Impact of Cover Crop Usage on Soilborne Diseases in Field Nursery Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experimental Design and Layout
2.2. Cover Crop Application
2.3. Soil Moisture and Temperature Measurement
2.4. Soil Sampling
2.5. Fungal Culture and Pathogen Inoculum Preparation
2.6. Red Maple Seed Collection and Planting
2.7. Greenhouse Bioassays
2.7.1. Evaluation of Red Maple Crop Health
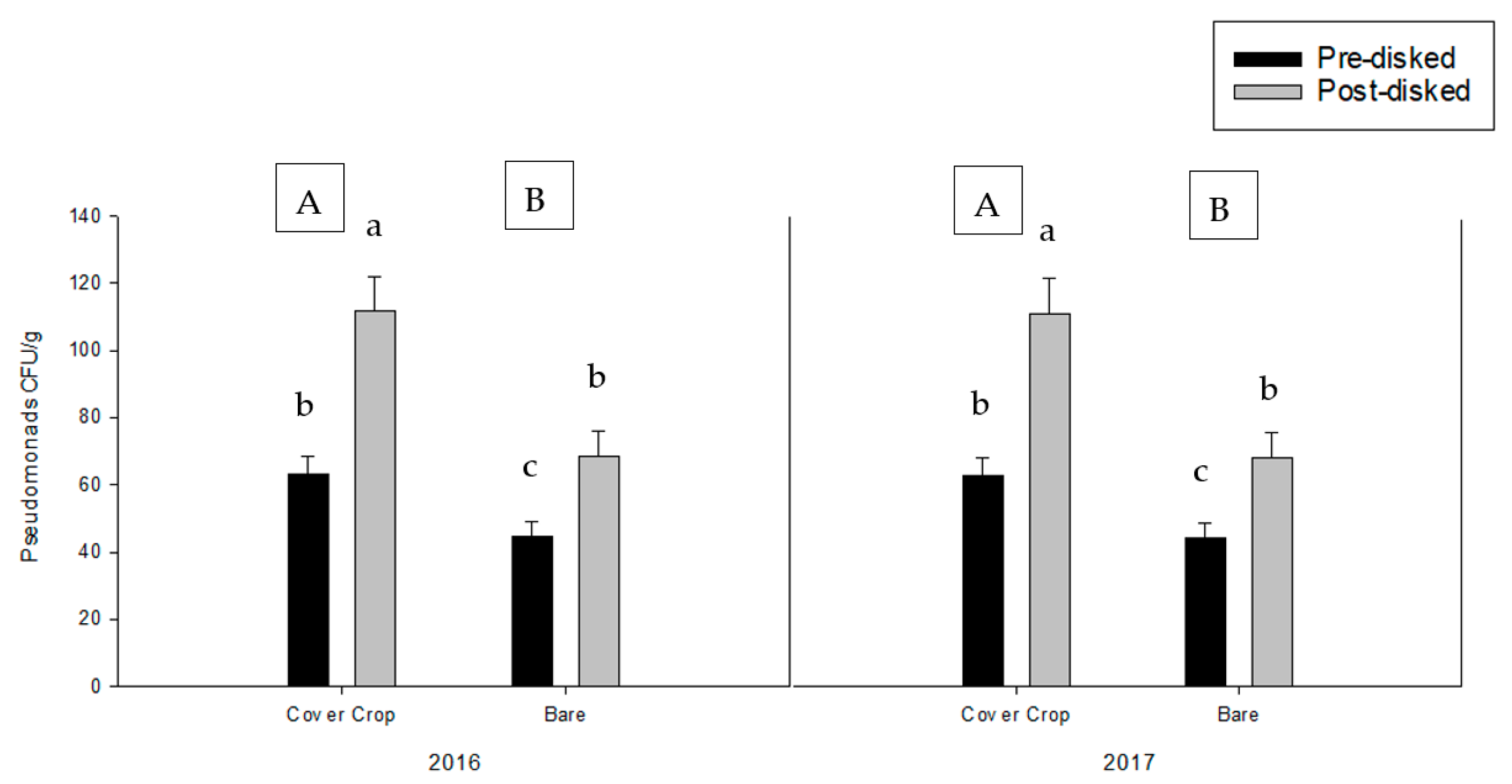
2.7.2. Pseudomonad Colonies
2.8. Statistical Analysis
3. Results
3.1. Greenhouse Bioassay without the Addition of Pathogen Inoculum
