Melatonin Stimulates Activities and Expression Level of Antioxidant Enzymes and Preserves Functionality of Photosynthetic Apparatus in Hickory Plants (Carya cathayensis Sarg.) under PEG-Promoted Drought
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Setup
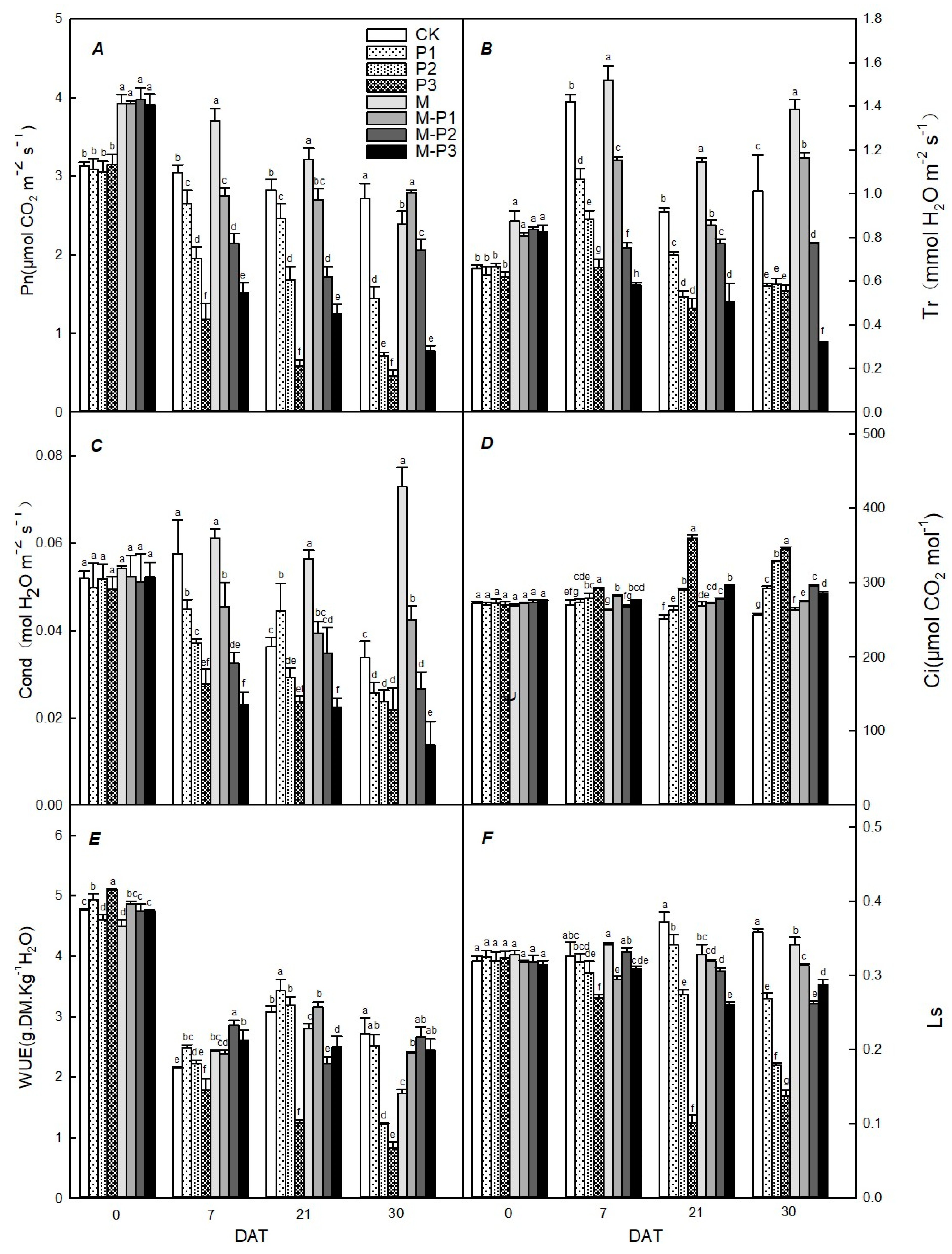
2.3. Determination of Leaf Gas Exchange Parameters
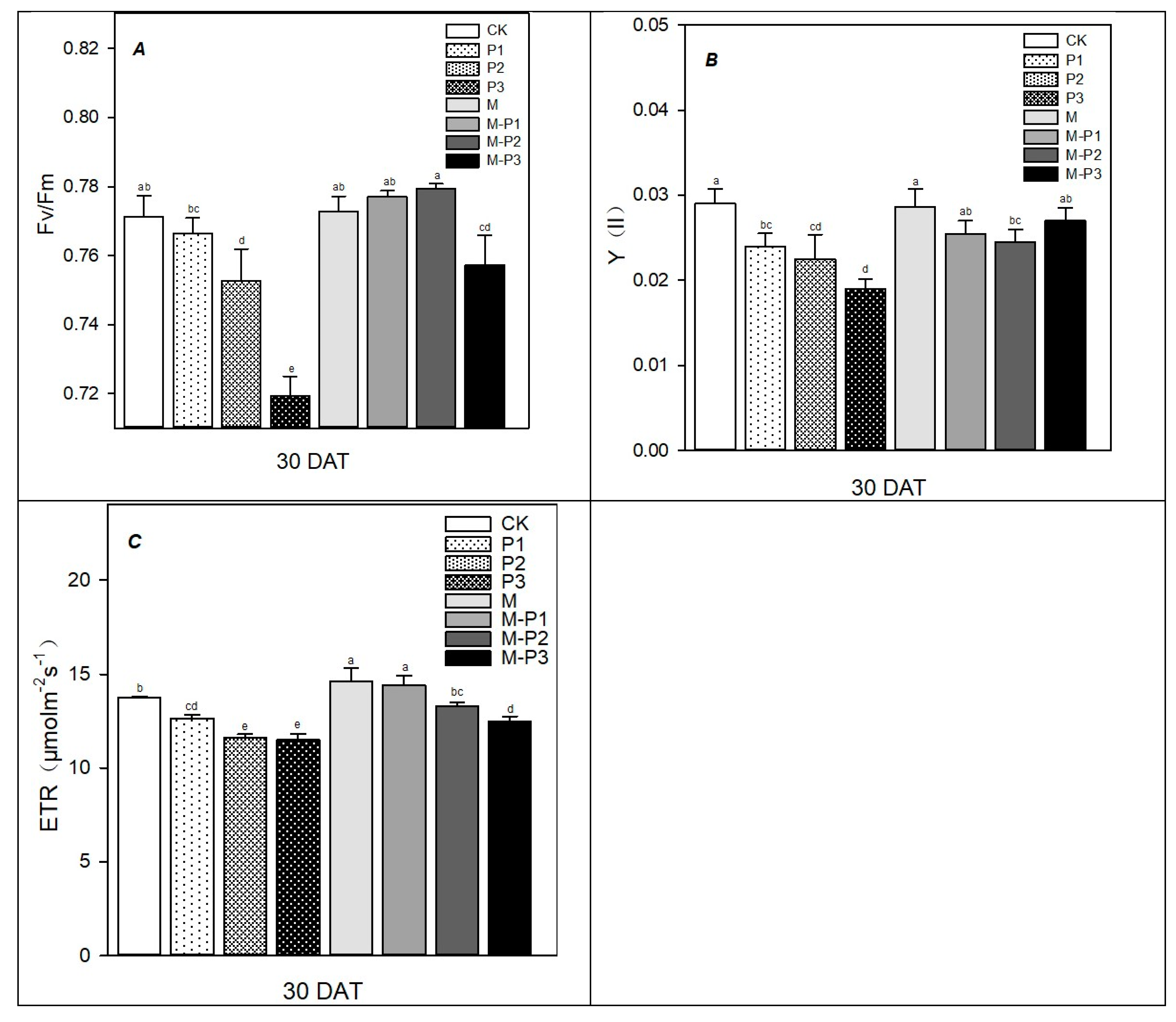
2.4. Determination of Chlorophyll Fluorescence
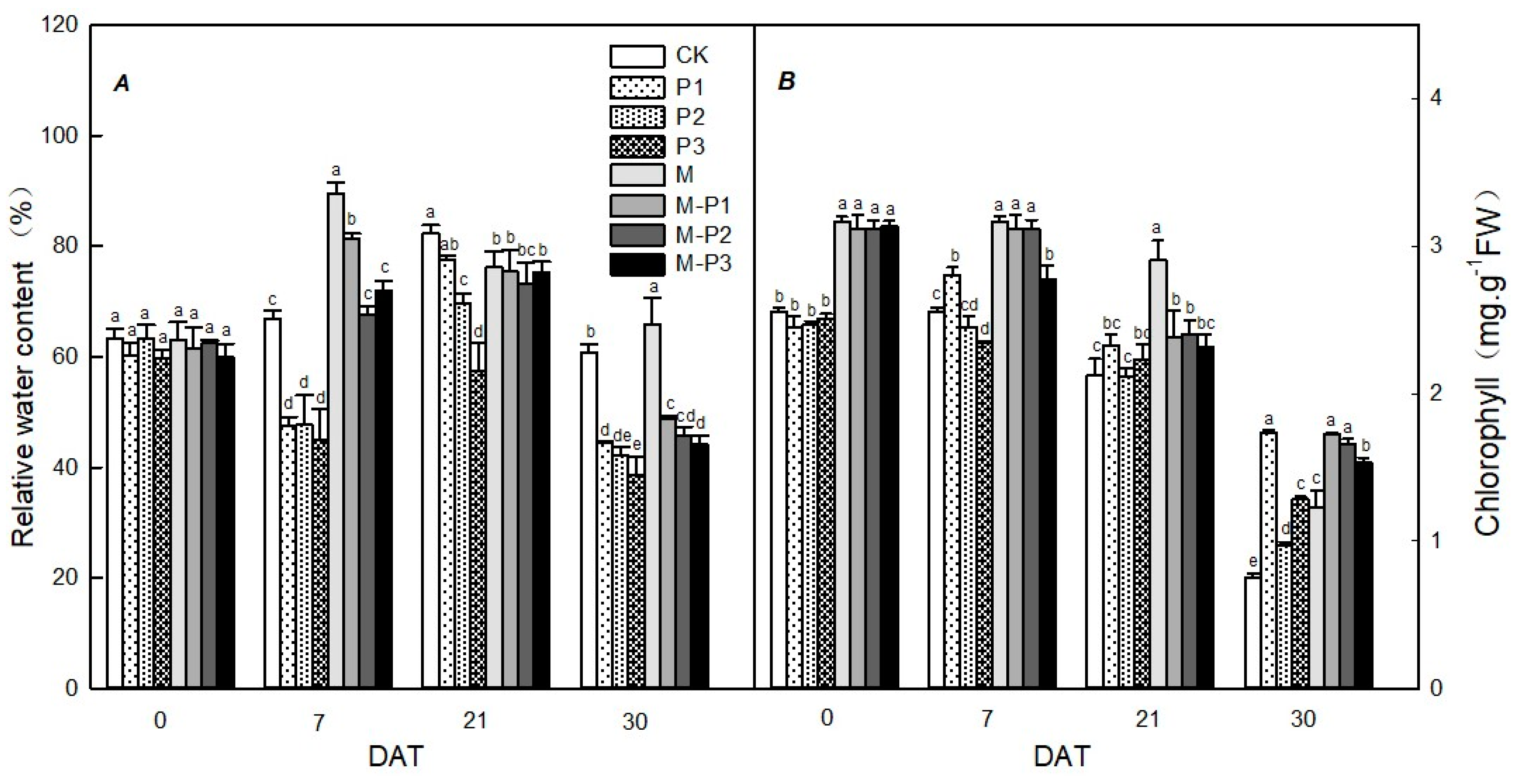
2.5. Relative Water Content of Leaves
2.6. Total Chlorophyll Content
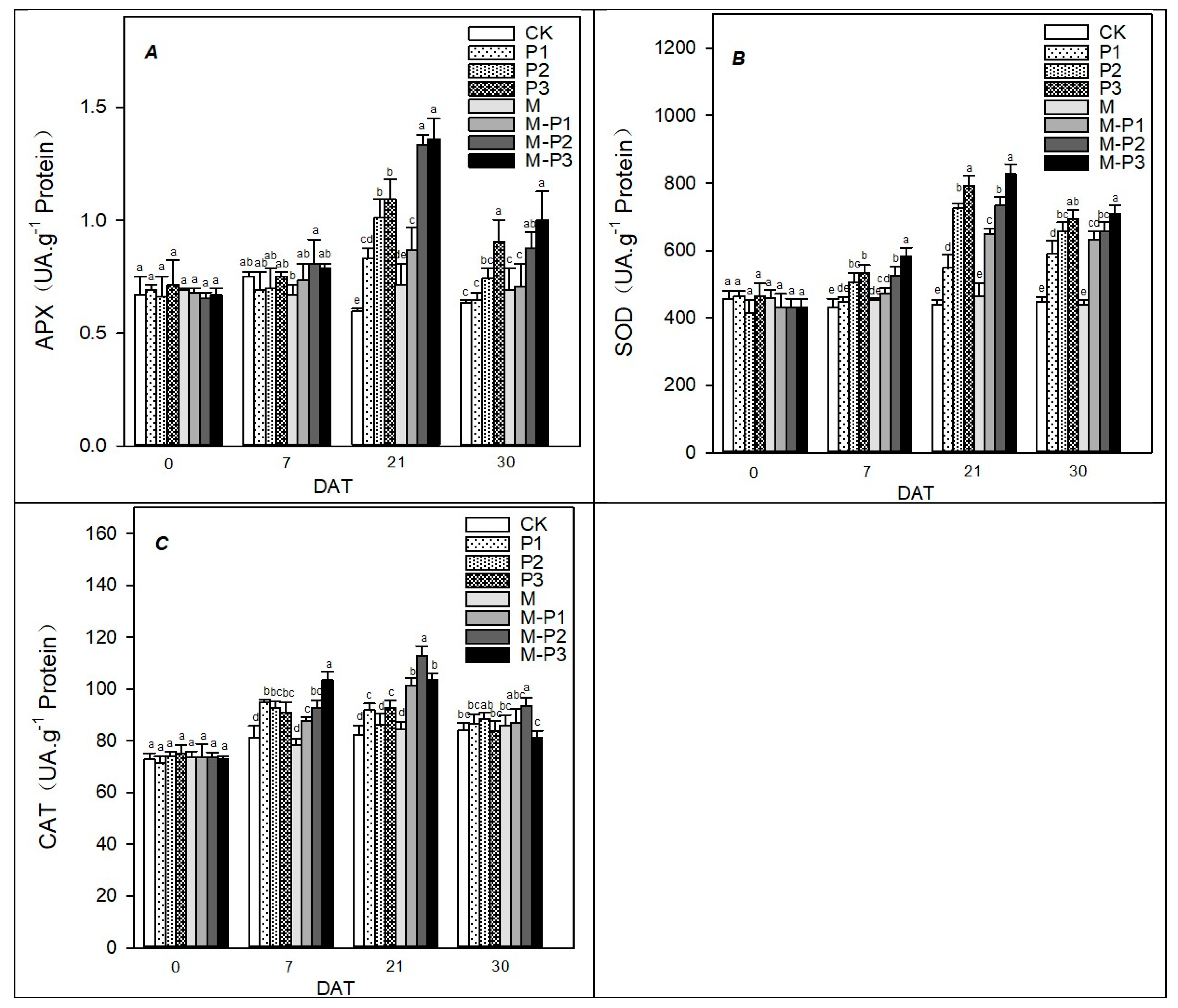
2.7. Antioxidant Enzyme Activity
2.8. Malondialdehyde (MDA) and Proline (Pro) Concentration
2.9. Quantitative Real-Time PCR
2.10. Statistical Analysis
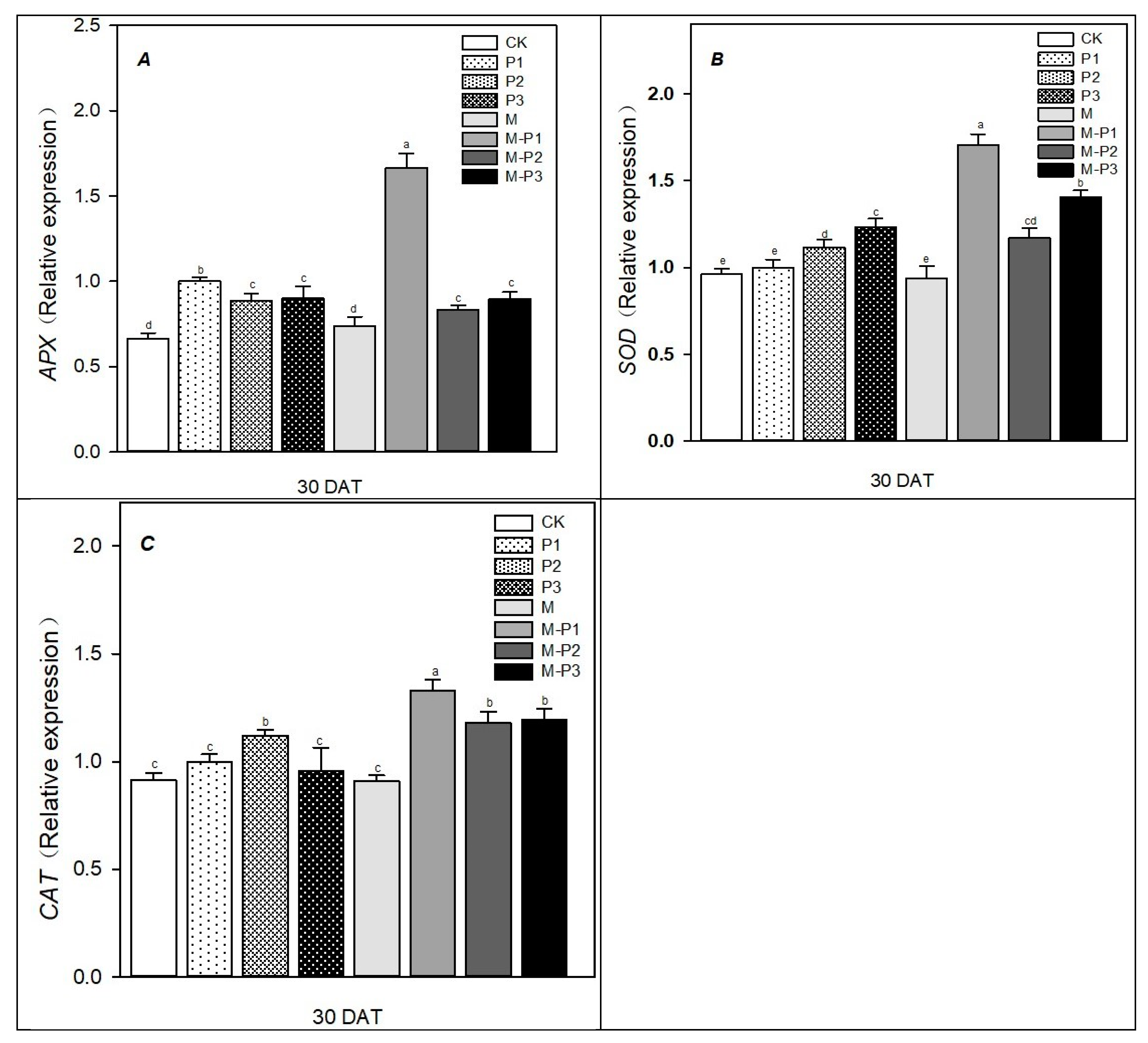
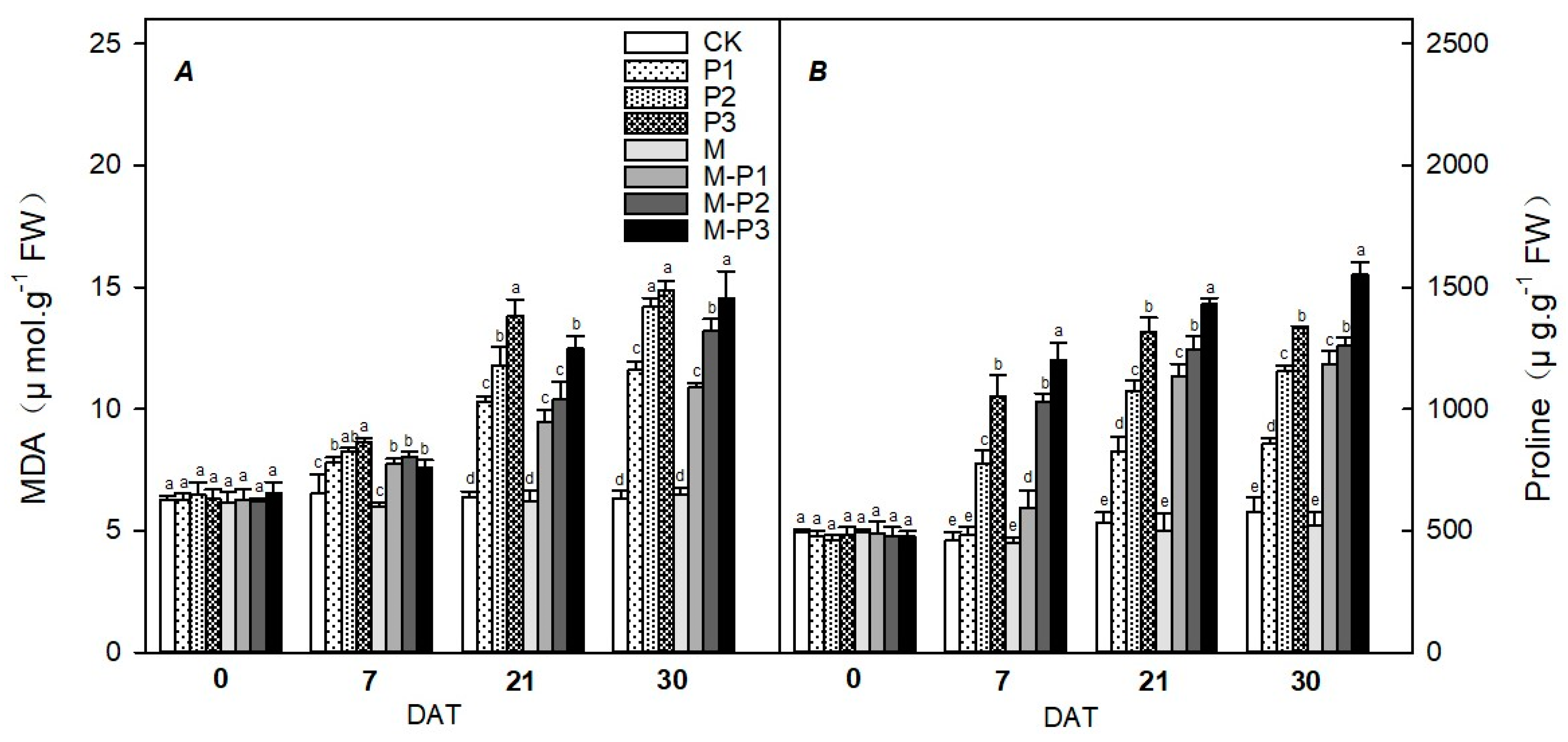
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, H.; Zhao, L.; Chen, J.; Yang, Y.; Xu, D.; Tao, S.; Zheng, S.; Shen, Y.; He, Y.; Shen, C. Identification and expression profiling of the Aux/IAA gene family in Chinese hickory ( Carya cathayensis Sarg.) during the grafting process. Plant Physiol. Biochem. 2018, 127, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wu, J.; Cao, Y.; Yang, X.; Wang, Z.; Huang, J.; Xia, G.; Zhang, Q.; Hu, Y. Photosynthetic characteristics of leaves and fruits of Hickory (Carya cathayensis Sarg.) and Pecan (Carya illinoensis K.Koch) during fruit development stages. Trees 2016, 30, 1523–1534. [Google Scholar] [CrossRef]
