Marker-Assisted Development of a Blue-Grained Substitution Line Carrying the Thinopyrum ponticum Chromosome 4Th(4D) in the Spring Bread Wheat Saratovskaya 29 Background
Abstract
1. Introduction
2. Materials and Methods
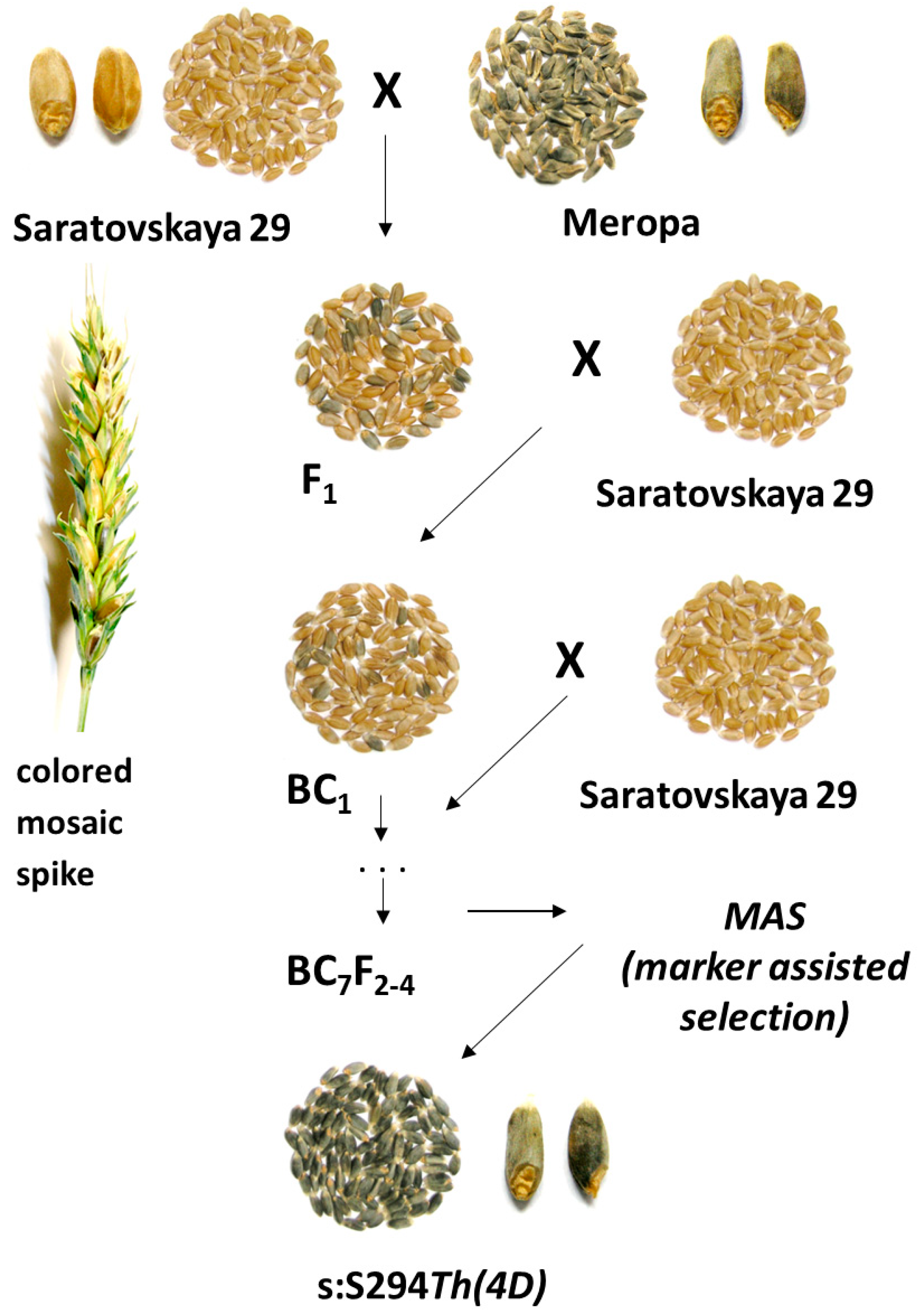
2.1. Plant Materials and Backcross Program
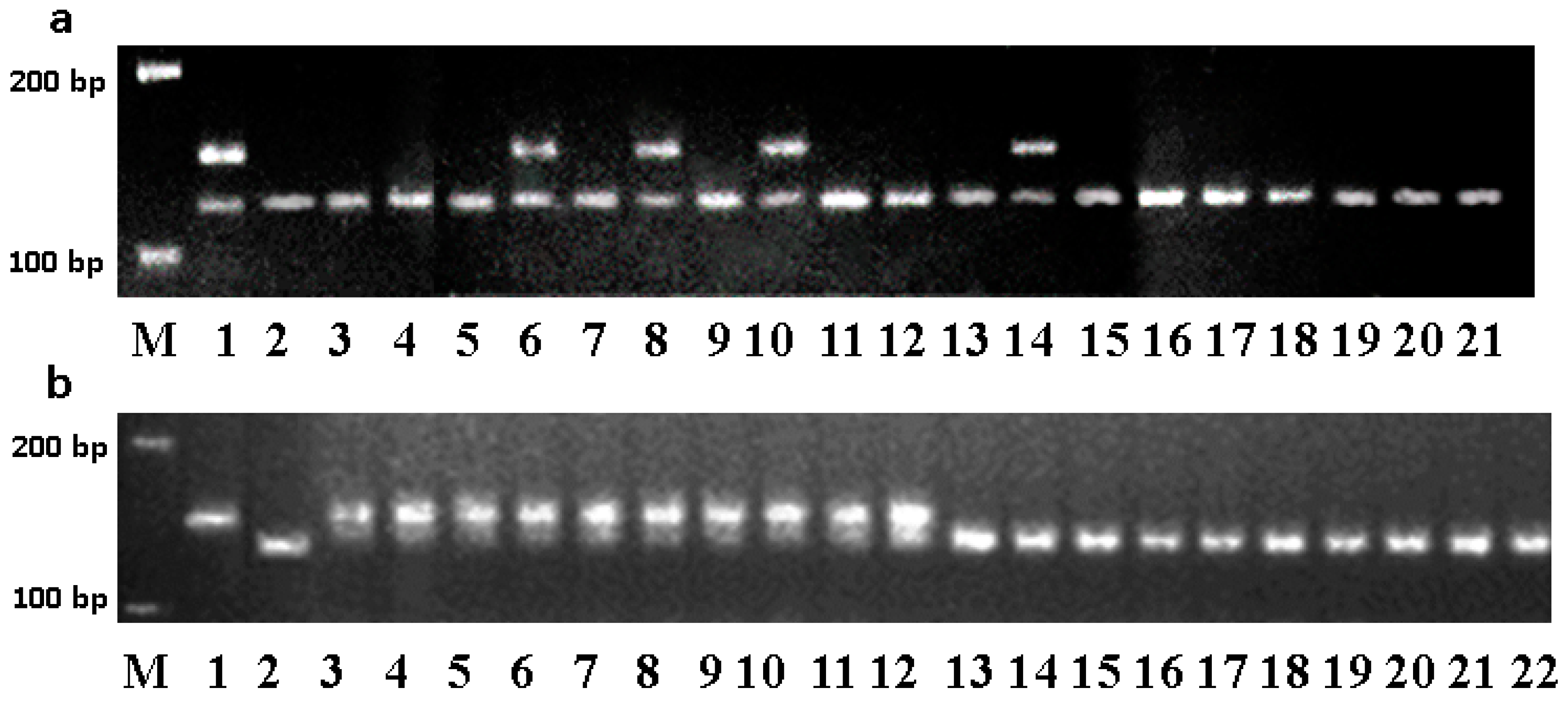
2.2. DNA Extraction and Microsatellite Analysis
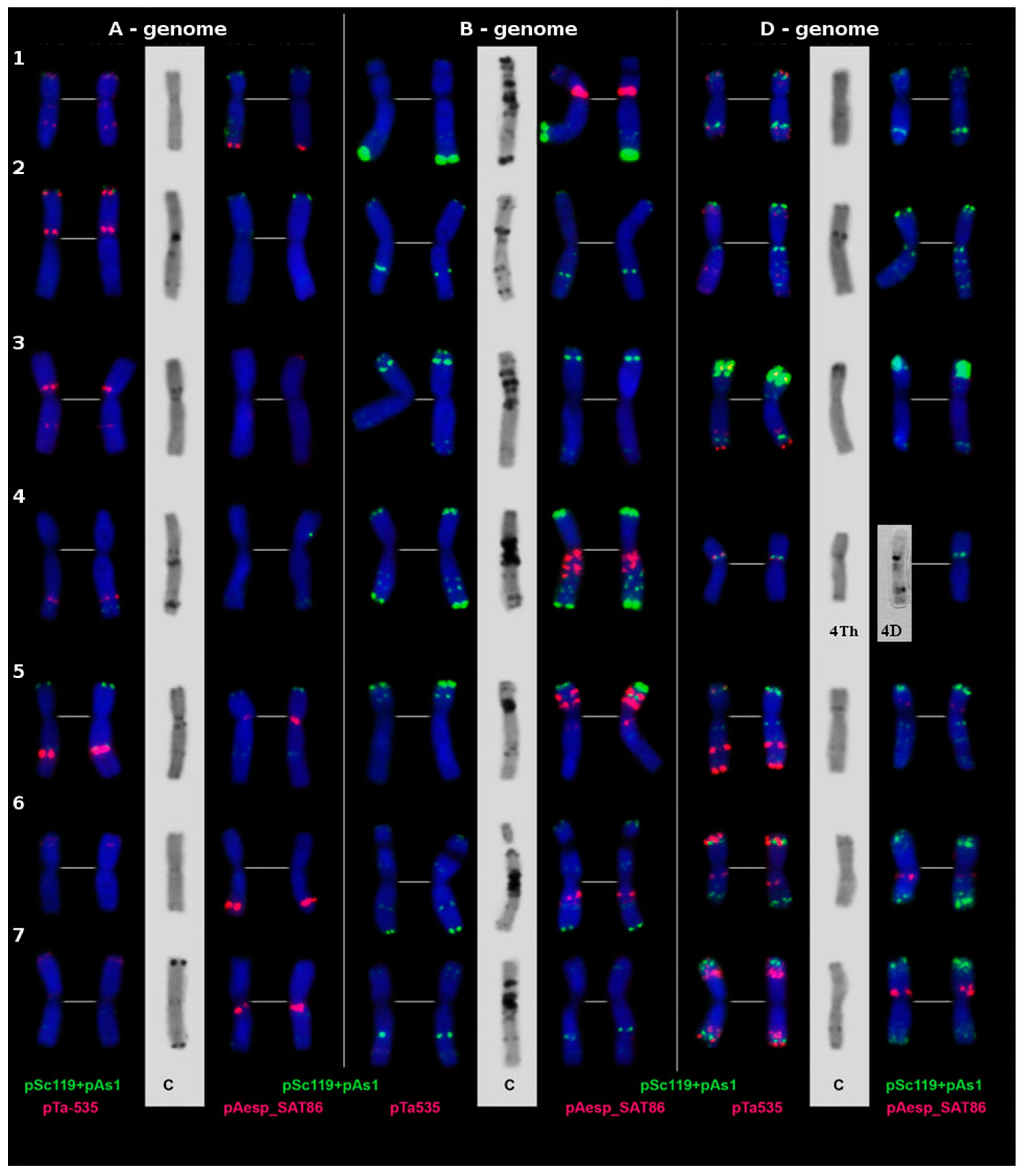
2.3. Cytological Examination
2.4. Analysis of Total Anthocyanin Content and Antioxidant Activity
2.5. Analysis of the Grain Quality
3. Results
3.1. Marker-Assisted Development of the Blue-Grained Line
3.2. Cytological Examination of the Developed Line
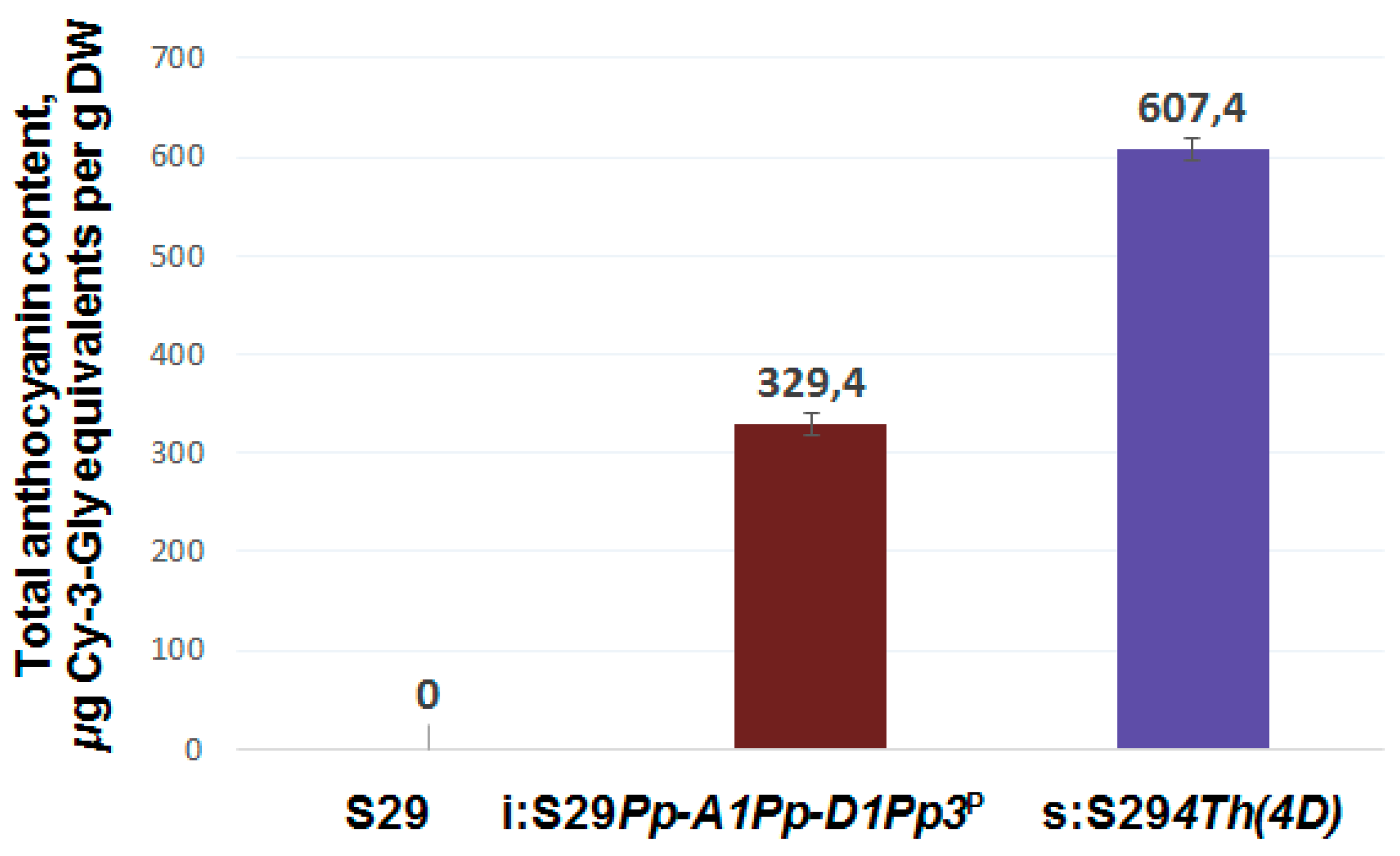
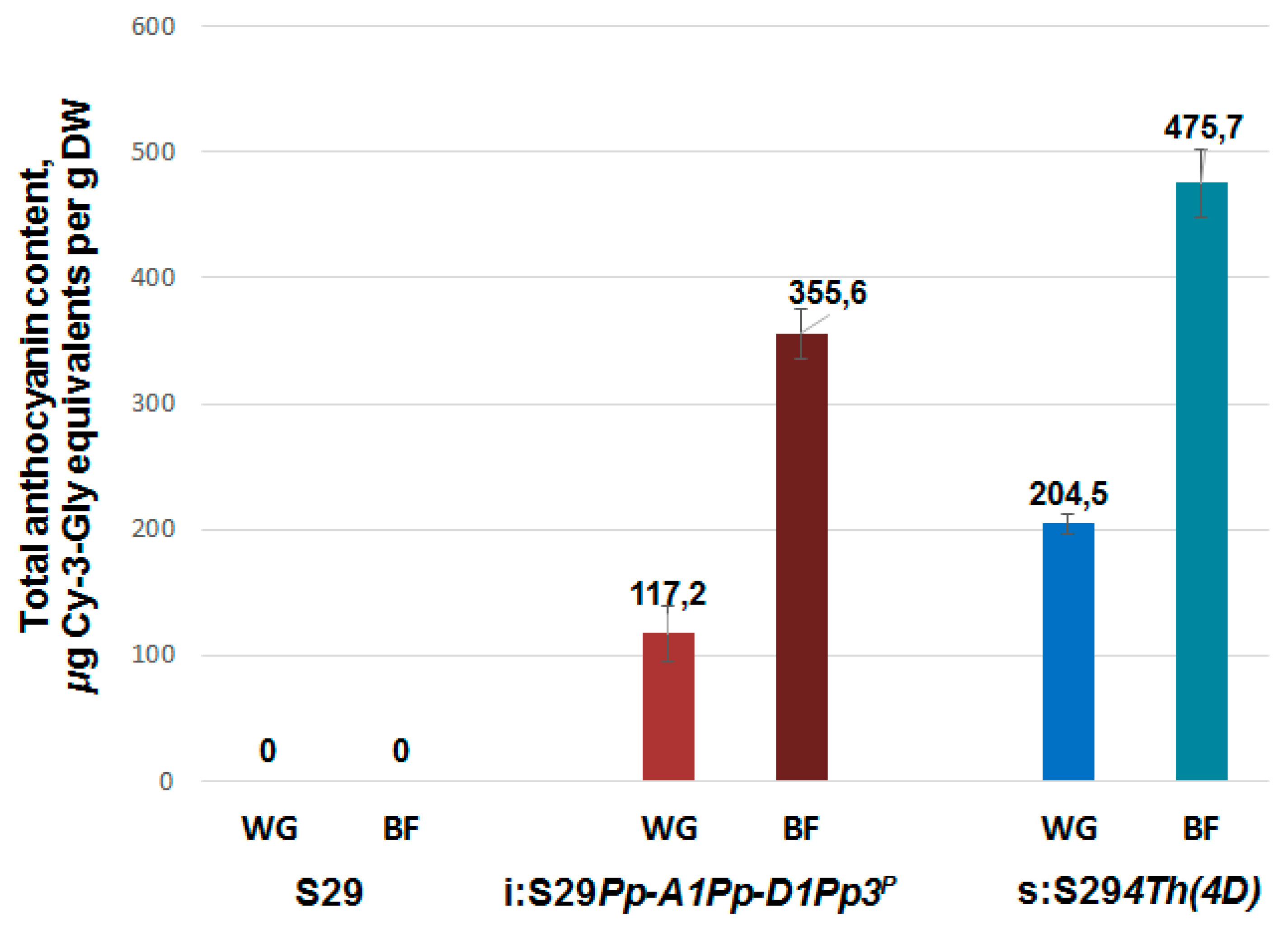
3.3. Total Anthocyanin Content in Whole Grains and Brans
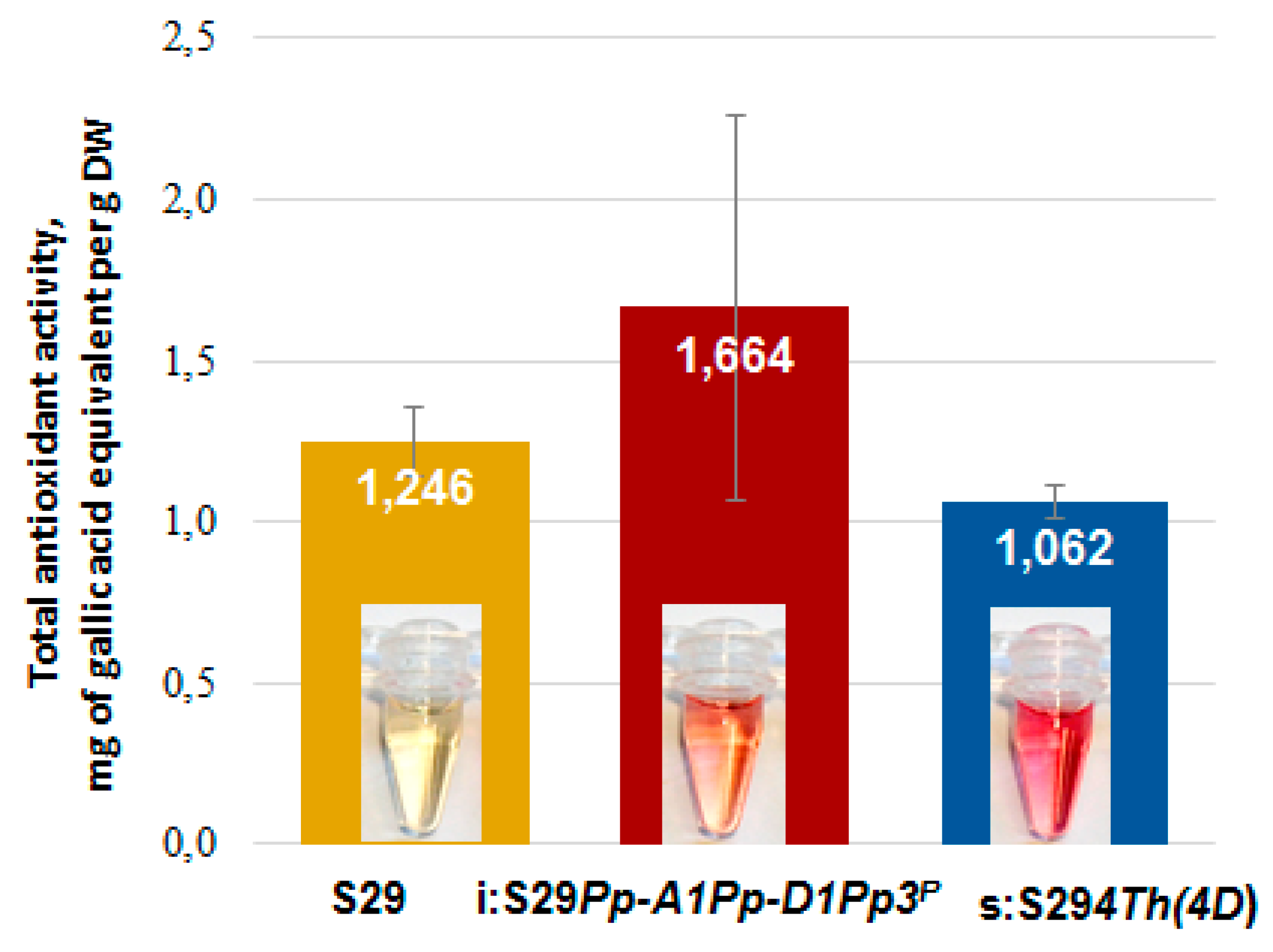
3.4. Antioxidant Activity of Bran Extracts
3.5. Analysis of Grain Quality and Other Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.; Rodrigo-García, J.; Martínez-Ruiz, N.; Cárdenas-Robles, A.; Mendoza-Díaz, S.; Alvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Anthocyanins in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Knievel, D.C.; Abdel-Aal, E.S.; Rabalski, I.; Nakamura, T.; Hucl, P. Grain color development and the inheritance of high anthocyanin blue aleurone and purple pericarp in spring wheat (Triticum aestivum L.). J. Cereal Sci. 2009, 50, 113–120. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; De Simone, V.; Colecchia, S.A.; Pecorella, I.; Platani, C.; Nigrom, F.; Finocchiaro, F.; Papa, R.; De Vita, P. Genetic variability in anthocyanin composition and nutritional properties of blue, purple, and red bread (Triticum aestivum L.) and durum (Triticum turgidum L. ssp. turgidum convar. durum) wheats. J. Agric. Food Chem. 2014, 62, 8686–8695. [Google Scholar] [CrossRef]
