Understanding the Interactions between Biomass, Grain Production and Grain Protein Content in High and Low Protein Wheat Genotypes under Controlled Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions and N Treatment
2.2. Experimental Design
2.3. Red, Green, Blue (RGB) Image Capture and Image Analysis
2.4. N Measurements
2.5. Statistical Analysis
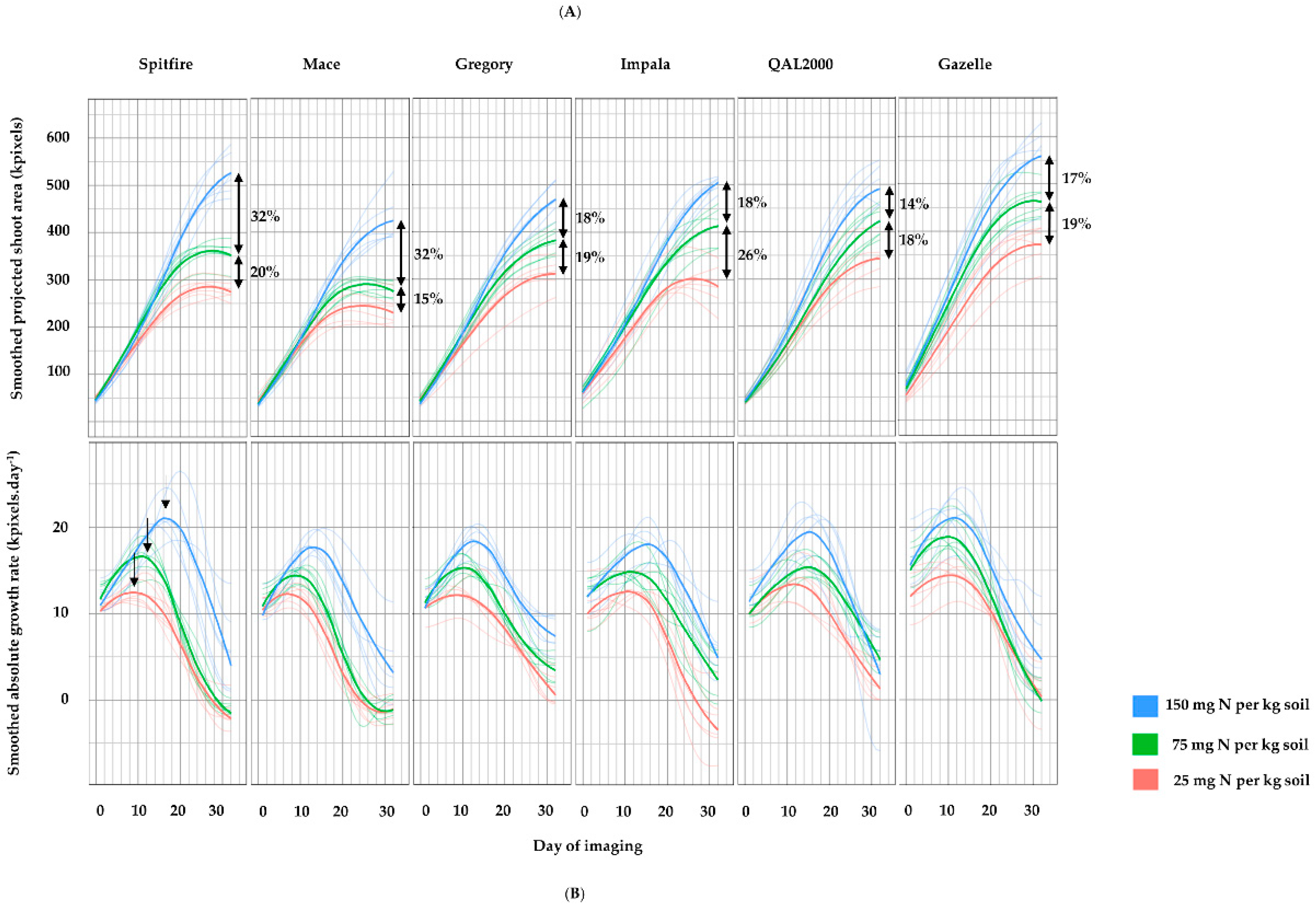
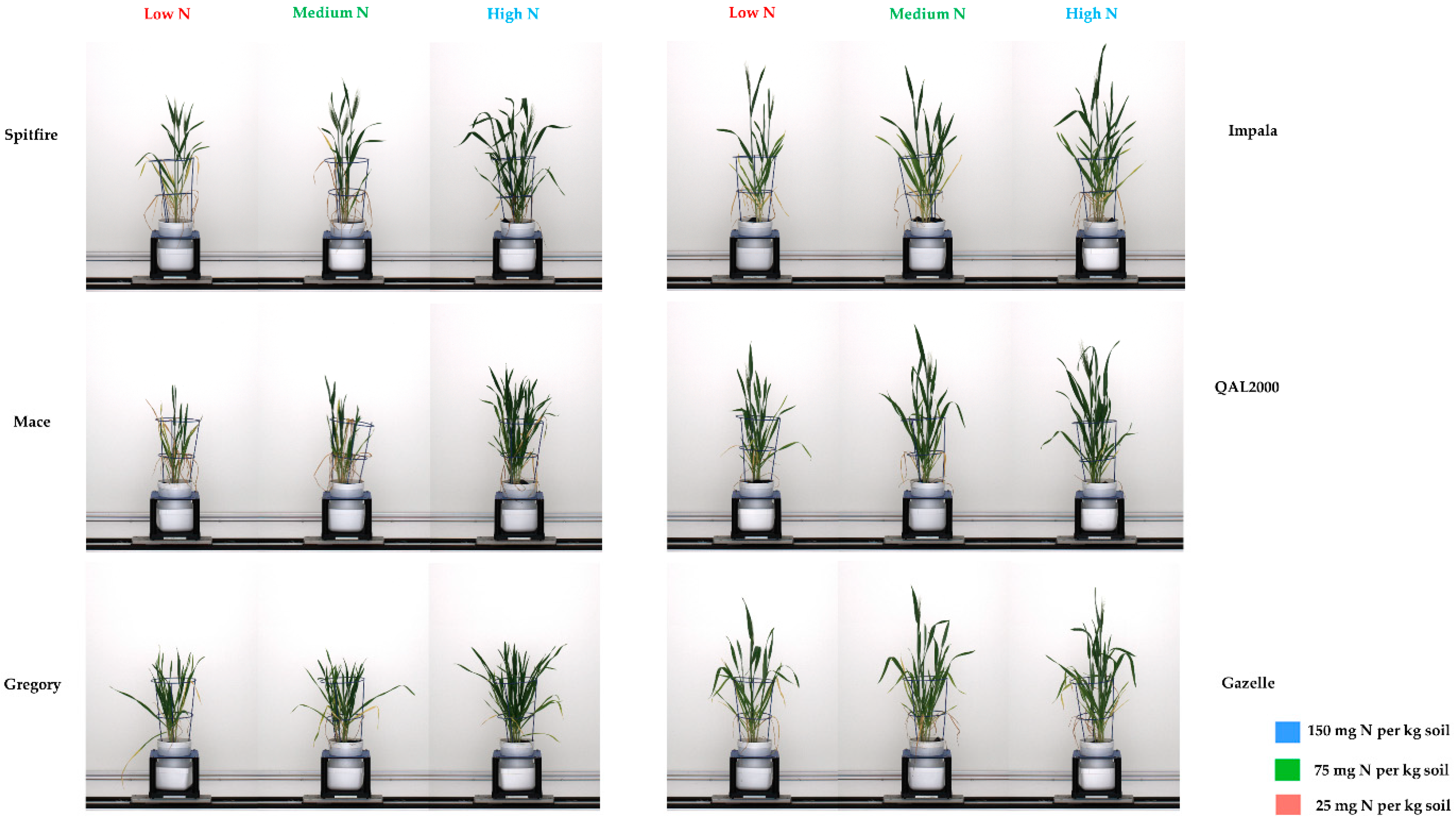
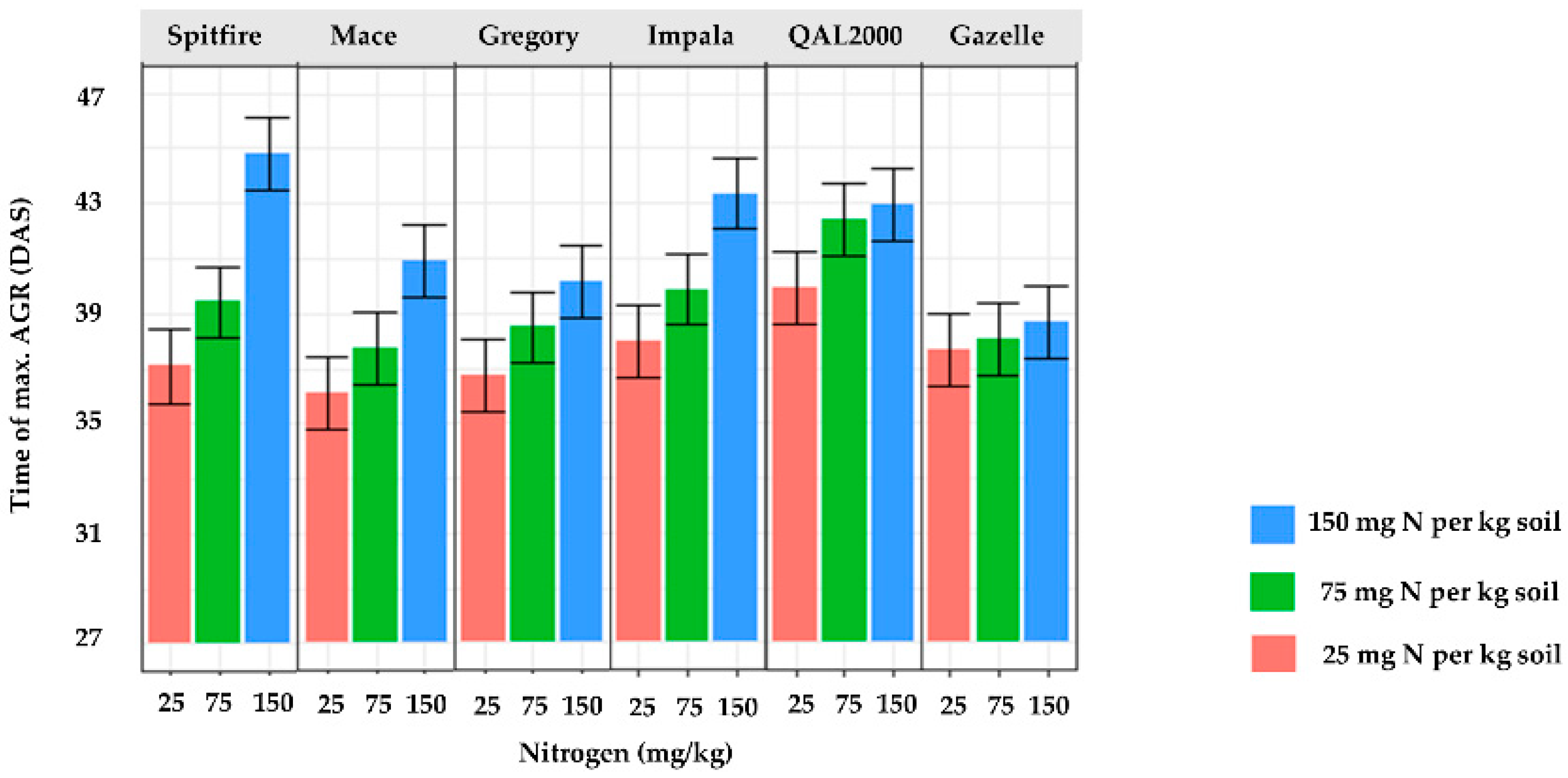
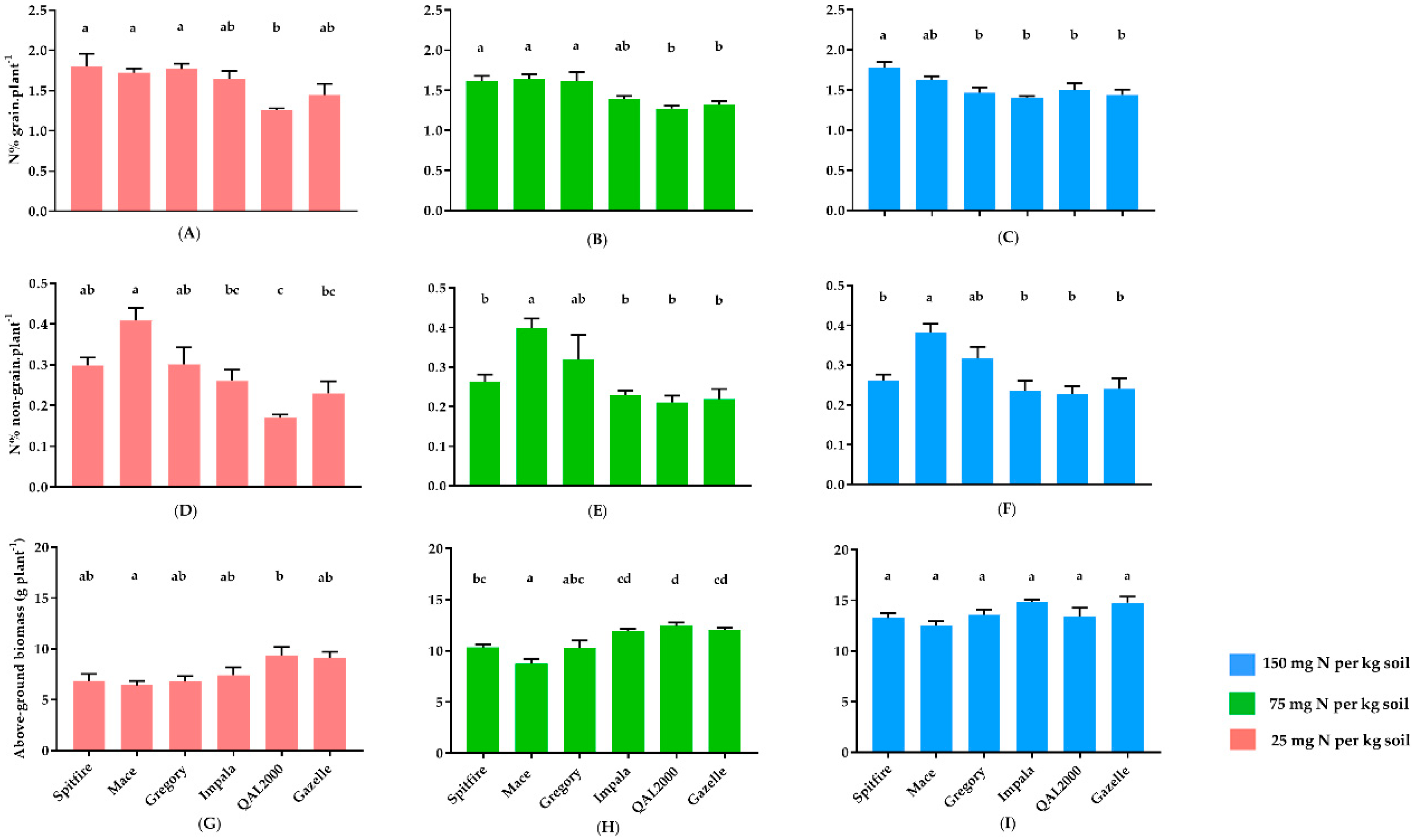
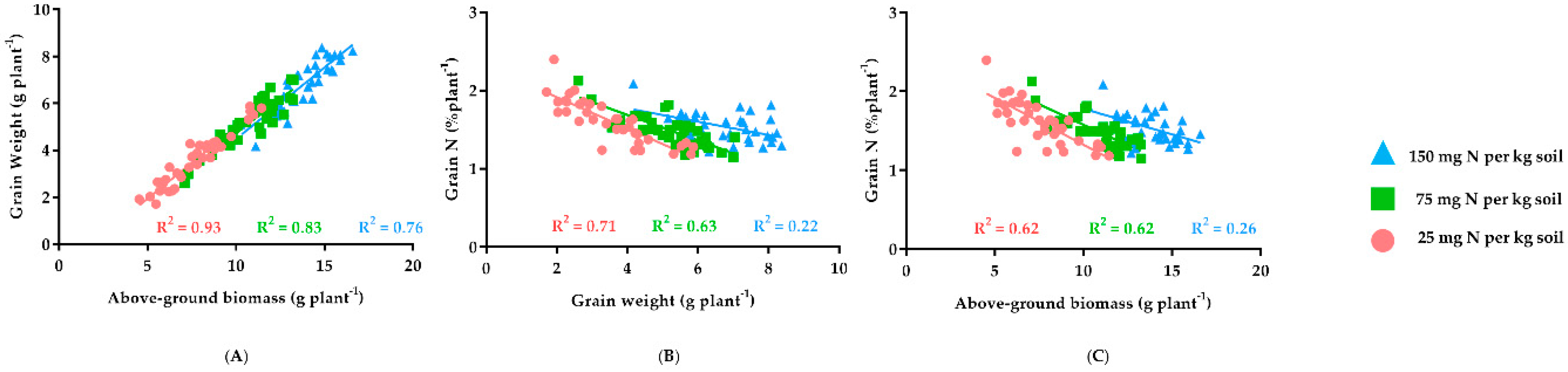
3. Results
4. Discussion
4.1. Biomass Can Indicate the Impact of Nitrogen on Grain Weight
4.2. Differences in Nitrogen Responsiveness between High and Low GPC Genotypes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGR | Absolute growth rate |
| DAS | Days after sowing |
| GPC | Grain protein content |
| N | Nitrogen |
| NUE | Nitrogen use efficiency |
| NVT | National variety trials |
| PSA | Projected shoot area |
| t-maxAGR | Time to maximum growth rate |
References
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Husenov, B.; Makhkamov, M.; Garkava-Gustavsson, L.; Muminjanov, H.; Johansson, E. Breeding for wheat quality to assure food security of a staple crop: The case study of tajikistan. Agric. Food Secur. 2015, 4, 9. [Google Scholar] [CrossRef]
- Kuhn, J.C.; Stubbs, T.L.; Carter, A.H. Effect of the gpc-b1 allele in hard red winter wheat in the us pacific northwest. Crop Sci. 2016, 56, 1009–1017. [Google Scholar] [CrossRef]
- Hong, B.H.; Rubenthaler, G.L.; Allan, R.E. Wheat pentosans. 1. Cultivar variation and relationship to kernel hardness. Cereal Chem. 1989, 66, 369–373. [Google Scholar]
- Bettge, A.D.; Morris, C.F. Relationships among grain hardness, pentosan fractions, and end-use quality of wheat. Cereal Chem. 2000, 77, 241–247. [Google Scholar] [CrossRef]