- Naresh, P.S.; Pramod, K.P.; Sandeep, K.V. Morpho-physiological characterization of Indian wheat genotypes and their evaluation under drought condition. Afr. J. Biotechnol. 2014, 13, 2022–2027. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019, 135, 295–303. [Google Scholar] [CrossRef]
- Shivakrishna, P.; Reddy, K.A.; Rao, D.M. Effect of PEG-6000 Imposed drought stress on RNA content, Relative water content (RWC), and Chlorophyll content in peanut leaves and roots. Saudi J. Biol. Sci. 2018, 25, 285. [Google Scholar]
- Karamanos, A.J. Water stress and leaf growth of field beans (Vicia faba L.) in the field: Leaf number and total leaf area. Ann. Bot. 1978, 42, 1393–1402. [Google Scholar] [CrossRef]
- Chaves, M.M. Effects of Water Deficits on Carbon Assimilation. J. Exp. Bot. 1991, 42, 1–16. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 Plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Chaves, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef]
- Aruoma, O.; Halliwell, B.; J Laughton, M.; Quinlan, G.; Gutteridge, J.M.C. The Mechanism of Initiation of Lipid Peroxidation. Evidence against a Requirement for an Iron (II)-Iron (III) Complex. Biochem. J. 1989, 258, 617–620. [Google Scholar] [CrossRef]