- Zeven, A.C. Wheats with purple and blue grains: A review. Euphytica 1991, 56, 243–258. [Google Scholar] [CrossRef]
- Dobrovolskaya, O.B.; Arbuzova, V.S.; Lohwasser, U.; Röder, M.S.; Börner, A. Microsatellite mapping of complementary genes for purple grain colour in bread wheat (Triticum aestivum L.). Euphytica 2006, 150, 355–364. [Google Scholar] [CrossRef]
- Khlestkina, E.K.; Röder, M.S.; Börner, A. Mapping genes controlling anthocyanin pigmentation on the glume and pericarp in tetraploid wheat (Triticum durum L.). Euphytica 2010, 171, 65–69. [Google Scholar] [CrossRef]
- Tereshchenko, O.; Gordeeva, E.; Arbuzova, V.; Börner, A.; Khlestkina, E. The D genome carries a gene determining purple grain colour in wheat. Cereal Res. Commun. 2012, 40, 334–341. [Google Scholar] [CrossRef]
- Gordeeva, E.I.; Shoeva, O.Y.; Khlestkina, E.K. Marker-assisted development of bread wheat near-isogenic lines carrying various combinations of purple pericarp (Pp) alleles. Euphytica 2015, 203, 469–476. [Google Scholar] [CrossRef]
- Shoeva, O.; Gordeeva, E.; Khlestkina, E. The regulation of anthocyanin synthesis in the wheat pericarp. Molecules 2014, 19, 20266–20279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, T.; Nan, W.; Jeewani, D.C.; Niu, Y.; Li, C.; Wang, Y.; Shi, X.; Wang, C.; Wang, J.; et al. Two transcription factors TaPpm1 and TaPpb1 co-regulate anthocyanin biosynthesis in purple pericarps of wheat. J. Exp. Bot. 2018, 69, 2555–2567. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Li, B.; Mu, S.; Zhou, H.; Li, Z. Physical mapping of the blue-grained gene(s) from Thinopyrum ponticum by GISH and FISH in a set of translocation lines with different seed colors in wheat. Genome 2006, 49, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Arbuzova, V.S.; Badaeva, E.D.; Efremova, T.T.; Osadchaya, T.S.; Trubacheeva, N.V.; Dobrovolskaya, O.B. A cytogenetic study of the blue-grain line of the common wheat cultivar Saratovskaya 29. Rus. J. Genet. 2012, 4, 785–791. [Google Scholar] [CrossRef]