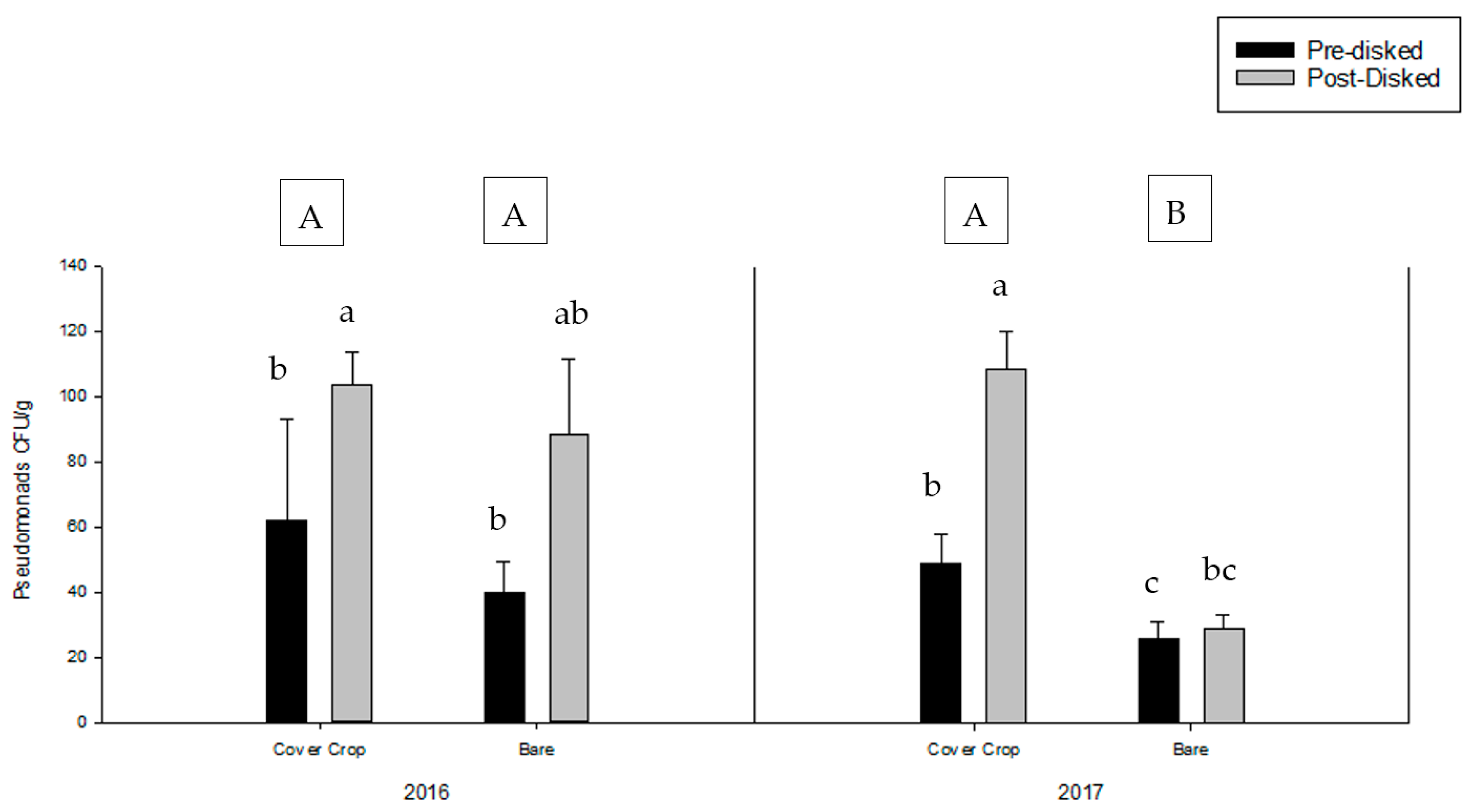
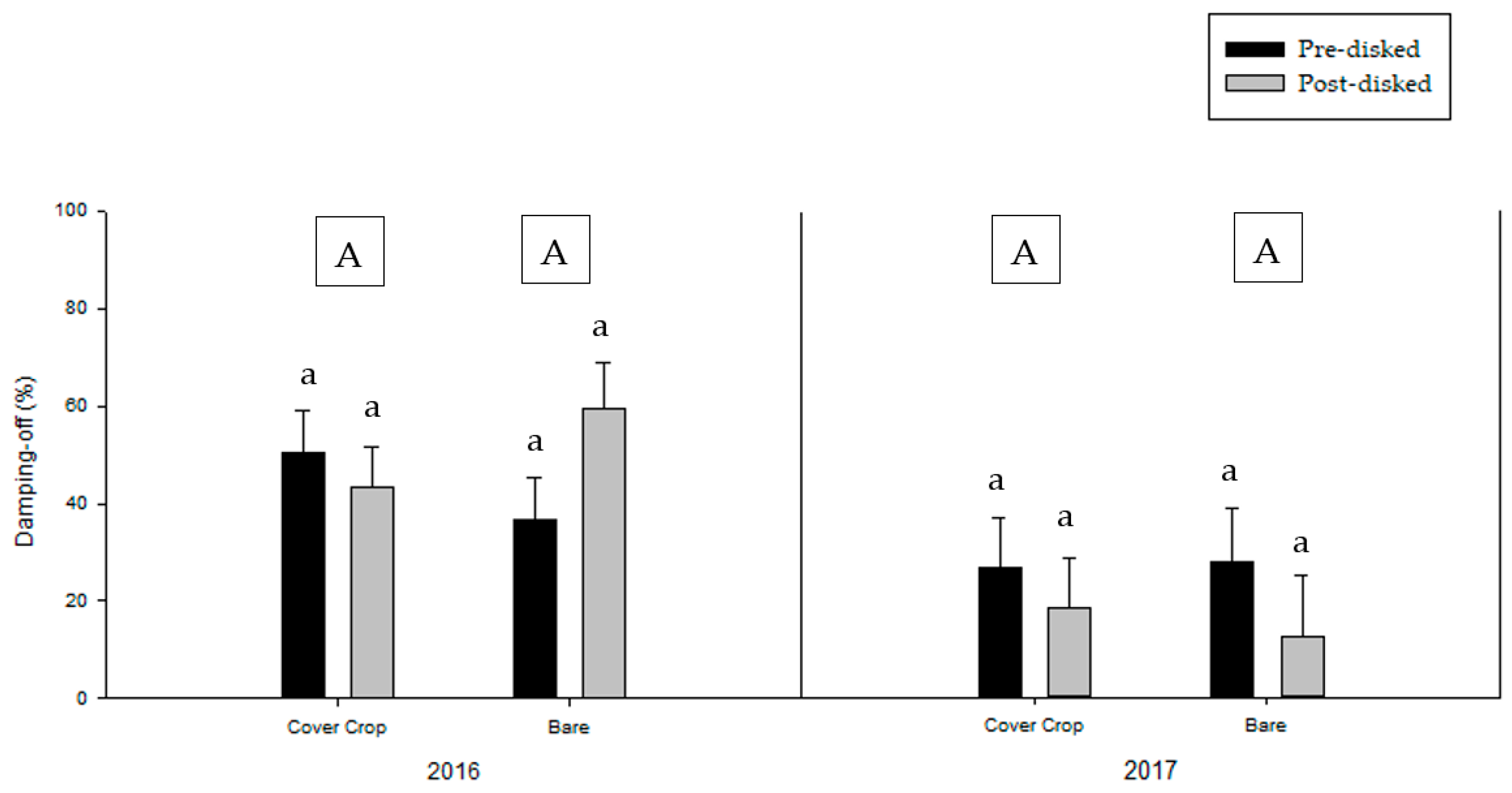
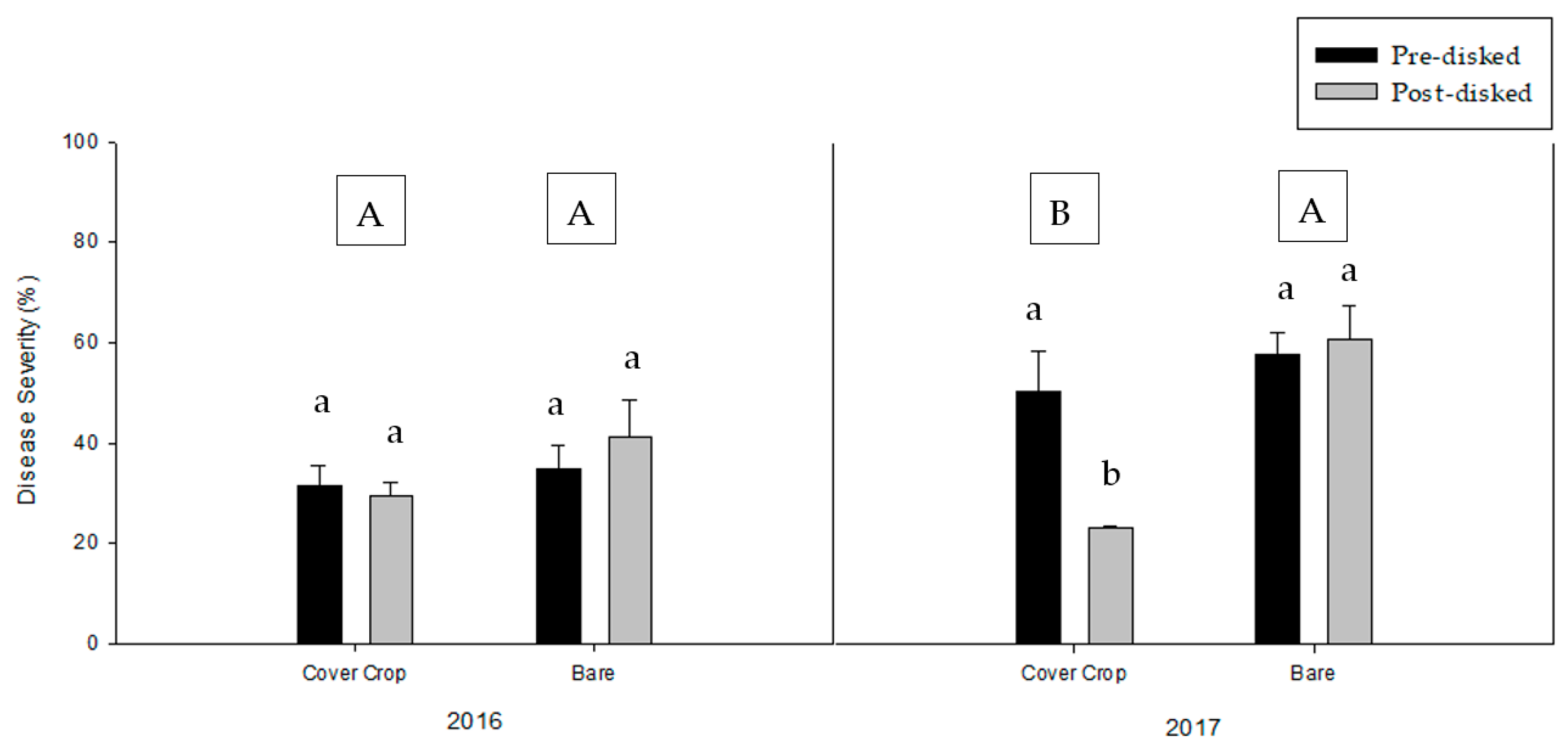
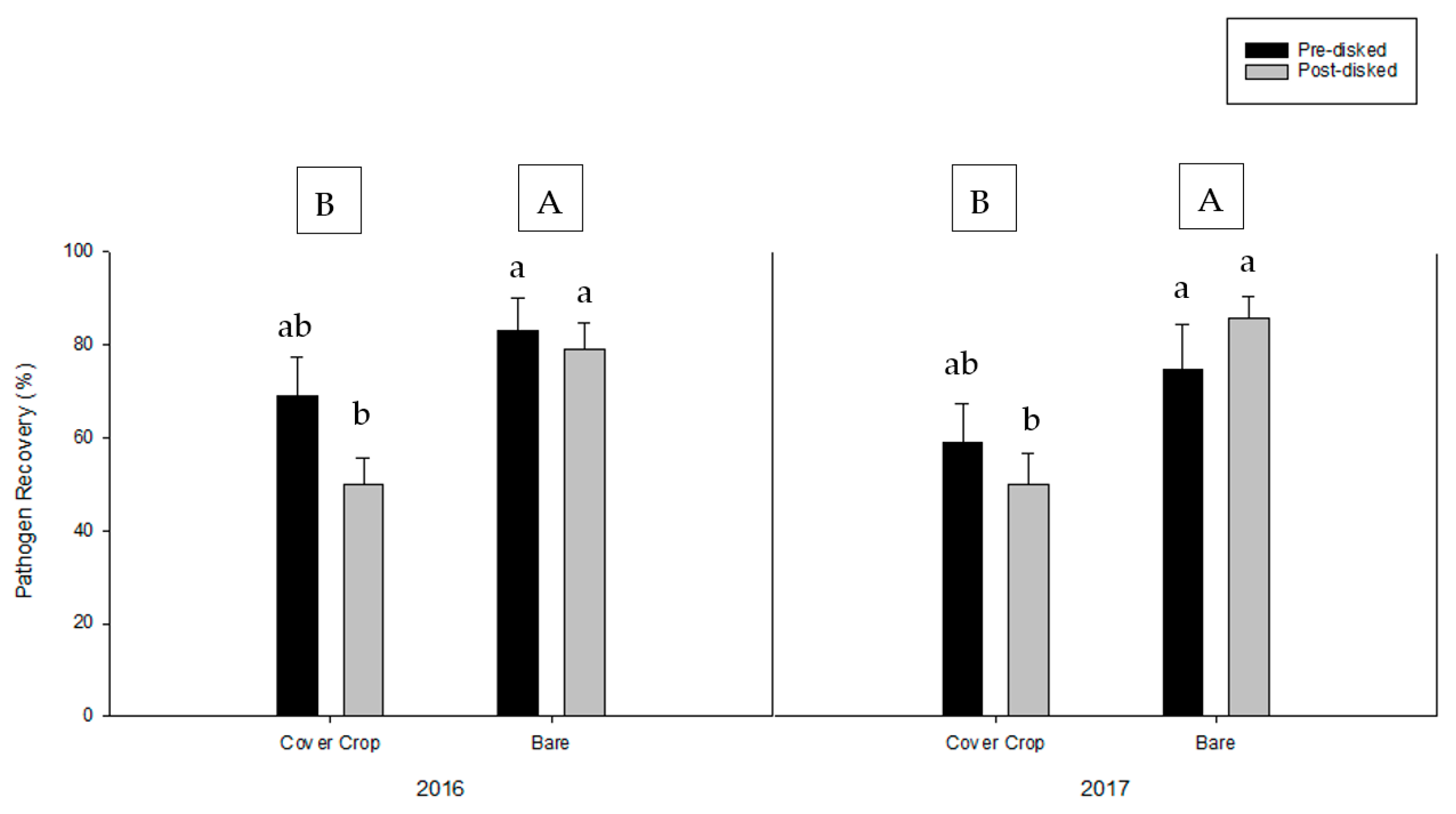
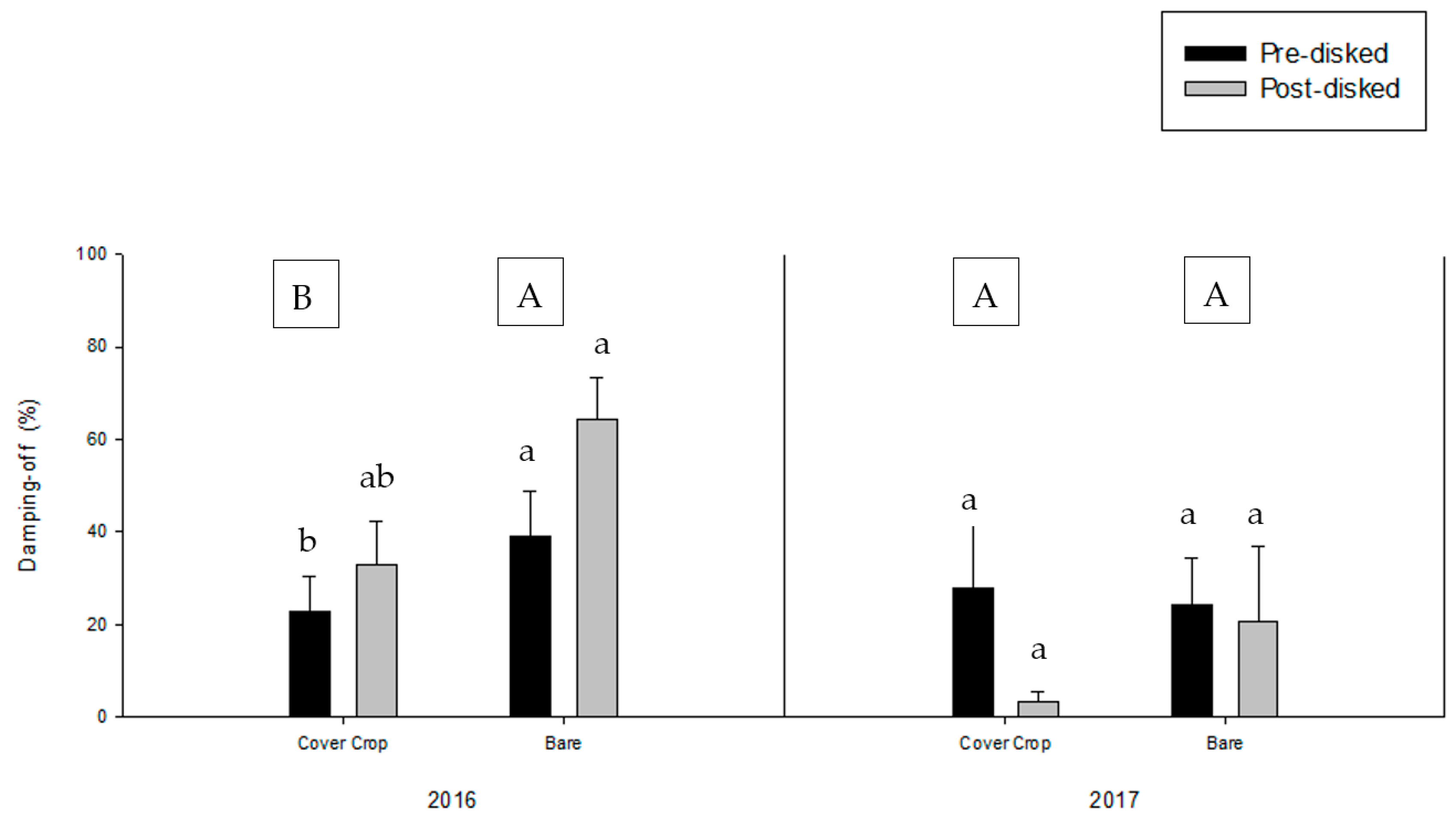
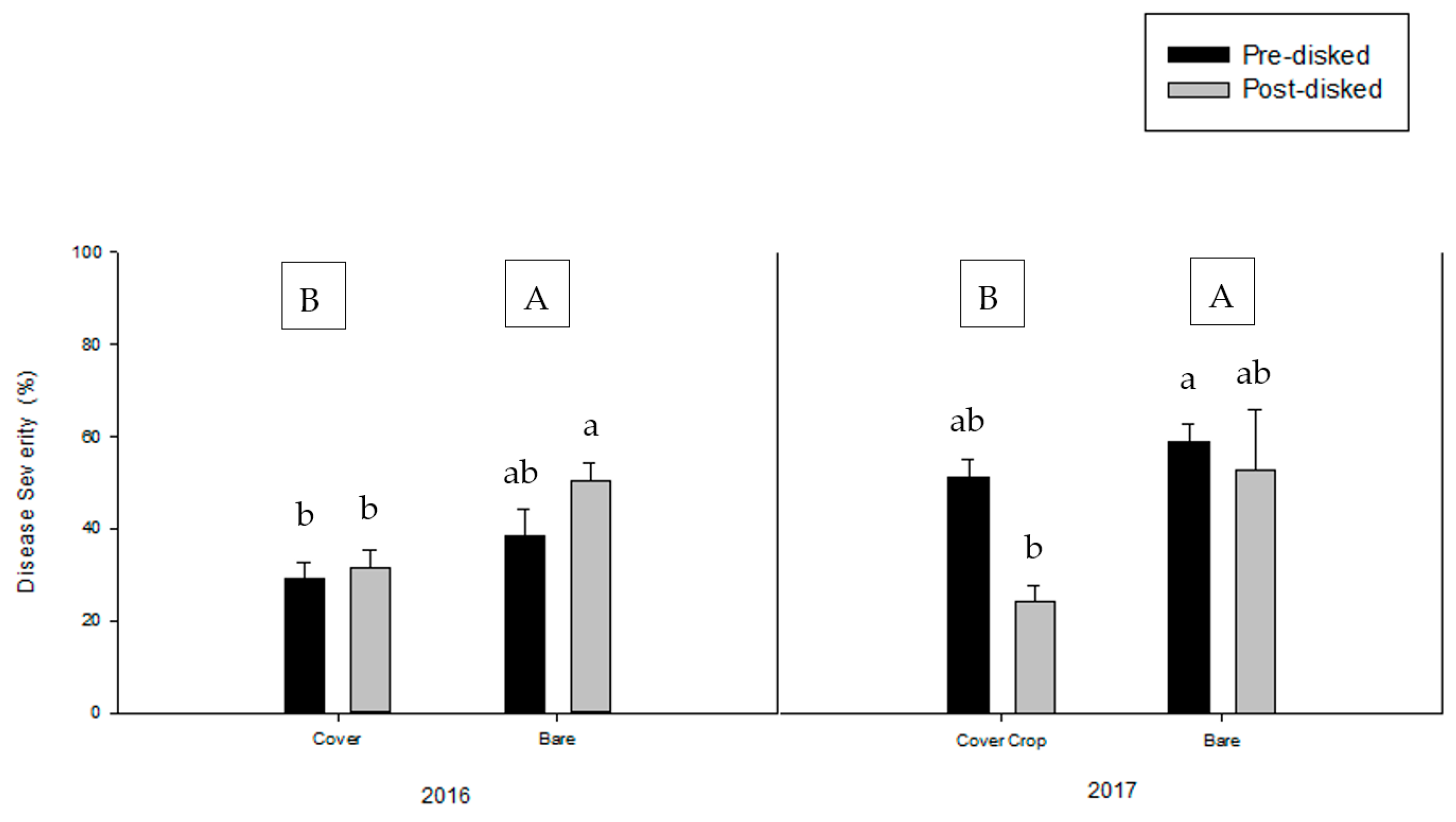
