50 t/ha. Finally, on 12 August 2018, new tomato seedlings were planted to establish E3. Harvesting of commercially ripe tomato fruit commenced in 17 October 2018, and the crop was terminated on 20 January 2019.
In E1, self-rooted seedlings of the commercial tomato hybrid “Elpida F1” were used to establish the experiment. However, due to a severe infection by root-knot nematodes in E1, the commercial tomato hybrid “Ekstasis F1” was grafted onto the commercial rootstock Maxifort F1 (Solanum lycopersicum × Solanum habrochaites) was cultivated in E2 and the hybrid “Elpida F1” was grafted onto “Maxifort” in E3. The plant density was 2.13 plants m−2 in all three experiments. The tomato and faba bean plants were drip-irrigated with drippers set 50 cm and 20 cm apart, respectively, while the cowpea plants were overhead-irrigated using sprinklers. During the cropping period, no additional fertilizers were provided to the plants in E1 and E3 in all treatments. However, in E2, due to the occurrence of N deficiency symptoms seven weeks after crop establishment, extra fertilization via the drip irrigation system was applied in all treatments at four dates, particularly on April 25 and 29 and on May 2 and 6, using an organic fertilizer based on amino acids, containing 14% N. The total fertilizer application rate was 16 g/m2 (i.e., 22.4 kg N ha−1) in all treatments.
2.2. Growth, Mineral Analysis, and Nitrogen Fixation by Legumes
In all plots of the cowpea and faba bean crops, root samples were collected from soil cores using a 1 L cylindrical metal auger. All soil samples were placed for 24 h in a “Calgon” solution (dispersing agent) prepared by adding 40 g (NaPO3)6 and 10 g Na2CO3 per 1000 mL of water. Subsequently, the roots were carefully washed out over a sieve and the number of nodules was measured after detaching them from the roots. The root dry weight was also determined after drying the samples for 48 h at 65 °C. To determine the aboveground fresh and dry biomass, shoots from a 1 m2 area of each plot center were fresh-weighed before their incorporation into the soil, and subsequently tissue subsamples were oven-dried at 65 °C to a constant weight.
In E1, the aboveground part of three cowpea plants per plot was sampled at 23, 35, 52, and 63 days after sowing. In E2, the aboveground parts of three faba bean plants per plot were sampled at 31, 43, 53, and 77 days after sowing. Similarly, in E3, the aboveground parts of three cowpea plants per plot were sampled at 22, 37, and 52 days after sowing. All tissue samples were oven-dried at 65 °C to a constant weight, powdered using a ball mill, and passed through a sieve (0.5 mm). Organic C and total N in plant tissue samples were determined by high temperature combustion using an elemental analyzer (Unicube, Elementar Analysensysteme GmbH, Hanau, Germany). Total P concentrations in plant tissues were determined by ashing at 550 °C for 8 h, dissolving the soluble salts in 4 M HCl, and quantifying P in the extracts using a spectrophotometer (U-2000, Hitachi, Tokyo, Japan) following the molybdate blue method [
14]. Potassium was determined in the same aqueous extract using a flame photometer (Sherwood Model 410, Cambridge, UK).
The N derived from the atmosphere in the aboveground part of cowpea and faba plants bean was determined by applying a method based on the natural abundance of
15N in plant tissues relative to the air [
15,
16,
17]. To apply this method, the stable N isotopic composition of legume tissue samples was determined using an Isoprime 100 continuous flow isotope ratio mass spectrometer coupled to a Vario Isotope Select elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). The δ-values were calibrated relative to air by means of a three-point calibration using standard reference materials IAEA-N1, IAEA-600, and IAEA-N2. Measurement uncertainty was monitored by repeated measurements of internal laboratory standards and standard reference materials. Precision was determined to be ± 0.19‰ based on repeated measurements of calibration standards and internal laboratory standards. Accuracy was determined to be ± 0.19‰ on the basis of the difference between the observed and known δ values of check standards and their standard deviations. The total analytical uncertainty was estimated to be ± 0.27‰ for δ
15N. The δ
15Ν values were estimated as parts per thousand (‰) deviations relative to the nominated international standard of atmospheric N
2 (0.3663%), using the following equation [
18]:
Subsequently, the proportion of N derived from the atmosphere (%Ndfa) was estimated by substituting the δ
15N (‰) of the N
2-fixing legume and a non-N
2-fixing reference plant grown in the same soil, as calculated using Equation (1), into the following equation suggested by Unkovich et al. [
15]:
where “B” is the δ
15N in shoots of cowpea or faba bean plants grown on an inert medium and starved of N throughout their life, thereby being fully dependent on N
2 fixation. The B values used in the current study were −1.61 for cowpea and −0.50 for faba bean, as suggested by Unkovich et al. [
15]. The reference plant used in this study to determine the corresponding δ
15Ν values was the grass weed
Digitaria sanguinalis (L.).