- Liu, L.; Luo, Q.; Li, H.; Li, B.; Li, Z.; Zheng, Q. Physical mapping of the blue-grained gene from Thinopyrum ponticum chromosome 4Ag and development of blue-grain-related molecular markers and a FISH probe based on SLAF-seq technology. Theor. Appl. Genet. 2018, 131, 2359–2370. [Google Scholar] [CrossRef]
- Li, N.; Li, S.; Zhang, K.; Chen, W.; Zhang, B.; Wang, D.; Liu, D.; Liu, B.; Zhang, H. ThMYC4E, candidate Blue aleurone 1 gene controlling the associated trait in Triticum aestivum. PLoS ONE 2017, 12, e0181116. [Google Scholar] [CrossRef]
- Strygina, K.V.; Khlestkina, E.K. MYC gene family in cereals: Transformations during evolution of hexaploid bread wheat and its relatives. Mol. Biol. 2017, 51, 674–680. [Google Scholar] [CrossRef]
- Dubcovsky, J.; Luo, M.C.; Zhong, G.Y.; Bransteitter, R.; Desai, A.; Kilian, A.; Dvořák, J. Genetic map of diploid wheat, Triticum monococcum L., and its comparison with maps of Hordeum vulgare L. Genetics 1996, 143, 983–999. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1207354/ (accessed on 30 May 2019).
- Singh, K.; Ghai, M.; Garg, M.; Chhuneja, P.; Kaur, P.; Schnurbusch, T.; Keller, B.; Dhaliwal, H.S. An integrated molecular linkage map of diploid wheat based on a Triticum boeoticum× T. monococcum RIL population. Theor. Appl. Genet. 2007, 115, 301. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, J.; Zhuang, L.; Wang, Y.; Pu, J.; Feng, Y.; Qi, Z. Physical localization of a novel blue-grained gene derived from Thinopyrum bessarabicum. Mol. Breed. 2013, 31, 195–204. [Google Scholar] [CrossRef]