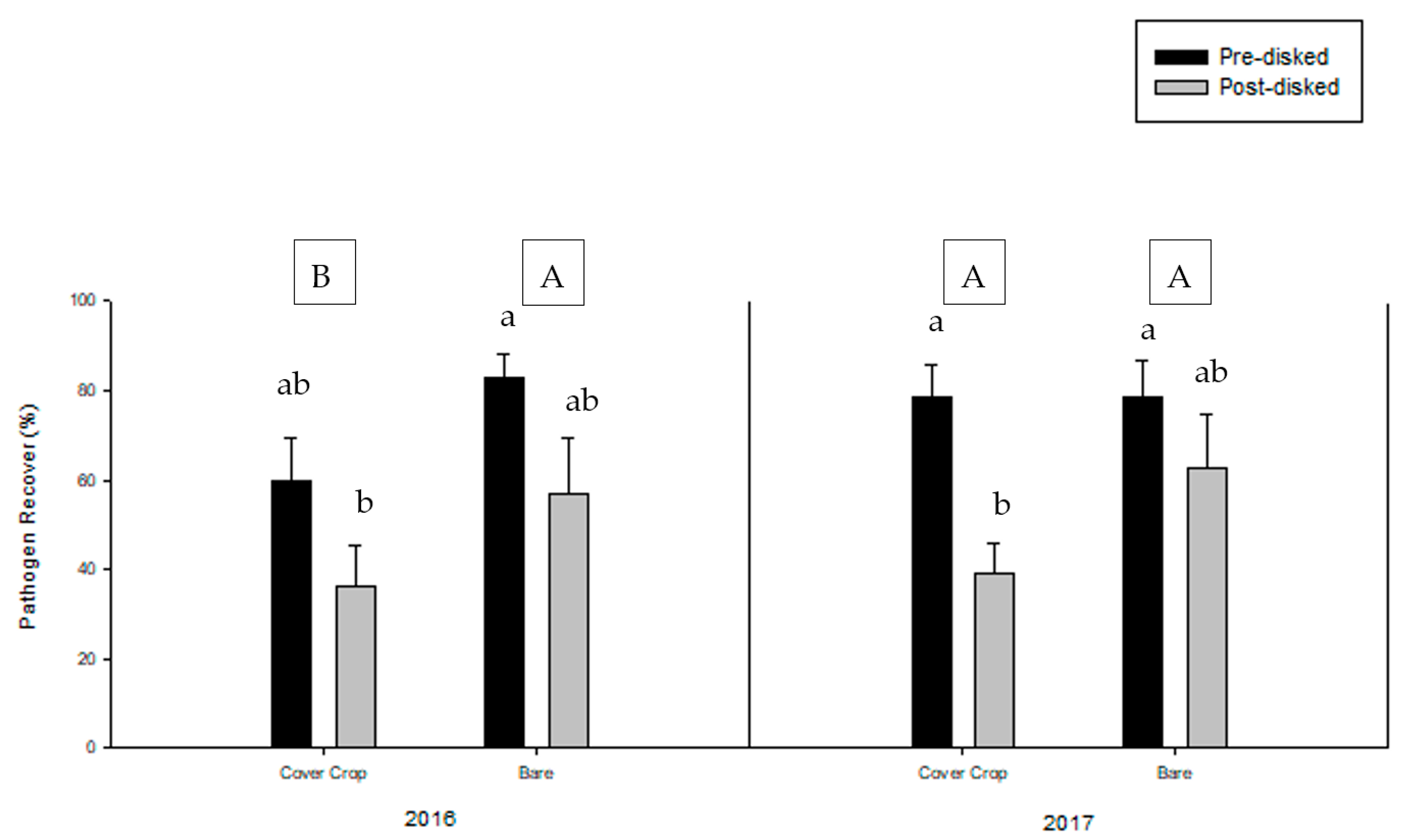
3.2. Greenhouse Bioassay with Rhizoctonia solani
3.3. Greenhouse Bioassay with Phytophthora nicotianae
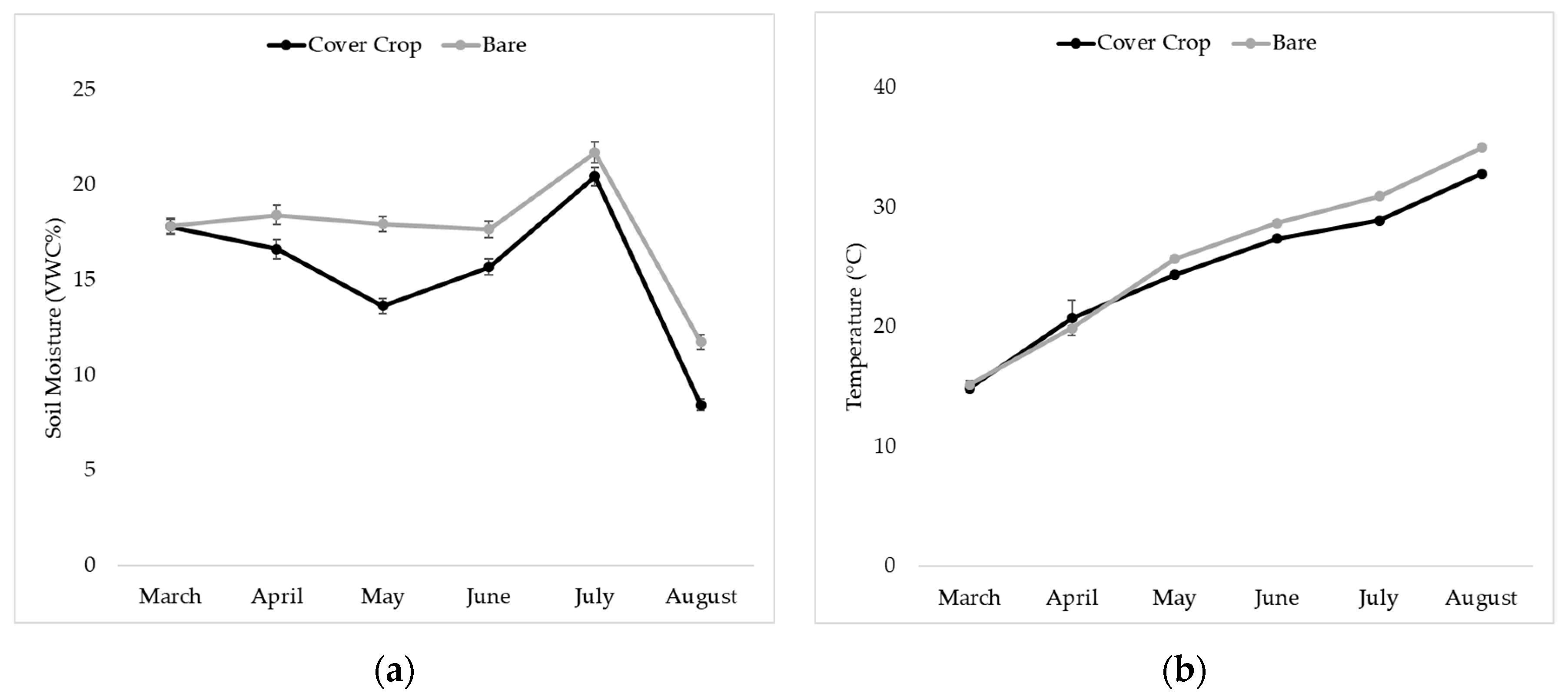
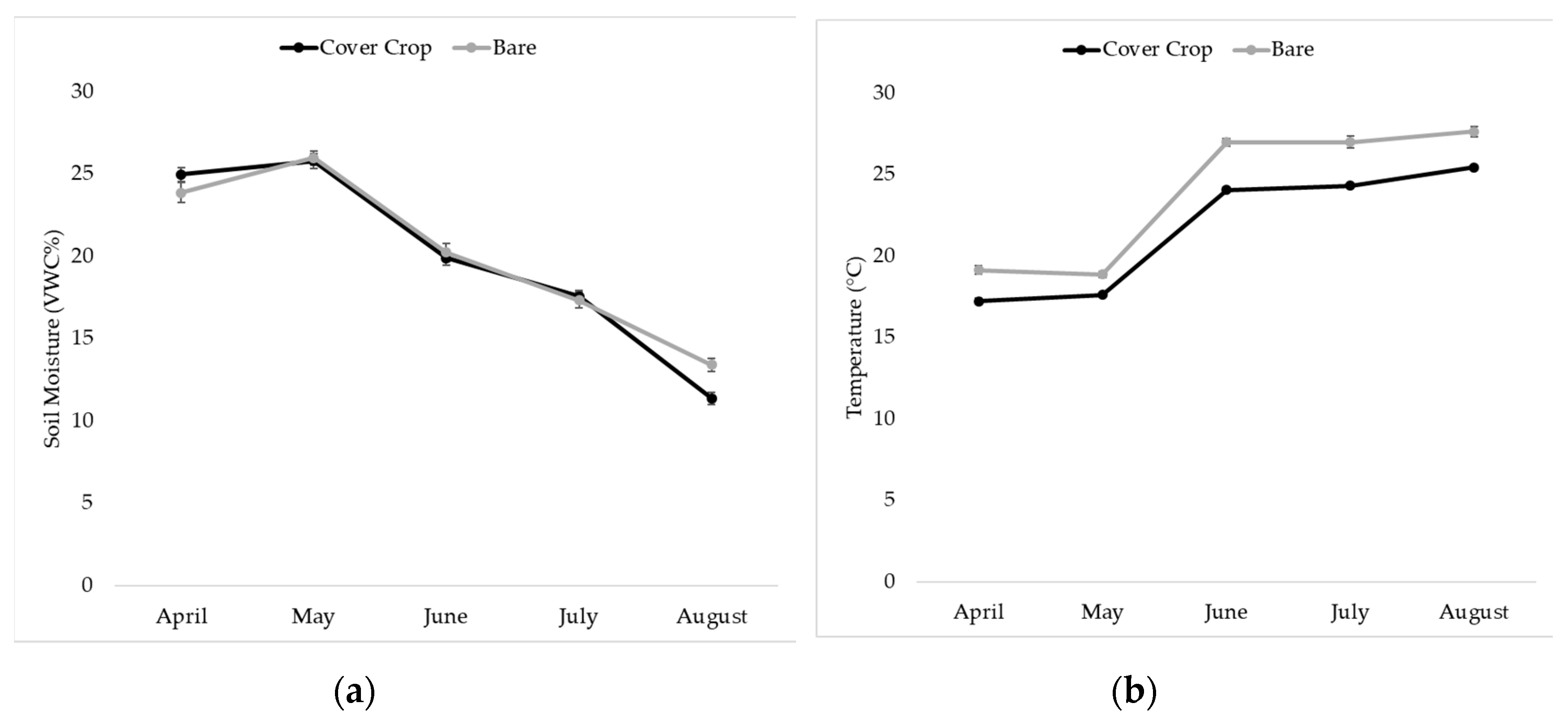
3.4. Soil Moisture and Temperature
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USDA. Census of Horticultural Specialties. Nursery Stock Sold. 2012 Census of Agriculture. 2016. Available online: https://www.nass.usda.gov/Publications/AgCensus/2012/Online_Resources/Census_of_Horticulture_Specialties/hortic_1_016_018.pdf (accessed on 14 October 2019).