- Kaur, P.; Bali, S.; Sharma, A.; Kohli, S.K.; Vig, A.P.; Bhardwaj, R.; Thukral, A.K.; Abd-Allah, E.F.; Wijaya, L.; Alyemeni, M.N. Cd induced generation of free radical species in Brassica juncea is regulated by supplementation of earthworms in the drilosphere. Sci. Total Environ. 2019, 655, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment-A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Kanwar, M.; Thukral, A.; Bhardwaj, R. Ameliorating imidacloprid induced oxidative stress by 24-epibrassinolide in Brassica juncea L. Russ. J. Plant Physiol. 2017, 64, 509–517. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; Manivannan, P.; Ines, J.; Al-Juburi, H.J.; Chang-Xing, Z.; Hong-Bo, S.; Panneerselvam, R. Antioxidant defense responses: Physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant. 2009, 31, 427–436. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Semane, B.; Cuypers, A.; Smeets, K.; Van Belleghem, F.; Horemans, N.; Schat, H.; Vangronsveld, J. Cadmium responses in Arabidopsis thaliana: Glutathione metabolism and antioxidative defence system. Physiol. Plant. 2007, 129, 519–528. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Kumar, R.; Shahzad, B.; Thukral, A.K.; Bhardwaj, R. Brassinosteroid-mediated pesticide detoxification in plants: A mini-review. Cogent Food Agric. 2018, 4, 1436212. [Google Scholar] [CrossRef]