- Burešová, V.; Kopecký, D.; Bartoš, J.; Martinek, P.; Watanabe, N.; Vyhnánek, T.; Doležel, J. Variation in genome composition of blue-aleurone wheat. Theor. Appl. Genet. 2015, 128, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, Y.; Wang, R.R.C. Characterization of genomes and chromosomes in partial amphiploids of the hybrid Triticum aestivum × Thinopyrum ponticum by in situ hybridization, isozyme analysis, and RAPD. Genome 1996, 39, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Abou-Arab, A.A.; Gamel, T.H.; Hucl, P.; Young, J.C.; Rabalski, I. Fractionation of blue wheat anthocyanin compounds and their contribution to antioxidant properties. J. Agric. Food Chem. 2008, 56, 11171–11177. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Hucl, P.; Shipp, J.; Rabalski, I. Compositional Differences in Anthocyanins from Blue-and Purple-Grained Spring Wheat Grown in Four Environments in Central Saskatchewan. Cereal Chem. 2016, 93, 32–38. [Google Scholar] [CrossRef]
- Khlestkina, E.K.; Usenko, N.I.; Gordeeva, E.I.; Stabrovskaya, O.I.; Sharfunova, I.B.; Otmakhova, Y.S. Evaluation of wheat products with high flavonoid content: Justification of importance of marker-assisted development and production of flavonoid-rich wheat cultivars. Vavilov J. Genet. Breed. 2017, 21, 545–553. [Google Scholar] [CrossRef]
- Usenko, N.I.; Khlestkina, E.K.; Asavasanti, S.; Gordeeva, E.I.; Yudina, R.S.; Otmakhova, Y.S. Possibilities of enriching food products with anthocyanins by using new forms of cereals. Foods Raw Mater. 2018, 6, 128–135. [Google Scholar] [CrossRef]
- Plaschke, J.; Ganal, M.W.; Röder, M.S. Detection of genetic diversity in closely related bread wheat using microsatellite markers. Theor. Appl. Genet. 1995, 91, 1001–1007. [Google Scholar] [CrossRef]