- Saint Pierre, C.; Peterson, C.J.; Ross, A.S.; Ohm, J.B.; Verhoeven, M.C.; Larson, M.; Hoefer, B. White wheat grain quality changes with genotype, nitrogen fertilization, and water stress. Agron. J. 2008, 100, 414–420. [Google Scholar] [CrossRef]
- Kibite, S.; Evans, L. Causes of negative correlations between grain yield and grain protein concentration in common wheat. Int. J. Plant. Breed. 1984, 33, 801–810. [Google Scholar] [CrossRef]
- Simmonds, N.W. The relation between yield and protein in cereal grain. J. Sci. Food Agric. 1995, 67, 309–315. [Google Scholar] [CrossRef]
- Bogard, M.; Allard, V.; Brancourt-Hulmel, M.; Heumez, E.; Machet, J.-M.; Jeuffroy, M.-H.; Gate, P.; Martre, P.; Le Gouis, J. Deviation from the grain protein concentration–grain yield negative relationship is highly correlated to post-anthesis n uptake in winter wheat. J. Exp. Bot. 2010, 61, 4303–4312. [Google Scholar] [CrossRef]
- Ortiz-Monasterio, R.J.I.; Sayre, K.D.; Rajaram, S.; McMahon, M. Genetic progress in wheat yield and nitrogen use efficiency under four nitrogen rates. Crop Sci. 1997, 37, 898. [Google Scholar] [CrossRef]
- Huebner, F.R.; Bietz, J.A.; Nelsen, T.; Bains, G.S.; Finney, P.L. Soft wheat quality as related to protein composition. Cereal Chem. 1999, 76, 650–655. [Google Scholar] [CrossRef]
- Moss, H.J. Quality standards for wheat varieties. J. Aust. Inst. Agric. Sci. 1973, 39, 109–115. [Google Scholar]
- Anderson, W.K.; Sawkins, D. Production practices for improved grain yield and quality of soft wheats in western australia. Aust. J. Exp. Agric. 1997, 37, 173–180. [Google Scholar] [CrossRef]
- Triboi, E.; Abad, A.; Michelena, A.; Lloveras, J.; Ollier, J.L.; Daniel, C. Environmental effects on the quality of two wheat genotypes: 1. Quantitative and qualitative variation of storage proteins. Eur. J. Agron. 2000, 13, 47–64. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Rufty, T.W. Nitrogen and water resources commonly limit crop yield increases, not necessarily plant genetics. Glob. Food Secur. 2012, 1, 94–98. [Google Scholar] [CrossRef]