- Alonso, R.; Elvira, S.; Castillo, F.; Gimeno, B. Interactive effects of ozone and drought stress on pigments and activities of antioxidative enzymes in Pinus halepensis. Plant Cell Environ. 2001, 24, 905–916. [Google Scholar] [CrossRef]
- Silva, E.N.; Ribeiro, R.V.; Ferreira-Silva, S.L.; Vieira, S.A.; Ponte, L.F.; Silveira, J.A. Coordinate changes in photosynthesis, sugar accumulation and antioxidative enzymes improve the performance of Jatropha curcas plants under drought stress. Biomass Bioenergy 2012, 45, 270–279. [Google Scholar] [CrossRef]
- Lu, S.; Su, W.; Li, H.; Guo, Z. Abscisic acid improves drought tolerance of triploid bermudagrass and involves H2O2-and NO-induced antioxidant enzyme activities. Plant Physiol. Biochem. 2009, 47, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Tarkow, H.; Feist, W.C.; Southerland, C.F. Interaction of wood with polymeric materials. Penetration versus molecular size. For. Prod. J. 1966, 16, 61–65. [Google Scholar]
- Emmerich, W.E.; Hardegree, S.P. Polyethylene glycol solution contact effects on seed germination. Agron. J. 1990, 82, 1103–1107. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.-D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef]