- Gullino, M.L.; Garibaldi, A. Critical aspects in management of fungal diseases of ornamental plants and directions in research. Phytopathol. Mediterr. 2007, 46, 135–149. [Google Scholar]
- IPM. Pest Management Strategic Plan for Container and Field-Produced Nursery Crops. 2009. Available online: http://www.ipmcenters.org/pmsp/pdf/GA-KY-NC-SC TNnurserycropsPMSP.pdf (accessed on 18 September 2019).
- Mihajlović, M.; Rekanović, E.; Hrustić, J.; Tanović, B. Methods for management of soilborne plant pathogens. Pestic. Ifitomed. 2017, 32, 9–24. [Google Scholar] [CrossRef]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; American Phytopathological Society: St. Paul, MN, USA, 1996. [Google Scholar]
- Agrios, G. Plant Pathology, 5th ed.; Elsevier Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- Baysal-Gurel, F.; Liyanapathiranage, P.; Mullican, J. Biofumigation: Opportunities and challenges for control of soilborne diseases in nursery production. Plant Health Prog. 2018, 19, 332–337. [Google Scholar] [CrossRef]
- Cline, E.T.; Farr, D.F.; Rossman, A.Y. A synopsis of Phytophthora with accurate scientific names, host range, and geographic distribution. Plant Health Prog. 2008, 9, 32. [Google Scholar] [CrossRef]
- Benson, D.M.; Cartwright, D.K. Ornamental diseases incited by Rhizoctonia spp. In Rhizoctonia Species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control; Sneh, B., Jabaji-Hare, S., Neate, S.M., Dijst, G., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 303–314. [Google Scholar]
- Parry, D.W. Plant Pathology in Agriculture; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Triky-Dotan, S.; Westerdahl, B.; Martin, F.N.; Subbarao, K.; Koike, S.T.; Ajwa, H.A. Fumigant dosages below maximum label rate control some soilborne pathogens. Calif. Agric. 2016, 70, 130–136. [Google Scholar] [CrossRef][Green Version]
- Matthiessen, J.N.; Kirkegaard, J.A. Biofumigation and enhanced biodegradation: Opportunity and challenge in soilborne pest and disease management. Crit. Rev. Plant Sci. 2006, 25, 235–265. [Google Scholar] [CrossRef]
- Bowman, G.; Shirley, C.; Cramer, C. Managing cover crops profitably. In Sustainable Agric. Network Handb. Ser. Book 3, 2nd ed.; Sustainable Agric Network, Natl Agric Library: Beltsville, MD, USA, 1998; pp. 1–112. [Google Scholar]
- Vukicevich, E.; Lowery, T.; Bowen, P.; Úrbez-Torres, J.R.; Hart, M. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron. Sustain. Dev. 2016, 36, 48. [Google Scholar] [CrossRef]
- Finney, D.M.; Buyer, J.S.; Kaye, J.P. Living cover crops have immediate impacts on soil microbial community structure and function. J. Soil Water Conserv. 2017, 72, 361–373. [Google Scholar] [CrossRef]
- Wu, H.S.; Yang, X.N.; Fan, J.Q.; Miao, W.G.; Ling, N.; Xu, Y.C.; Huang, Q.W.; Shen, Q.R. Suppression of Fusarium wilt of watermelon by a bio-organic fertilizer containing combinations of antagonistic microorganisms. Biocontrol 2009, 54, 287–300. [Google Scholar] [CrossRef]
- Ling, N.; Xue, C.; Huang, Q.W.; Yang, X.M.; Xu, Y.C.; Shen, Q.R. Development of a mode of application of bioorganic fertilizer for improving the biocontrol efficacy to Fusarium wilt. Biocontrol 2010, 55, 673–683. [Google Scholar] [CrossRef]
- Chen, L.H.; Yang, X.M.; Raza, W.; Luo, J.; Zhang, F.G.; Shen, Q.R. Solid-state fermentation of agro-industrial wastes to produce bioorganic fertilizer for the biocontrol of Fusarium wilt of cucumber in continuously cropped soil. Bioresour. Technol. 2011, 102, 3900–3910. [Google Scholar] [CrossRef] [PubMed]
- Couillerot, O.; Prigent-Combaret, C.; Caballero-Mellado, J.; Moënne-Loccoz, Y. Pseudomonas fluorescens and closely-related fluorescent pseudomonads as biocontrol agents of soil-borne phytopathogens. Lett. Appl. Microbiol. 2009, 48, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Panpatte, D.G.; Jhala, Y.K.; Shelat, H.N.; Vyas, R.V. Pseudomonas Fluorescens: A Promising Biocontrol Agent and PGPR for Sustainable Agriculture. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New Delhi, India, 2016; pp. 257–270. [Google Scholar]
- Le, C.N.; Hoang, T.K.; Thai, T.H.; Tran, T.L.; Phan, T.P.N.; Raaijmakers, J.M. Isolation, characterization and comparative analysis of plant-associated bacteria for suppression of soil-borne diseases of field-grown groundnut in Vietnam. Biol. Control 2018, 121, 256–262. [Google Scholar] [CrossRef]
- Suslow, T.V. Role of root colonizing bacteria in plant growth. In Phytopathogenic Prokaryotes; Mount, M.S., Lacy, G.H., Eds.; Academic Press: London, UK, 1982; Volume 1, pp. 187–223. [Google Scholar]
- Suslow, T.V.; Schroth, M.N. Rhizobacteria of sugarbeets: Effects of seed application and root colonization on yield [Fungal and bacterial phytopathogens]. Phytopathology 1982, 77, 199–206. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Schroth, M.N.; Miller, T.D. Effects of rhizosphere colonization by plant growth-promoting rhizobacteria on potato plant development and yield. Phytopathology 1980, 70, 1078–1082. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Hume, D.J.; Scher, F.M.; Singleton, C.; Tipping, B.; Laliberte, M.; Frauley, K.; Kutchaw, T.; Simonson, C.; Lifshitz, R.; et al. Plant growth-promoting rhizobacteria on canola (rapeseed). Plant Dis. 1988, 72, 42–46. [Google Scholar] [CrossRef]
- Cook, R.J. Assuring the safe use of microbial biocontrol agents: A need for policy based on real rather than perceived risks. Can. J. Plant Pathol. 1996, 18, 439–445. [Google Scholar] [CrossRef]
- Weller, D.M. Biological-control of soilborne plant-pathogens in the rhizosphere with bacteria. Annu. Rev. Phytopathol. 1988, 26, 379–407. [Google Scholar] [CrossRef]
- Weller, D.M. Pseudomonas biocontrol agents of soilborne pathogens: Looking back over 30 years. Phytopathology 2007, 97, 250–256. [Google Scholar] [CrossRef]