- Sylvester-Bradley, R.; Kindred, D.R. Analysing nitrogen responses of cereals to prioritize routes to the improvement of nitrogen use efficiency. J. Exp. Bot. 2009, 60, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.; Webb, J. A review of the effect of n fertilizer type on gaseous emissions. Adv. Agron. 2001, 73, 65–108. [Google Scholar]
- Bouwer, H. Agricultural contaminants: Problems and solutions. J. Water Environ. Technol. 1989, 1, 292–297. [Google Scholar]
- Sadras, V.O.; Hayman, P.T.; Rodriguez, D.; Monjardino, M.; Bielich, M.; Unkovich, M.; Mudge, B.; Wang, E. Interactions between water and nitrogen in australian cropping systems: Physiological, agronomic, economic, breeding and modelling perspectives. Crop Pasture Sci. 2016, 67, 1019–1053. [Google Scholar] [CrossRef]
- Birch, C.J.; Long, K.E. Effect of nitrogen on the growth, yield and grain protein-content of barley (Hordeum vulgare). Aust. J. Exp. Agric. 1990, 30, 237–242. [Google Scholar] [CrossRef]
- Sadras, V.O.; Richards, R.A. Improvement of crop yield in dry environments: Benchmarks, levels of organisation and the role of nitrogen. J. Exp. Bot. 2014, 65, 1981–1995. [Google Scholar] [CrossRef] [PubMed]
- Cormier, F.; Foulkes, J.; Hirel, B.; Gouache, D.; Moenne-Loccoz, Y.; Le Gouis, J. Breeding for increased nitrogen-use efficiency: A review for wheat (T. aestivum L.). Plant Breed. 2016, 135, 255–278. [Google Scholar] [CrossRef]
- Sanford, D.; MacKown, C. Variation in nitrogen use efficiency among soft red winter wheat genotypes. Int. J. Plant. Breed. Res. 1986, 72, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Bogard, M.; Jourdan, M.; Allard, V.; Martre, P.; Perretant, M.R.; Ravel, C.; Heumez, E.; Orford, S.; Snape, J.; Griffiths, S.; et al. Anthesis date mainly explained correlations between post-anthesis leaf senescence, grain yield, and grain protein concentration in a winter wheat population segregating for flowering time QTLS. J. Exp. Bot. 2011, 62, 3621–3636. [Google Scholar] [CrossRef] [PubMed]