- Sharma, A.; Zheng, B. Melatonin mediated regulation of drought stress: Physiological and molecular aspects. Plants 2019, 8, 190. [Google Scholar] [CrossRef]
- Sarropoulou, V.N.; Therios, I.N.; Dimassi-Theriou, K.N. Melatonin promotes adventitious root regeneration in in vitro shoot tip explants of the commercial sweet cherry rootstocks CAB-6P (Prunus cerasus L.), Gisela 6 (P. cerasus × P. canescens), and MxM 60 (P. avium × P. mahaleb). J. Pineal Res. 2012, 52, 38–46. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Wei, Z.; Liang, D.; Liu, C.; Yin, L.; Jia, D.; Fu, M.; Ma, F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef]
- Kostopoulou, Z.; Therios, I.; Roumeliotis, E.; Kanellis, A.K.; Molassiotis, A. Melatonin combined with ascorbic acid provides salt adaptation in Citrus aurantium L. seedlings. Plant Physiol. Biochem. 2015, 86, 155–165. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2012, 54, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.H.; Chun, C.W.; Ivanov, S.V.; Alexieva, V.; Chih, W.Y. Repetition of hydrogen peroxide treatment induces a chilling tolerance comparable to cold acclimation in mung bean. J. Am. Soc. Hortic. Sci. 2007, 132, 770–776. [Google Scholar]