- Agrawal, T.; Kotasthane, A.S.; Kosharia, A.; Kushwah, R.; Zaidi, N.W.; Singh, U.S. Crop specific plant growth promoting effects of ACCd enzyme and siderophore producing and cynogenic fluorescent Pseudomonas. 3 Biotech 2017, 7, 27. [Google Scholar]
- Dawadi, S. Cover Crop Usage for Pest Management in Red Maple Tree Production Systems. Master’s Thesis, Tennessee State University, Nashville, TN, USA, 2017. [Google Scholar]
- Dawadi, S.; Oliver, J.B.; O’Neal, P.A.; Addesso, K.M. Impact of cover cropping on non-target arthropod pests of red maple trees in nursery production. Fla. Entomol. 2019, 102, 187–193. [Google Scholar]
- Dawadi, S.; Oliver, J.B.; O’Neal, P.; Addesso, K.M. Management of flatheaded appletree borer (Chrysobothris femorata Olivier) in woody ornamental nursery production with a winter cover crop. Pest Manag. Sci. 2019, 75, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Yeager, T.; Bilderback, T.; Fare, D.; Gilliam, C.; Lea-Cox, J.; Niemiera, A.; Ruter, J.; Tilt, K.; Warren, S.; Whitwell, T.; et al. Best Management Practices: Guide for Producing Nursery Crops; Southern Nursery Assoc.: Atlanta, GA, USA, 2007. [Google Scholar]
- Ferguson, A.J.; Jeffers, S.N. Detecting multiple species of Phytophthora in container mixes from ornamental crop nurseries. Plant Dis. 1999, 83, 1129–1136. [Google Scholar] [CrossRef]
- Holmes, K.A.; Benson, D.M. Evaluation of Phytophthora parasitica var. nicotianae for biocontrol of Phytophthora parasitica on Catharanthus roseus. Plant Dis. 1994, 78, 193–199. [Google Scholar] [CrossRef]
- Baysal-Gurel, F.; Simmons, T.; Liyanapathiranage, P.; Kabir, M.N. Evaluation of Biorational Products and Fungicides for the Control of Rhizoctonia Root Rot of Viburnum; Plant Disease Management Report No. 11:OT003; Online publication; American Phytopathological Society: St. Paul, MN, USA, 2017. [Google Scholar]
- Gutierrez, W.A.; Shew, H.D.; Melton, T.A. A Semi-Selective Medium to Isolate Rhizoctonia Solani from Soil and Tissue; Plant Path. Extension, NC State Extension: Raleigh, NC, USA, 2001; pp. 1–2. [Google Scholar]
- Gould, W.D.; Hagedorn, C.; Bardinelli, T.R.; Zablotowicz, R.M. New selective media for enumeration and recovery of fluorescent Pseudomonad from various habitats. Appl. Environ. Microbiol. 1985, 49, 28–32. [Google Scholar]
- Langdale, G.W.; Blevins, R.L.; Karlen, D.L.; McCool, D.K.; Nearing, M.A.; Skidmore, E.L.; Williams, J.R. Cover crop effects on soil erosion by wind and water. In Proceedings of the Cover Crops for Clean Water, Jackson, TN, USA, 9–11 April 1991; pp. 15–22. [Google Scholar]
- Gómez, J.A.; Campos, M.; Guzmán, G.; Castillo-Llanque, F.; Vanwalleghem, T.; Lora, Á.; Giráldez, J.V. Soil erosion control, plant diversity, and arthropod communities under heterogeneous cover crops in an olive orchard. Environ. Sci. Pollut. Res. 2018, 25, 977–989. [Google Scholar] [CrossRef]
- Kunz, C.; Sturm, D.J.; Varnholt, D.; Walker, F.; Gerhards, R. Allelopathic effects and weed suppressive ability of cover crops. Plant Soil Environ. 2016, 62, 60–66. [Google Scholar]
- Madsen, H.; Talgre, L.; Eremeev, V.; Alaru, M.; Maeorg, E.; Luik, A. Winter cover crops decrease weediness in organic cropping systems. In Proceedings of the NJF Seminar 495-4th organic Conference: Organics for Tomorrow’s Food Systems, Mikkeli, FL, USA, 19–21 June 2017; Volume 13, pp. 35–37. [Google Scholar]
- Crusciol, C.A.; Nascente, A.S.; Borghi, E.; Soratto, R.P.; Martins, P.O. Improving soil fertility and crop yield in a tropical region with palisadegrass cover crops. Agron. J. 2015, 107, 2271–2280. [Google Scholar] [CrossRef]
- Tiecher, T.; Calegari, A.; Caner, L.; dos Santos Rheinheimer, D. Soil fertility and nutrient budget after 23-years of different soil tillage systems and winter cover crops in a subtropical Oxisol. Geoderma 2017, 308, 78–85. [Google Scholar] [CrossRef]
- Ristaino, J.B.; Parra, G.; Campbell, C.L. Suppression of Phytophthora blight in bell pepper by a no-till wheat cover crop. Phytopathology 1997, 87, 242–249. [Google Scholar] [CrossRef]
- Ristaino, J.B.; Johnston, S.A. Ecologically based approaches to management of Phytophthora blight on bell pepper. Plant Dis. 1999, 83, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Hausbeck, M.K.; Lamour, K.H. Phytophthora capsici on vegetable crops: Research progress and management challenges. Plant Dis. 2004, 88, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, U.; Morra, M.J.; Knudsen, G.R.; James, R.L. Isothiocyanates produced by Brassicaceae species as inhibitors of Fusarium oxysporum. Plant Dis. 2003, 87, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Lee-Marzano, S.; Ortiz-Ribbing, L.M.; Gruver, J.; Hartman, G.L.; Eastburn, D.M. Suppression of soilborne diseases of soybean with cover crops. Plant Dis. 2017, 101, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Baysal-Gurel, F.; Kabir, M.N.; Liyanapathiranage, P. Effect of organic inputs and solarization for the suppression of Rhizoctonia solani in woody ornamental plant production. Plants 2019, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Antignani, V.; Pane, C.; Scala, F. Suppression of soilborne fungal diseases with organic amendments. J. Plant Pathol. 2007, 89, 311–324. [Google Scholar]
- Sanford, G.B. Studies on Rhizoctonia solani kühn.: IV. Effect of soil temperature and moisture on virulence. Can. J. Res. 1938, 16c, 203–213. [Google Scholar] [CrossRef]
- Schnürer, J.; Clarholm, M.; Boström, S.; Rosswall, T. Effects of moisture on soil microorganisms and nematodes: A field experiment. Microb. Ecol. 1996, 12, 217–230. [Google Scholar] [CrossRef]
- Banerjee, S.; Helgason, B.; Wang, L.; Winsley, T.; Ferrari, B.C.; Siciliano, S.D. Legacy effects of soil moisture on microbial community structure and N2O emissions. Soil Biol. Biochem. 2016, 95, 40–50. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Soil moisture as a factor affecting the microbiological and biochemical activity of soil. Plant Soil Environ. 2016, 62, 250–255. [Google Scholar] [CrossRef]