- Hitz, K.; Clark, A.J.; Van Sanford, D.A. Identifying nitrogen-use efficient soft red winter wheat lines in high and low nitrogen environments. Field Crops Res. 2017, 200, 1–9. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Reynolds, M.P.; Royo, C. Plant breeding and drought in c3 cereals: What should we breed for? Ann. Bot. 2002, 89, 925–940. [Google Scholar] [CrossRef]
- Tardieu, F.; Tuberosa, R. Dissection and modelling of abiotic stress tolerance in plants. Curr. Opin. Plant Biol. 2010, 13, 206–212. [Google Scholar] [CrossRef]
- Dunbabin, V. Simulating the role of rooting traits in crop-weed competition. Field Crops Res. 2007, 104, 44–51. [Google Scholar] [CrossRef]
- Hansen, N.J.S.; Plett, D.; Berger, B.; Garnett, T. Tackling nitrogen use efficiency in cereal crops using high-throughput phenotyping. In Engineering Nitrogen Utilization in Crop Plants; Shrawat, A., Zayed, A., Lightfoot, D.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 121–139. [Google Scholar]
- Sharma, R. Selection for biomass yield in wheat. Int. J. Plant Breed. 1993, 70, 35–42. [Google Scholar] [CrossRef]
- Oscarson, P. The strategy of the wheat plant in acclimating growth and grain production to nitrogen availability. J. Exp. Bot. 2000, 51, 1921–1929. [Google Scholar] [CrossRef][Green Version]
- Alzueta, I.; Abeledo, L.G.; Mignone, C.M.; Miralles, D.J. Differences between wheat and barley in leaf and tillering coordination under contrasting nitrogen and sulfur conditions. Eur. J. Agron. 2012, 41, 92–102. [Google Scholar] [CrossRef]
- Richards, R.A. Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 2000, 51, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Furbank, R.T.; Tester, M. Phenomics—Technologies to relieve the phenotyping bottleneck. Trends Plant Sci. 2011, 16, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Berger, B.; De Regt, B.; Tester, M. High-throughput phenotyping of plant shoots. In High-throughput Phenotyping in Plants; Normanly, J., Ed.; Humana Press: Totowa, NJ, USA, 2012; Volume 918. [Google Scholar]