- Röder, M.S.; Korzun, V.; Wendehake, K.; Plaschke, J.; Tixier, M.H.; Leroy, P.; Ganal, M.W. A microsatellite map of wheat. Genetics 1998, 149, 2007–2023. [Google Scholar]
- Ganal, M.W.; Röder, M.S. Microsatellite and SNP markers in wheat breeding. In Genomics-Assisted Crop Improvement; Springer: Dordrecht, Germany, 2007; pp. 1–24. [Google Scholar]
- Badaeva, E.D.; Badaev, N.S.; Filatenko, A.A. Intraspecific karyotype divergence in Triticum araraticum. Plant Syst. Evol. 1994, 192, 117–145. Available online: https://www.jstor.org/stable/23674610 (accessed on 30 May 2019). [CrossRef]
- Gill, B.S.; Friebe, B.; Endo, T.R. Standard karyotype and nomenclature system for description of chromosome bands and structural aberrations in wheat (Triticum aestivum). Genome 1991, 34, 830–839. [Google Scholar] [CrossRef]
- Friebe, B.; Gill, B.S. Chromosome banding and genome analysis in diploid and cultivated polyploid wheats. In Methods of genome analusis in plants; Jauhar, P.P., Ed.; CRC Press: Boca Raton, FL, USA, 1996; pp. 39–60. [Google Scholar]
- Badaeva, E.D.; Ruban, A.S.; Aliyeva-Schnorr, L.; Municio, C.; Hesse, S.; Houben, A. In situ hybridization to plant chromosomes. In Fluorescence in Situ Hybridization (FISH); Liehr, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 477–494. [Google Scholar]
- Bedbrook, R.J.; Jones, J.; O’Dell, M.; Thompson, R.J.; Flavell, R.B. A molecular description of telomeric heterochromatin in Secale species. Cell 1980, 19, 545–560. [Google Scholar] [CrossRef]
- Rayburn, A.L.; Gill, B.S. Isolation of a D-genome specific repeated DNA sequence from Aegilops squarrosa. Plant Mol. Biol. Rep. 1986, 4, 104–109. [Google Scholar] [CrossRef]