- Moussa, H.R.; Algamal, S.M.A. Does Exogenous application of melatonin ameliorate boron toxicity in spinach plants? Int. J. Veg. Sci. 2017, 23, 233–245. [Google Scholar] [CrossRef]
- Sarafi, E.; Tsouvaltzis, P.; Chatzissavvidis, C.; Siomos, A.; Therios, I. Melatonin and resveratrol reverse the toxic effect of high boron (B) and modulate biochemical parameters in pepper plants (Capsicum annuum L.). Plant Physiol. Biochem. 2017, 112, 173–182. [Google Scholar] [CrossRef]
- Tan, D.X. Phytoremediative Capacity of Plants Enriched with Melatonin. Plant Signal. Behav. 2007, 2, 514–516. [Google Scholar] [CrossRef]
- Zhao, Y.; Qi, L.W.; Wang, W.M.; Saxena, P.K.; Liu, C.Z. Melatonin improves the survival of cryopreserved callus of Rhodiola crenulata. J. Pineal Res. 2011, 50, 83–88. [Google Scholar] [CrossRef]
- García, J.J.; López-Pingarrón, L.; Almeida-Souza, P.; Tres, A.; Escudero, P.; García-Gil, F.A.; Tan, D.X.; Reiter, R.J.; Ramírez, J.M.; Bernal-Pérez, M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J. Pineal Res. 2014, 56, 225–237. [Google Scholar] [CrossRef]
- Allegra, M.; Reiter, R.J.; Tan, D.X.; Gentile, C.; Tesoriere, L.; Livrea, M.A. The chemistry of melatonin’s interaction with reactive species. J. Pineal Res. 2010, 34, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.P.; Scott, E.R.; Liu, J.W.; Guo, S. Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in Camellia sinensis L. Molecules 2018, 23, 165. [Google Scholar] [CrossRef]