The total amounts of biologically-fixed N
2 by cowpea and faba bean per cultivated area unit (BNF, kg/ha
−1) were estimated using the following equation [
19]:
where DB is the total dry biomass of the shoot, Nt is the total N concentration (% w/w) in the aboveground dry biomass, and %Ndfa are the values obtained from (2).
2.3. Tomato Tissue Sampling and Mineral Analysis
To assess the amounts of nutrients removed through harvesting of ripe fruit in the tomato crops, four ripe fruits from the 2nd cluster of 4 plants per plot were collected. The fruits were chopped and oven-dried at 65 °C for at least 3 days to a constant weight. Then, they were powdered using a ball mill, sieved (0.5 mm), homogenized, and chemically analyzed for total N, P, and K, as described above.
To determine the nutritional status of the plants, samples of the youngest fully expanded leaves were collected from all plots in all three experiments. The leaves were washed with distilled water, chopped, and oven-dried at 65 °C for at least 2 days until they reached constant weight, were powdered using a ball mill, and passed through a 40 mesh sieve. Subsequently, 0.5 g of powdered material was dry ashed in a muffle furnace at 550 °C for 5 h, and chemically analyzed for total N, P, and K, as described above.
Due to an unexpected nematode infection by
Meloidogyne spp. in E1, the severity of the infection was estimated as described by Bridge and Page [
20], based on visual observation of the tomato plants. At the termination of the experiment, the root systems of 10 plants from each plot were used for yield determination, which were dug from the soil and indexed for root galls using a 0–10 scale (0 = no root galls, 1 = few small galls, difficult to find, 2 = small galls but main roots clean, …, 10 = all roots severely galled).
2.4. Soil Analysis
Soil samples were collected from the central square of each plot (dimensions 2 × 2.5 m). In each plot, 5 soil cores weighing about 400 g were collected from the root zone of 5 plants at a depth of 0–20 cm. The samples were oven-dried at 40 °C for at least 3 days until their weight stabilized to a constant level. Subsequently, the samples were sieved (2 mm diameter), homogenized, and analyzed to determine the organic C, total N, NO
3-N, NH
4-N, and plant-available P and K concentrations. Total C and N in soil samples were determined by high temperature combustion using an elemental analyzer (Unicube, Elementar Analysensysteme GmbH, Hanau, Germany). Since soil pH was 7.5 due to the presence of carbonates, organic C was determined in soil aliquots that were pretreated with HCl to remove inorganic C before elemental analysis. To determine the concentration of mineral nitrogen (N-min, i.e., NO
3—N+ NH
4 + -N) in the soil, each sample of sieved soil was extracted using a KCl solution, as described by Keeney and Nelson [
21]. Subsequently, the nitrate and ammonium concentrations in the sample extracts were determined by applying the cadmium reduction to NO
2− and the indophenol blue methods, respectively [
21], using a Spectronic Helios spectrophotometer (Thermo Electron Corporation, Mercers Row, Cambridge CB5 8HY, UK). Plant-available phosphorus was determined using the Olsen method [
22] and quantified by molybdate colorimetry [
23]. Exchangeable soil K was determined using a flame photometer (Sherwood Model 420, Sherwood Scientific, Cambridge, UK) following extraction with an ammonium acetate solution.
2.5. Tomato Growth and Yield
The impact of the experimental treatments on crop yield was assessed by harvesting all ripe tomatoes from 10 plants of the plot center twice per week, counting them, and weighing them on a commercial scale.
2.6. Statistical Analysis
All experiments were set as randomized block designs with 4 replicates. The data were statistically analyzed by applying ANOVA using the STATISTICA software package, version 12.0 for Windows. A Duncan’s multiple range test was performed when the ANOVA was significant at p < 0.05 level. Data are presented in graphs as means ± SE of four replicates, or in tables.