- Badaeva, E.D.; Amosova, A.V.; Goncharov, N.P.; Macas, J.; Ruban, A.S.; Grechishnikova, I.V.; Zoshchuk, S.A.; Houben, A. A set of cytogenetic markers allows the precise identification of all A-genome chromosomes in diploid and polyploid wheat. Cytogenet. Genome Res. 2015, 146, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Komuro, S.; Endo, R.; Shikata, K.; Kato, A. Genomic and chromosomal distribution patterns of various repeated DNA sequences in wheat revealed by a fluorescence in situ hybridization procedure. Genome 2013, 56, 131–137. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Z.; Fu, S. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J. Appl. Genet. 2014, 55, 313–318. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P. A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Yashin, Y.I.; Nemzer, B.V.; Ryzhnev, V.Y.; Yashin, A.Y.; Chernousova, N.I.; Fedina, P.A. Creation of a databank for content of antioxidants in food products by an amperometric method. Molecules 2010, 15, 7450–7466. [Google Scholar] [CrossRef]
- Sortoispytaniye zernovykh i krupyanykh kul’tur [Variety testing of cereals and groats]. In Metodika Gosudarstvennogo Sortoispytaniya Sel’skokhozyaystvennykh Kul’tur [Methods of State Variety Testing of Crops], 2nd ed.; Fedin, M.A., Ed.; Gosagroprom: Moscow, Russia, 1989; pp. 5–23. (in Russian) [Google Scholar]
- Griffin, W.B. Outcrossing in New Zealand wheats measured by occurrence of purple grain. N. Z. J. Agric. Res. 1987, 30, 287–290. [Google Scholar] [CrossRef]
- Martinek, P.; Škorpík, M.; Chrpová, J.; Fučík, P.; Schweiger, J. Development of the new wheat variety Skorpion with blue grain. Czech J. Genet. Plant Breed. 2013, 49, 90–94. [Google Scholar] [CrossRef]
- Musilová, M.; Trojan, V.; Vyhnánek, T.; Havel, L. Genetic variability for coloured caryopses in common wheat varieties determined by microsatellite markers. Czech J. Genet. Plant Breed. 2013, 49, 116–122. [Google Scholar] [CrossRef]
- Soliman, K.M.; Qualset, C.O. Evaluation of alien chromosome addition and recombinant isolines of wheat. Crop Sci. 1984, 24, 142–147. [Google Scholar] [CrossRef]