- Koelewijn, H.P. Rapid change in relative growth rate between the vegetative and reproductive stage of the life cycle in plantago coronopus. New Phytol. 2004, 163, 67–76. [Google Scholar] [CrossRef]
- Kozłowski, J. Optimal allocation of resources to growth and reproduction: Implications for age and size at maturity. Trends Ecol. Evol. 1992, 7, 15–19. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Kopriva, S.; De Kok, L.J. Nutrient Use Efficiency in Plants: Concepts and Approaches; Springer International Publishing: Cham, Switzerland, 2014; Volume 10. [Google Scholar]
- Marschner, H.; Marschner, P.; Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier Academic Press: London, UK, 2012. [Google Scholar]
- Neilson, E.H.; Edwards, A.M.; Blomstedt, C.K.; Berger, B.; Moller, B.L.; Gleadow, R.M. Utilization of a high-throughput shoot imaging system to examine the dynamic phenotypic responses of a c-4 cereal crop plant to nitrogen and water deficiency over time. J. Exp. Bot. 2015, 66, 1817–1832. [Google Scholar] [CrossRef]
- Shi, P.-J.; Men, X.-Y.; Sandhu, H.S.; Chakraborty, A.; Li, B.-L.; Ou-Yang, F.; Sun, Y.-C.; Ge, F. The “general” ontogenetic growth model is inapplicable to crop growth. Ecol. Model. 2013, 266, 1–9. [Google Scholar] [CrossRef]
- Al-Tamimi, N.; Brien, C.; Oakey, H.; Berger, B.; Saade, S.; Ho, Y.S.; Schmöckel, S.M.; Tester, M.; Negrão, S. Salinity tolerance loci revealed in rice using high-throughput non-invasive phenotyping. Nat. Commun. 2016, 7, 13342. [Google Scholar] [CrossRef]
- Brien, C.J. Dae: Functions Useful in the Design and Anova of Experiments. R Package Version 3.0-32. Available online: https://cran.at.r-project.org/package=dae (accessed on 17 June 2019).