4. Discussion
As postulated by Atkinson and Watson [
24], organic farming is characterized by a complexity of relationships between different system components, and thus the sustainability of the system is dependent upon the functioning of a whole integrated and inter-related system. The results of the present study provide a good example of the complexity of factors governing yield performance in organic crops. Thus, in E1, the tomato fruit yield decreased significantly when cowpea fresh biomass was incorporated into the soil as green manure (GM) in addition to FYM, despite the significantly higher levels of soil NO
3-N compared to sole FYM application, because the local cowpea variety used as GM proved to be a good host of
Meloidogyne incognita. As a result, the subsequent tomato crop was more severely affected by the root-knot nematode when cowpea fresh biomass grown in the same plots was incorporated into the soil prior to tomato transplanting. Watson et al. [
11] already pointed out that despite the benefits obtained from incorporation of green manures on N management, this cultural practice may be associated with disease risks. However, in E2 and E3, the yield was increased by the incorporation of legume biomass to the soil when the nematode infection was effectively controlled. Grafting onto “Maxifort” provides substantial protection against root-knot nematodes [
25]. Furthermore, nontoxic agents allowed for organic tomato production, such as
Bacillus firmus and
Purpureocillium lilacinus strain 251, can provide additional protection [
26,
27]. Thus, the use of tomato seedlings grafted onto “Maxifort” and the application of biological control agents against nematodes effectively controlled the nematode infection in E2 and E3, thereby eliminating its interference with crop performance and yield. As a result, the tomato crop benefited from the higher soil NO
3-N levels originating from the legume treatments, as indicated by the significantly higher fruit production, and this effect is reasonable given that N represents the primary nutrient-limiting yield in organic cropping systems [
28,
29].
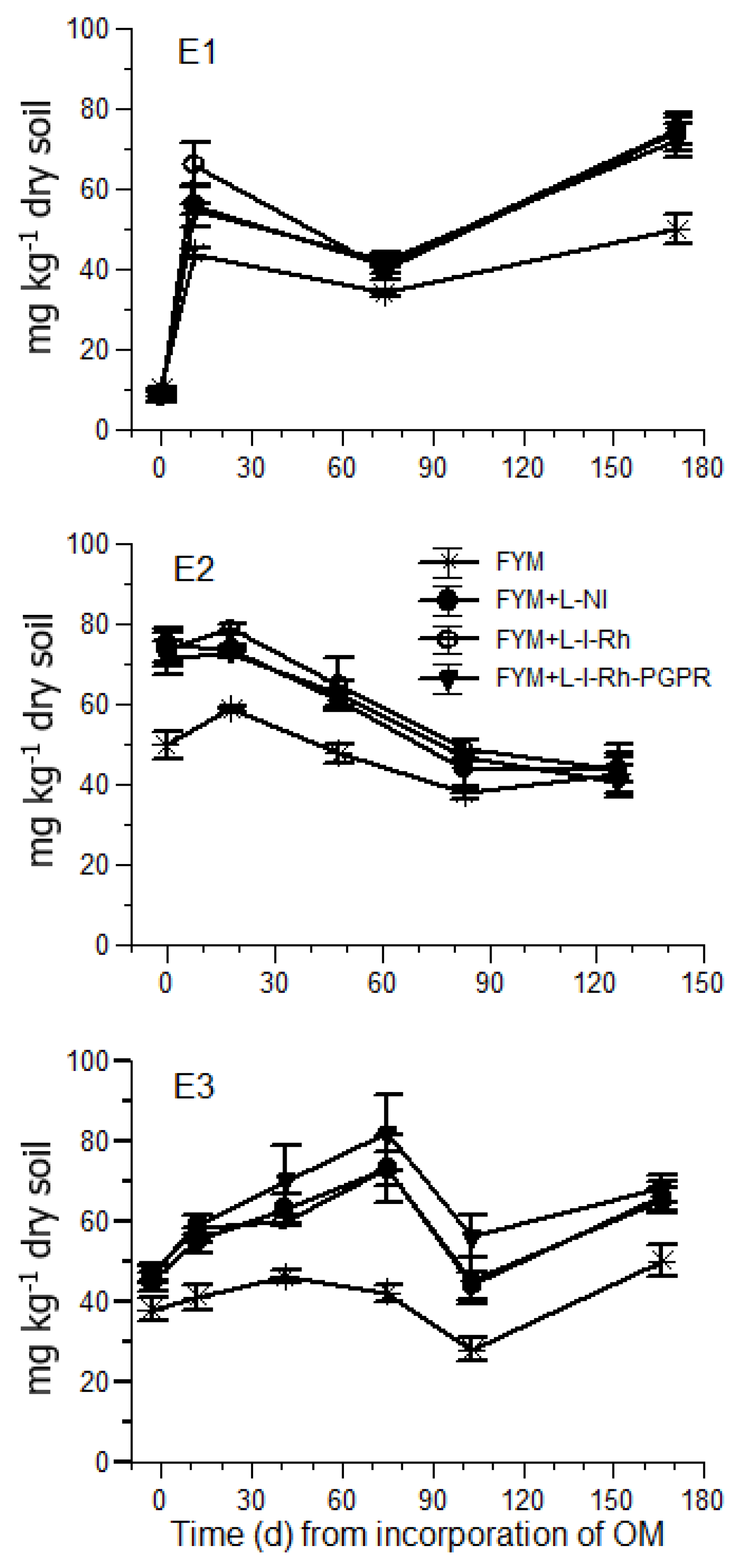
In E1, three weeks after incorporation of FYM and legume biomass to the soil, the mean NO
3-N concentrations ranged from 55 to 66 mg kg
−1 in the plots treated with both FYM and cowpea GM, and from 41 to 46 in the plots receiving only FYM. The NO
3-N levels recorded in all plots treated with cowpea GM at that stage of cultivation are considered adequate for tomato [
30,
31]. Nevertheless, in October 2017, the soil NO
3-N in E1 decreased to levels close to or below 40 mg kg
−1 in all treatments, which are considered insufficient for tomato plants carrying a heavy fruit load [
8,
32]. In agreement with this consideration, N deficiency symptoms were observed in tomato plants by the end of October in E1. However, in E3, which was conducted one year later in the same season, the soil NO
3-N was maintained at sufficient levels for tomato production throughout the cropping period, especially when cowpea GM inoculated with rhizobia and PGPR was applied. In contrast, in the plots treated solely with FYM, the concentration of NO
3-N in the soil ranged within insufficient levels in both E1 and E3 (<50 mg kg
−1) according to Sainju et al. [
8]. The significant increase of the soil NO
3-N when cowpea was applied as GM compared to sole application of FYM indicates that GM with legumes is an efficient tool to increase the soil N levels in organic tomato crops in greenhouses. However, the benefits of cowpea GM with respect to the soil NO