- Mu, S.M.; Li, Z.S.; Zhou, H.P.; Yu, L. Cytogenetic identification of blue-grained wheat. Acta Genet. Sin. 1986, 13, 259–261. [Google Scholar]
- Garg, M.; Chawla, M.; Chunduri, V.; Kumar, R.; Sharma, S.; Sharma, N.K.; Kaur, N.; Kumar, A.; Mundey, J.K.; Saini, M.K.; et al. Transfer of grain colors to elite wheat cultivars and their characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Siebenhandl, S.; Grausgruber, H.; Pellegrini, N.; Del Rio, D.; Fogliano, V.; Pernice, R.; Berghofer, E. Phytochemical profile of main antioxidants in different fractions of purple and blue wheat, and black barley. J. Agric. Food. Chem. 2007, 55, 8541–8547. [Google Scholar] [CrossRef]
- Syed Jaafar, S.N.; Baron, J.; Siebenhandl-Ehn, S.; Rosenau, T.; Böhmdorfer, S.; Grausgruber, H. Increased anthocyanin content in purple pericarp× blue aleurone wheat crosses. Plant Breed. 2013, 132, 546–552. [Google Scholar] [CrossRef]
- Shchukina, L.V.; Pshenichnikova, T.A.; Khlestkina, E.K.; Misheva, S.; Kartseva, T.; Abugalieva, A.; Börner, A. Chromosomal location and mapping of quantitative trait locus determining technological parameters of grain and flour in strong-flour bread wheat cultivar Saratovskaya 29. Cereal Res. Commun. 2018, 46, 628–638. [Google Scholar] [CrossRef]
| Chromosome | Markers |
|---|---|
| 2A | Xgwm: 0294, 0312, 0636 |
| 2D | Xgwm: 0261 |
| 4A | Xgwm: 0610, 1091 |
| 4B | Xgwm: 0066, 0251, 0910 |
| 4D | Xgwm: 0624, 1163, 1397, 1706, 3156, 4001, 4083, 4736 |
| 5A | Xgwm: 0291 |
| 5B | Xgwm: 0540 |
| 5D | Xgwm: 0174 |
| 7A | Xgwm: 0060, 0282, 0631, 0748 |
| 7B | Xgwm: 0046, 0537 |
| 7D | Xgwm: 0044, 0111, 0885, 1044 |
| Parameters | S29 | s:S294Th(4D) |
|---|---|---|
| 1000 grains weight, g | 25.7 ± 0.6 | 26.9 ± 4.6 |