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing, R Development Core Team: Vienna, Austria, 2009.
- Butler, D.G.; Cullis, B.R.; Gilmour, A.R.; Gogel, B.J.; Thompson, R. Asreml-R Reference Manual. Version 4. Available online: http://asreml.org (accessed on 2 February 2018).
- Brien, C.J. Asremlplus: Augments Asreml-R in Fitting Mixed Models and Packages Generally in Exploring Prediction Differences. R Package Version 4.1-28. Available online: https://cran.at.r-project.org/package=asremlPlus (accessed on 17 June 2019).
- Davis, L.; Ware, A.; Cook, A. Port. Kenny, Franklin Harbour, Elliston and Wharminda Wheat and Barley Variety Trials in 2015; PIRSA: Adelaide, SA, Australia, 2016. [Google Scholar]
- Mahjourimajd, S.; Kuchel, H.; Langridge, P.; Okamoto, M. Evaluation of australian wheat genotypes for response to variable nitrogen application. Int. J. Plant Soil Relatsh. 2016, 399, 247–255. [Google Scholar] [CrossRef]
- Eagles, H.A.; McLean, R.; Eastwood, R.F.; Appelbee, M.J.; Cane, K.; Martin, P.J.; Wallwork, H. High-yielding lines of wheat carrying Gpc-B1 adapted to mediterranean-type environments of the south and west of australia. Crop Pasture Sci. 2014, 65, 854–861. [Google Scholar] [CrossRef]
- Mosleth, E.F.; Wan, Y.; Lysenko, A.; Chope, G.A.; Penson, S.P.; Shewry, P.R.; Hawkesford, M.J. A novel approach to identify genes that determine grain protein deviation in cereals. Plant Biotechnol. J. 2015, 13, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Cowling, S.A.; Field, C.B. Environmental control of leaf area production: Implications for vegetation and land-surface modeling. Glob. Biogeochem. Cycles 2003, 17, 1–14. [Google Scholar] [CrossRef]
- White, E.M.; Wilson, F.E.A. Responses of grain yield, biomass and harvest index and their rates of genetic progress to nitrogen availability in ten winter wheat varieties. Ir. J. Agric. Food Res. 2006, 45, 85–101. [Google Scholar]
- Kato, Y. Grain nitrogen concentration in wheat grown under intensive organic manure application on andosols in central japan. Plant Prod. Sci. 2012, 15, 40–47. [Google Scholar] [CrossRef]
- Jones, C.; Olson-Rutz, K. Practices to Increase Wheat Grain Protein; Extension; Montana State University: Bozeman, MT, USA, 2012. [Google Scholar]
- McKenzie, R.H.; Bremer, E.; Grant, C.A.; Johnston, A.M.; DeMulder, J.; Middleton, A.B. In-crop application effect of nitrogen fertilizer on grain protein concentration of spring wheat in the canadian prairies. Crop Appl. Eff. Nitrogen Fertil. Grain Protein Conc. Spring Wheat Can. Prairies 2006, 86, 565–572. [Google Scholar] [CrossRef]
- Yau, S.K.; Thurling, N. Variation in nitrogen response among spring rape (brassica napus) cultivars and its relationship to nitrogen uptake and utilization. Field Crops Res. 1987, 16, 139–155. [Google Scholar] [CrossRef]
- Ulas, A.; Behrens, T.; Wiesler, F.; Horst, W.J.; Erley, G.S.A. Does genotypic variation in nitrogen remobilisation efficiency contribute to nitrogen efficiency of winter oilseed-rape cultivars (Brassica napus L.)? Plant Soil 2013, 371, 463–471. [Google Scholar] [CrossRef]
- Johnson, V.A.; Dreier, A.F.; Grabousk, P. Yield and protein responses to nitrogen fertilizer of two winter wheat varieties differing in inherent protein content of their grain. Agron. J. 1973, 65, 259–263. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Broadley, M.R.; Escobar-Gutiérrez, A.J.; Burns, A.; Burns, I.G. What are the effects of nitrogen deficiency on growth components of lettuce? New Phytol. 2000, 147, 519–526. [Google Scholar] [CrossRef]
- Xiao, J.; Yin, X.; Ren, J.; Zhang, M.; Tang, L.; Zheng, Y. Complementation drives higher growth rate and yield of wheat and saves nitrogen fertilizer in wheat and faba bean intercropping. Field Crops Res. 2018, 221, 119–129. [Google Scholar] [CrossRef]
- Cox, M.C.; Qualset, C.O.; Rains, D.W. Genetic variation for nitrogen assimilation and translocation in wheat. I. Dry matter and nitrogen accumulation. Genet. Var. Nitrogen Assim. Translocat. Wheat. I. Dry Matter Nitrogen Accumul. 1985, 25, 430–435. [Google Scholar]
- Brancourt-Hulmel, M.; Heumez, E.; Pluchard, P.; Beghin, D.; Le Gouis, C.; Depatureaux, A.; Giraud, J. Indirect versus direct selection of winter wheat for low-input or high-input levels. Crop Sci. 2005, 45, 1427–1431. [Google Scholar] [CrossRef]