3-N levels were more profound in E3. This is reasonable, as in organic crops fertility management relies on a long-term integrated approach [
11], because the release of nutrients from organic biomass incorporated into the soil is a long-lasting process exceeding crop life.
In E2, a winter legume (faba bean) was applied as the intercrop in the preceding tomato crop to deliver atmospheric N
2 to the soil, since E2 took place during spring–summer, and thus the preceding legume crop had to take place during late autumn and winter. Furthermore, E2 was conducted immediately after the autumn–winter tomato crop of E1 in the same plots, in an attempt to assess whether a legume plant cultivated as an intercrop in an autumn–winter tomato crop is beneficial to a subsequent tomato crop cultivated in spring–summer season in terms of N supply. This would allow for two subsequent organic tomato crops in the same year and concomitantly for an increase in the grower’s income. Faba bean was selected to serve the above mentioned role because several studies have shown that the incorporation of legume residues arising from the preceding crop into the soil (including their application as green manure) increases growth and yield of many crops, such as canola, maize, potato. and wheat [
33,
34]. The results in
Figure 3 show that the soil NO
3-N levels in E2 were significantly higher at the beginning of the tomato crop when faba bean biomass originating from intercropping with the previous tomato crop was incorporated into the soil. However, the levels and the difference in soil NO
3-N were similar to those found at the end of the tomato crop in E1 before incorporation of faba bean residues to the soil (
Figure 3). Thus, the large difference in soil NO
3-N between the plots treated solely with FYM and those treated additionally with faba in E2, especially at the beginning of the crop, seem to be directly or indirectly (e.g., by favoring the mineralization of FYM) related to the significant N inputs from cowpea incorporation during the previous cropping period. During the cropping period, this difference tended to decrease and finally diminished by the end of the tomato crop in E2. These results indicate that faba bean did not contribute substantially to the N needs of tomato in E2. As reported by Amanuel et al. [
35] and Neugschwandtner et al. [
36], faba bean is an efficient N
2-fixing legume plant, as in crops aiming to produce edible pods, this legume plant was capable of contributing from 139 to 210 kg N ha
−1 and from 63 to 219 kg N ha
−1, respectively, through BNF. In agreement with those results, Ntatsi et al. [
16] found that faba bean contributed up to 190 kg N ha
−1 through BNF when cultivated for fresh pod production. However, in the current study, faba bean was cultivated as an intercrop, which dictated a much lower plant density and less light availability than in open-field crops, while it was incorporated into the soil at a much earlier growth stage compared to the studies reported by Amanuel et al. [
35], Neugschwandtner et al. [
36], and Ntatsi et al. [
16]. Therefore, the net contribution of the faba bean intercrop to soil N through BNF in the current study did not exceed 17 kg N ha
−1, as shown in
Table 4. These results indicate that intercropping of faba bean in a previous tomato crop provides no substantial benefit in terms of N delivery via BNF in a subsequent tomato crop.