| Grain vitreousness, % | 67.3 ± 15.2 | 60.8 ± 9.5 |
| Mean flour particle diameter, μm | 19.9 ± 1.6 | 20.8 ± 1.8 |
| Flour particle specific area, cm2/g | 1795.5 ± 166.2 | 1942.5 ± 163.3 * |
| Raw gluten content in grain, % | 36.8 ± 0.7 | 38.1 ± 1.9 |
| Flour strength, e.a. | 573.0 ± 84,9 | 414.0 ± 101.8 * |
| Dough tenacity, mm | 163.5 ± 0.7 | 143.0 ± 26.9 |
| Dough extensibility, mm | 80.5 ± 9.2 | 78.0 ± 12.7 |
| Test balance | 2.0 ± 0.2 | 1.9 ± 0.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordeeva, E.; Badaeva, E.; Yudina, R.; Shchukina, L.; Shoeva, O.; Khlestkina, E. Marker-Assisted Development of a Blue-Grained Substitution Line Carrying the Thinopyrum ponticum Chromosome 4Th(4D) in the Spring Bread Wheat Saratovskaya 29 Background. Agronomy 2019, 9, 723. https://doi.org/10.3390/agronomy9110723
Gordeeva E, Badaeva E, Yudina R, Shchukina L, Shoeva O, Khlestkina E. Marker-Assisted Development of a Blue-Grained Substitution Line Carrying the Thinopyrum ponticum Chromosome 4Th(4D) in the Spring Bread Wheat Saratovskaya 29 Background. Agronomy. 2019; 9(11):723. https://doi.org/10.3390/agronomy9110723
Chicago/Turabian StyleGordeeva, Elena, Ekaterina Badaeva, Rimma Yudina, Lyudmila Shchukina, Olesya Shoeva, and Elena Khlestkina. 2019. "Marker-Assisted Development of a Blue-Grained Substitution Line Carrying the Thinopyrum ponticum Chromosome 4Th(4D) in the Spring Bread Wheat Saratovskaya 29 Background" Agronomy 9, no. 11: 723. https://doi.org/10.3390/agronomy9110723
APA StyleGordeeva, E., Badaeva, E., Yudina, R., Shchukina, L., Shoeva, O., & Khlestkina, E. (2019). Marker-Assisted Development of a Blue-Grained Substitution Line Carrying the Thinopyrum ponticum Chromosome 4Th(4D) in the Spring Bread Wheat Saratovskaya 29 Background. Agronomy, 9(11), 723. https://doi.org/10.3390/agronomy9110723