- Shepherd, K.D.; Cooper, P.J.M.; Allan, A.Y.; Drennan, D.S.H.; Keatinge, J.D.H. Growth, water-use and yield of barley in mediterranean-type environments. J. Agric. Sci. 1987, 108, 365–378. [Google Scholar] [CrossRef]
- Nord, E.A.; Lynch, J.P. Plant phenology: A critical controller of soil resource acquisition. J. Exp. Bot. 2009, 60, 1927–1937. [Google Scholar] [CrossRef]
- Thies, J.E.; Singleton, P.W.; Bohlool, B.B. Phenology, growth, and yield of field-grown soybean and bush bean as a function of varying modes of n-nutrition. Soil Biol. Biochem. 1995, 27, 575–583. [Google Scholar] [CrossRef]
- Aerts, R. Nutrient resorption from senescing leaves of perennials: Are there general patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Gobert, S.; Lejeune, P.; Lepoint, G.; Bouquegneau, J.M. C, n, p concentrations and requirements of flowering posidonia oceanica shoots. Hydrobiologia 2005, 533, 253–259. [Google Scholar] [CrossRef]
- Milla, R.; Castro-Diez, P.; Maestro-Martinez, M.; Montserrat-Marti, G. Relationships between phenology and the remobilization of nitrogen, phosphorus and potassium in branches of eight mediterranean evergreens. New Phytol. 2005, 168, 167–178. [Google Scholar] [CrossRef]
- Lynch, J.; White, J.W. Shoot nitrogen dynamics in tropical common bean. Crop Sci. 1992, 32, 392–397. [Google Scholar] [CrossRef]
- Dechorgnat, J.; Kaiser, B.N. Grain nitrogen filling in two contrasting australian wheat cultivars. In COMBIO Symposium; Adelaide, 2 October 2017. [Google Scholar]
- Fettell, N.; Brill, R.; Gardner, M.; McMullen, G. Yield and Protein Relationships in Wheat; Grains Research & Development Corporation: Canberra, Australia, 2012. [Google Scholar]
- Savin, R.; Slafer, G.A.; Cossani, C.M.; Abeledo, L.G.; Sadras, V.O. Cereal yield in mediterranean-type environments: Challenging the paradigms on terminal drought, the adaptability of barley vs. wheat and the role of nitrogen fertilization. In Crop Physiology, 2nd ed.; Sadras, V.O., Calderini, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 141–158. [Google Scholar]
- Dhugga, K.S.; Waines, J.G. Analysis of nitrogen accumulation and use in bread and durum wheat. Crop Sci. 1989, 29, 1232–1239. [Google Scholar] [CrossRef]
- Anderson, W.K.; Hamza, M.A.; Sharma, D.L.; D’Antuono, M.F.; Hoyle, F.C.; Hill, N.; Shackley, B.J.; Amjad, M.; Zaicou-Kunesch, C. The role of management in yield improvement of the wheat crop—A review with special emphasis on western australia. Aust. J. Agric. Res. 2005, 56, 1137–1149. [Google Scholar] [CrossRef]
- Rao, A.C.S.; Smith, J.L.; Jandhyala, V.K.; Papendick, R.I.; Parr, J.F. Cultivar and climatic effects on the protein content of soft white winter wheat. Agron. J. 1993, 85, 1023–1028. [Google Scholar] [CrossRef]
- Sullivan, D.M.; Bary, A.I.; Cogger, C.G.; Shearin, T.E. Predicting biosolids application rates for dryland wheat across a range of northwest climate zones. Commun. Soil Sci. Plant Anal. 2009, 40, 1770–1789. [Google Scholar] [CrossRef]
- Cogger, C.G.; Bary, D.M.; Sullivan, A.I.; Kropf, J.A. Matching plant-available nitrogen from biosolids with dryland wheat needs. J. Prod. Agric. 1998, 11, 41–47. [Google Scholar] [CrossRef]
- Hunter, A.; Gerard, C.; Waddoups, H.; Hall, W.; Cushman, H.; Alban, L. The effect of nitrogen fertilizers on the relationship between increases in yields and protein content of pastry-type wheats. Agron. J. 1958, 50, 311. [Google Scholar] [CrossRef]
- Mahler, R.L.; Guy, S.O. Soft wheat spring wheat. In Northern Idaho Fertilizer Guide; University of Idaho: Moscow, ID, USA, 2007. [Google Scholar]
- Stewart, W.M. Fertilizer for better bread. Better Crops 2003, 87, 15–17. [Google Scholar]
- Swanston, J.S.; Newton, A.C.; Brosnan, J.M.; Fotheringham, A.; Glasgow, E. Determining the spirit yield of wheat varieties and variety mixtures. J. Cereal Sci. 2005, 42, 127–134. [Google Scholar] [CrossRef]
- Matthews, P.; McCaffery, D.; Jenkins, L. Winter Crop Variety Sowing Guide 2017; Department of Primary Industries: Orange, NSW, Australia, 2017. [Google Scholar]
- Fuller, P. New wheat varieties meet the quality challenge. In Grain Business; Glencore Grain Pty Ltd.: Melbourne, VIC, Australia, 2013. [Google Scholar]