The significant increase of N derived from atmospheric N
2-fixation (% Ndfa) in the shoots of cowpea inoculated with
Bradyrhizobium sp. VULI11 in E1 (
Figure 1) show that efficient indigenous rhizobia strains suitable for cowpea were not present in the greenhouse soil used for this experiment. This is corroborated by the appreciably higher number and mean individual dry weight of nodules collected from cowpea plants inoculated with rhizobia compared to those measured in the roots of noninoculated plants. As reported by Soares et al. [
37], inoculation of cowpea with
Bradyrhizobium strains characterized by high nitrogen-fixing capacity in symbiosis with cowpea can substantially increase the ability of this plant species to fix atmospheric N
2. Although cowpea is considered a promiscuous species capable of establishing efficient symbiosis with diverse symbiotic bacteria [
38], the most efficient symbiotic relationships are achieved with
Bradyrhizobium species [
39], especially in nonalkaline soils [
12]. In the current study, the indigenous
Bradyrhizobium strain VULI11 [
40] was used as inoculum, which exhibited high N
2-fixing ability for cowpea, as confirmed by the results of E1. Nevertheless, in E3 the %Ndfa was similar in inoculated and noninoculated cowpea plants, which indicates that the inoculum applied during E1 was capable of persisting and spreading out throughout the field trial area, and was likely present at high populations in the soil in all plots one year later in E3. The similar %Ndfa values in inoculated and noninoculated cowpea plants in E3 are in line with the similar number of nodules per root segment and individual nodule dry weight, which were measured shortly before incorporation of faba bean to the soil. Thus, inoculation with
Bradyrhizobium sp. did not increase the BNF of cowpea plants and concomitantly provided no benefit to the subsequent tomato crop in E3. These results indicate that inoculation of cowpea with rhizobia is beneficial mainly in fields where this plant had been not cultivated in the recent years, and thus efficient rhizobia strains for cowpea were not present in the soil. Several other investigators found no benefit from rhizobia inoculation of legumes when efficient indigenous rhizobia strains for that particular legume species were present in the soil [
41,
42,
43]. Furthermore, the significant decrease of the %Ndfa in E3 compared to E1 in the inoculated treatments is ascribed to the notably higher soil nitrate concentrations in E3. Indeed, as shown by other researchers [
44,
45], high NO
3-N concentrations in the root zone of legume plants are associated with reduced nodulation and N
2-fixation.
The %Ndfa in faba bean fresh biomass ranged from 73% to 78% at crop termination, while inoculation of faba bean with
Rhizobium sp. VFBL1 had no significant impact on %Ndfa (
Figure 1B), or on fresh biomass, tissue N concentration, or BNF (
Table 4). The similar %Ndfa, tissue N, and BNF values between treatments in E2 are in agreement with the lack of any significant differences in the number and mean size of nodules, which were high in all treatments. These results indicate that indigenous rhizobia strains that are capable of nodulating faba bean and efficiently fixing atmospheric N
2 were present in the greenhouse soil, and thus inoculation with
Rhizobium sp. VFBL1 did not provide any benefit to the plants. This finding is in agreement with results found in a previous study [
16], in which the %Ndfa in faba bean plants cultivated for fresh pod production in an open field ranged from 79% to 91%, although the plants were not inoculated with rhizobia. Neugschwandtner et al. [
36] also found that faba bean was capable of fixing high amounts of atmospheric N
2 (219 kg/ha
−1 on average), although the plant was not inoculated with any rhizobia.
In nonacidic, oxic topsoils with high microbial activity, NH4-N derived from organic matter mineralization is rapidly converted into NO3-N by nitrification. Therefore, whereas similar trends in soil NH4-N concentrations were observed in all plots in all three experiments, regardless of legume application as green manure or intercrop, differences in soil NO3-N concentrations between treatments better reflected the differences in net organic N mineralization (under nonleaching conditions typical of the greenhouse environment).