- Zhou, W.P.; Shen, W.J.; Li, Y.E.; Hui, D.F. Interactive effects of temperature and moisture on composition of the soil microbial community. Eur. J. Soil Sci. 2017, 68, 909–918. [Google Scholar] [CrossRef]
- Dorrance, A.E.; Kleinhenz, M.D.; McClure, S.A.; Tuttle, N.T. Temperature, moisture, and seed treatment effects on Rhizoctonia solani root rot of soybean. Plant Dis. 2003, 87, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Gravuer, K.; Bossange, A.V.; Mitchell, J.; Scow, K. Long-term use of cover crops and no-till shift soil microbial community life strategies in agricultural soil. PLoS ONE 2018, 13, e0192953. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.L.; Lazarovits, G. Suppressing soilborne diseases with residue management and organic amendments. Soil Tillage Res. 2003, 72, 169–180. [Google Scholar] [CrossRef]
- Blok, W.J.; Lamers, J.G.; Termorshuizen, A.J.; Bollen, G.J. Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 2000, 90, 253–259. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Capodilupo, M.; Scala, F. Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biol. Biochem. 2010, 42, 136–144. [Google Scholar] [CrossRef]
- Mitsuboshi, M.; Kioka, Y.; Noguchi, K.; Asakawa, S. Evaluation of suppressiveness of soils exhibiting soil-borne disease suppression after long-term application of organic amendments by the co-cultivation method of pathogenic Fusarium oxysporum and indigenous soil microorganisms. Microbes Environ. 2018, 33, 58–65. [Google Scholar] [CrossRef]
- Elad, Y.; Chet, I. Possible role of competition for nutrients in biocontrol of Pythium damping-off by bacteria. Phytopathology 1987, 77, 190–195. [Google Scholar] [CrossRef]
- Parke, J.L.; Rand, R.E.; Joy, A.E.; King, E.B. Biological-control of Pythium damping-off and Aphanomyces root-rot of peas by application of Pseudomonas cepacia or P. fluorescens to seed. Plant Dis. 1991, 75, 987–992. [Google Scholar] [CrossRef]
- Ramamoorthy, V.; Samiyappan, R. Induction of defense-related genes in Pseudomonas fluorescens treated chilli plants in response to infection by Colletotrichum Capsici. J. Mycol. Plant Pathol. 2001, 31, 146–155. [Google Scholar]
- Mathivanan, N.; Prabavathy, V.R.; Vijayanandraj, V.R. Application of talc formulations of Pseudomonas fluorescens Migula and Trichoderma viridae Pers. ex SF Gray decrease the sheath blight disease and enhance the plant growth and yield in rice. J. Phytopathol. 2005, 153, 697–701. [Google Scholar] [CrossRef]
- Marimuthu, S.; Ramamoorthy, V.; Samiyappan, R.; Subbian, P. Intercropping system with combined application of Azospirillum and Pseudomonas fluorescens reduces root rot incidence caused by Rhizoctonia bataticola and increases seed cotton yield. J. Phytopathol. 2013, 161, 405–411. [Google Scholar] [CrossRef]
- Mazzola, M.; Gu, Y.H. Impact of wheat cultivation on microbial communities from replant soils and apple growth in greenhouse trials. Phytopathology 2000, 90, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, M. Mechanisms of natural soil suppressiveness to soilborne diseases. Antonie Van Leeuwenhoek Int. J. Gener. Mol. Microbiol. 2002, 81, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Patkowska, E.; Blazewicz-Wozniak, M.; Konopinski, M.; Wach, D. The effect of cover crops on the fungal and bacterial communities in the soil under carrot cultivation. Plant Soil Environ. 2016, 62, 237–242. [Google Scholar] [CrossRef]
- Patkowska, E.; Konopinski, M. Effect of cover crops on the microorganisms communities in the soil under Scorzonera cultivation. Plant Soil Environ. 2013, 59, 460–464. [Google Scholar] [CrossRef]

| Treatment * | 2016 | 2017 | ||||
|---|---|---|---|---|---|---|
| Damping-off (%) | Pathogen Recovery of Rhizoctonia solani (%) | Pathogen Recovery of Phytophthora nicotianae (%) | Damping-off (%) | Pathogen Recovery of Rhizoctonia solani (%) | Pathogen Recovery of Phytophthora nicotianae (%) | |
| Cover crop | 10.1 ± 3.3 a ** | 12.0 ± 3.0 a | 9.5 ± 2.8 a | 5.3 ± 2.1 a | 10.0 ± 2.5 a | 7.0 ± 2.4 a |
| Bare | 15.1 ± 4.2 a | 13.0 ± 2.5 a | 13.5 ± 3.7 a | 11.4 ± 4.6 a | 9.0 ± 3.1 a | 11.0 ± 3.3 a |
| p-value | 0.367 | 0.799 | 0.393 | 0.194 | 0.806 | 0.342 |
| Treatment * | 2016 | 2017 | ||||
|---|---|---|---|---|---|---|
| Damping-off (%) | Pathogen Recovery of Rhizoctonia solani (%) | Pathogen Recovery of Phytophthora nicotianae (%) | Damping-off (%) | Pathogen Recovery of Rhizoctonia solani (%) | Pathogen Recovery of Phytophthora nicotianae (%) | |
| Pre-disked cover crop | 16.7 ± 6.0 a ** | 13.0 ± 4.5 a | 12.0 ± 4.7 a | 5.4 ± 3.2 a | 9.0 ± 4.6 a | 7.0 ± 4.0 a |
| Post-disked cover crop | 3.8 ± 2.7 b | 11.0 ± 4.1 a | 7.0 ± 3.0 a | 5.1 ± 2.8 a | 11.0 ± 2.3 a | 7.0 ± 3.0 a |
| Pre-disked bare | 10.5 ± 4.6 ab | 16.0 ± 4.7 a | 16.0 ± 6.3 a | 7.9 ± 3.7 a | 8.0 ± 5.1 a | 11.0 ± 6.1 a |
| Post-disked bare | 19.3 ± 6.7 a | 10.0 ± 2.6 a | 11.0 ± 4.1 a | 15.5 ± 9.1 a | 10.0 ± 3.7 a | 11.0 ± 2.3 a |
| p-value | 0.036 | 0.308 | 0.286 | 0.867 | 0.623 | 1.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawadi, S.; Baysal-Gurel, F.; Addesso, K.M.; Oliver, J.B.; Simmons, T. Impact of Cover Crop Usage on Soilborne Diseases in Field Nursery Production. Agronomy 2019, 9, 753. https://doi.org/10.3390/agronomy9110753
Dawadi S, Baysal-Gurel F, Addesso KM, Oliver JB, Simmons T. Impact of Cover Crop Usage on Soilborne Diseases in Field Nursery Production. Agronomy. 2019; 9(11):753. https://doi.org/10.3390/agronomy9110753
Chicago/Turabian StyleDawadi, Sujan, Fulya Baysal-Gurel, Karla M. Addesso, Jason B. Oliver, and Terri Simmons. 2019. "Impact of Cover Crop Usage on Soilborne Diseases in Field Nursery Production" Agronomy 9, no. 11: 753. https://doi.org/10.3390/agronomy9110753
APA StyleDawadi, S., Baysal-Gurel, F., Addesso, K. M., Oliver, J. B., & Simmons, T. (2019). Impact of Cover Crop Usage on Soilborne Diseases in Field Nursery Production. Agronomy, 9(11), 753. https://doi.org/10.3390/agronomy9110753