- Lush, D.; Forknall, C.; Neate, S.; Sheedy, J. Queensland Wheat Varieties 2017; GRDC: Canberra, Australia; DAF: Brisbane, QLD, Australia, 2017. [Google Scholar]
- Wang, Q.; Li, F.R.; Zhao, L.; Zhang, E.H.; Shi, S.L.; Zhao, W.Z.; Song, W.X.; Vance, M.M. Effects of irrigation and nitrogen application rates on nitrate nitrogen distribution and fertilizer nitrogen loss, wheat yield and nitrogen uptake on a recently reclaimed sandy farmland. Plant Soil 2010, 337, 325–339. [Google Scholar] [CrossRef]
- Oliver, Y.M.; Robertson, M.J. Quantifying the benefits of accounting for yield potential in spatially and seasonally responsive nutrient management in a mediterranean climate. Aust. J. Soil Res. 2009, 47, 114. [Google Scholar] [CrossRef]
- Chen, W.; Bell, R.W.; Brennan, R.F.; Bowden, J.W.; Dobermann, A.; Rengel, Z.; Porter, W. Key crop nutrient management issues in the western australia grains industry: A review. Aust. J. Soil Res. 2009, 47, 1–18. [Google Scholar] [CrossRef]
- Simmonds, D.H. Wheat and Wheat Quality in Australia; CSIRO Publishing: Clayton, Australia, 1989. [Google Scholar]
- Mason, M.G. Nitrogen. In Soil Guide: A Handbook for Understanding and Managing Agricultural Soils; Moore, G., Ed.; Department of Agriculture and Food Western Australia: Kensington, Australia, 1998; Volume 4343, pp. 164–167. [Google Scholar]
- Girondé, A.; Poret, M.; Etienne, P.; Trouverie, J.; Bouchereau, A.; Le Cahérec, F.; Leport, L.; Orsel, M.; Niogret, M.-F.; Deleu, C.; et al. A profiling approach of the natural variability of foliar n remobilization at the rosette stage gives clues to understand the limiting processes involved in the low n use efficiency of winter oilseed rape. J. Exp. Bot. 2015, 66, 2461–2473. [Google Scholar] [CrossRef] [PubMed]
- Sorin, C.; Leport, L.; Cambert, M.; Bouchereau, A.; Mariette, F.; Musse, M. Nitrogen deficiency impacts on leaf cell and tissue structure with consequences for senescence associated processes in Brassica napus. Bot. Stud. 2016, 57, 11. [Google Scholar] [CrossRef] [PubMed]
- Presterl, T.; Seitz, G.; Landbeck, M.; Thiemt, E.M.; Schmidt, W.; Geiger, H.H. Improving nitrogen-use efficiency in european maize: Estimation of quantitative genetic parameters. Crop Sci. 2003, 43, 1259. [Google Scholar] [CrossRef]


| Spitfire | Mace | Gregory | Impala | QAL2000 | Gazelle | |
|---|---|---|---|---|---|---|
| Average of GPC from 11 years of NVT data | 13.4 | 11.3 | 12 | 11.6 | 10.9 | 10.9 |
| Average of grain N% in all N treatments | 1.7 | 1.7 | 1.6 | 1.5 | 1.3 | 1.4 |
| Average of non-grain N% in all N treatments | 0.3 | 0.4 | 0.3 | 0.2 | 0.2 | 0.2 |
| % total reduction of max-PSA from high to low N | 46 | 43 | 34 | 39 | 30 | 33 |
| % total reduction of tmax from high to low N | 44 | 35 | 25 | 32 | 19 | 8 |
| % total reduction of max-AGR from high to low N | 43 | 33 | 33 | 31 | 32 | 32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahimi Eichi, V.; Okamato, M.; Haefele, S.M.; Jewell, N.; Brien, C.; Garnett, T.; Langridge, P. Understanding the Interactions between Biomass, Grain Production and Grain Protein Content in High and Low Protein Wheat Genotypes under Controlled Environments. Agronomy 2019, 9, 706. https://doi.org/10.3390/agronomy9110706
Rahimi Eichi V, Okamato M, Haefele SM, Jewell N, Brien C, Garnett T, Langridge P. Understanding the Interactions between Biomass, Grain Production and Grain Protein Content in High and Low Protein Wheat Genotypes under Controlled Environments. Agronomy. 2019; 9(11):706. https://doi.org/10.3390/agronomy9110706
Chicago/Turabian StyleRahimi Eichi, Vahid, Mamoru Okamato, Stephan M. Haefele, Nathaniel Jewell, Chris Brien, Trevor Garnett, and Peter Langridge. 2019. "Understanding the Interactions between Biomass, Grain Production and Grain Protein Content in High and Low Protein Wheat Genotypes under Controlled Environments" Agronomy 9, no. 11: 706. https://doi.org/10.3390/agronomy9110706
APA StyleRahimi Eichi, V., Okamato, M., Haefele, S. M., Jewell, N., Brien, C., Garnett, T., & Langridge, P. (2019). Understanding the Interactions between Biomass, Grain Production and Grain Protein Content in High and Low Protein Wheat Genotypes under Controlled Environments. Agronomy, 9(11), 706. https://doi.org/10.3390/agronomy9110706