Apart from different N inputs, one of the main factors influencing the N availability for the crop as a result of organic N mineralization is the C/N ratio of the decomposing organic matter [
46]. This is because the C/N ratio determines the balance between the rates of microbial N immobilization and mineralization, and therefore the net supply of plant-available N [
47,
48]. Other factors determining the N mineralization rate, such as the soil type and the soil temperature [
49], were similar between treatments at the same time in the experiments of this study. Thus, the higher N supply observed in the plots receiving legume residues applied as green manure together with FYM with respect to those treated only with FYM, as indicated by the generally higher NO
3-N concentrations, may be ascribed to a combination of higher N inputs as well as lower C/N ratio of incorporated organic matter in the former.
The changes in the soil NO
3-N concentration over time seem to be influenced not only by the time and quantity of organic matter incorporation to the soil and the C/N ratio in the organic matter, but also by the soil temperature, which has a direct impact on N mineralization rates [
49] and plant uptake. Indeed, as reported by Bhogal et al. [
50], the amount of mineralized N is related to thermal time (i.e., the cumulative day degrees above 5 °C). Thus, since the tomato crop in E1 and E3 took place from August to January, it is assumed that the reduction in soil NO
3-N levels at a latter cropping stage was partly due to decreased soil temperature as the crop was aging, which gradually restricted the net N mineralization rates. The partial recovery of the soil NO
3-N in January in E1 and E3 is ascribed to reduced mineral N uptake by the tomato crop due to the low soil temperature. The optimal temperature for the nitrifying bacteria is 41 °C [
51], while for N uptake by tomato the optimal level in the roots is about 27 °C [
52]. Thus, it seems that the season-related gradual reduction of the ambient temperature in E1 and E3 initially restricted the conversion of organic N to NO
3-N, but in January, the further reduction of the ambient temperature also affected the N uptake, resulting in the small increase of soil NO
3-N at that stage of the crop. In contrast to E1, which was an autumn crop, in E2, which was a spring–summer crop, the soil NO
3-N tended to decline consistently with time in all treatments, presumably because the net N mineralization rate was lower than the rate of plant uptake. This is reasonable, given the relatively low amount of BNF contributed by the faba bean intercrop (
Table 4) and the increasing N needs by the crop with time as the climatic conditions in spring and early summer are favorable for plant growth.
The levels of plant-available P and K in the soil were not influenced by the incorporation of legumes to the soil, and thus their tissue concentrations were not affected by the treatments applied in the current experiments, with the exception of leaf P in E1. From a first approach, the lack of a treatment impact on P and K nutrition is reasonable, given that the legumes used as green manure utilize the available P and K of the soil to grow, and thus their incorporation to the soil does not result in a net input of these nutrients to the soil [
53]. In many cases, green manure may result in utilization of plant-available nutrient resources from deeper soil layers [
54], or nutrients that might be leached out through rainfall [
53] if the field were not cultivated by the green manure crop. However, in the current experiment, both legumes were cultivated for short periods, and thus they had no time to develop a deep root system, while the cultivation of tomato inside a greenhouse prevented any leaching of nutrients via rainfall. On the other hand, the green manure crop may immobilize part of the absorbed P and K for more than one year depending on the weather conditions [
55]. Nevertheless, as shown in
Table 5, the soil P and K reserves were high in the soil of the greenhouse used in the present experiments, and thus any reduction of their availability due to immobilization in the legume residues incorporated into the soil had no impact on tomato nutrition by K and P.
The increase of the soil organic C, total N, and plant-available P concentrations in E2 compared to E1 and their further increase in E3 indicate that the organic fertilization practices applied in the current study were capable of increasing the soil fertility as they increased the reserves of organic matter, characterized by a low C/N ratio. This is in line with the suggestions of Janzen et al. [
56] and Watson et al. [
11] that the primary advantage of organic management practices is the long-term replenishment of stable organic N reserves in the soil. The present study further showed that FYM is capable of increasing the soil P reserves in organic tomato crops. On the other hand, the reduction of the soil K in E2 compared to E1 and the further decrease of soil K in E3 show that in the long term, the K requirements of greenhouse organic tomato cannot be addressed merely by organic fertilization treatments [
57]. This finding stresses the necessity to apply inorganic forms of K compatible with organic agriculture in organic greenhouse tomato, such as potassium and magnesium sulphate.