- Sionit, N.; Mortensen, D.; Strain, B.; Hellmers, H. Growth response of wheat to CO2 enrichment and different levels of mineral nutrition. Agron. J. 1981, 73, 1023–1027. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hou, L.; Zhang, J.; Yao, C.; Liu, Y.; Zhang, C.; Xu, Y.; Cao, J. The structural and functional effects of fine particulate matter from cooking oil fumes on rat umbilical cord blood vessels. Environ. Sci. Poll. Res. 2016, 23, 16567–16578. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, S.; Zhou, L.; Sun, L. The potential liver, brain, and embryo toxicity of titanium dioxide nanoparticles on mice. Nanoscale Res. Lett. 2017, 12, 478. [Google Scholar] [CrossRef]
- Azevedo, R.; Alas, R.; Smith, R.; Lea, P. Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase-deficient mutant of barley. Physiol. Plant. 1998, 104, 280–292. [Google Scholar] [CrossRef]
- Zhao, S.J.; Xu, C.C.; Zou, Q.; Meng, Q.W. Improvements of method for measurement of malondialdehyde in plant tissues. Plant Physiol. Commun. 1994, 30, 207–210. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea maysL.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar] [CrossRef]
- Nativ, R.; Ephrath, J.E.; Berliner, P.R.; Saranga, Y. Drought resistance and water use efficiency in Acacia saligna. Aus. J. Bot. 1999, 47, 577–586. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhang, G.C.; Gu, S.Y.; Xia, J.B.; Zhao, J.K. Critical responses of photosynthetic efficiency of goldspur apple tree to soil water variation in semiarid loess hilly area. Photosynthetica 2010, 48, 589–595. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Maxwell, K. Chlorophyll fluorescence—A practical guide. J Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Woo, N.S.; Badger, M.R.; Pogson, B.J. A rapid, non-invasive procedure for quantitative assessment of drought survival using chlorophyll fluorescence. Plant Met. 2008, 4, 27. [Google Scholar] [CrossRef]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef]
- Gong, H.; Zhu, X.; Chen, K.; Wang, S.; Zhang, C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Souza, T.C.D.; Magalhães, P.C.; Castro, E.M.D.; Carneiro, N.P.; Padilha, F.A.; Júnior, C.C.G. ABA application to maize hybrids contrasting for drought tolerance: Changes in water parameters and in antioxidant enzyme activity. Plant Growth Regul. 2014, 73, 205–217. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Plummer, B.F.; Qi, W. Melatonin directly scavenges hydrogen peroxide: A potentially new metabolic pathway of melatonin biotransformation. Free Rad. Biol. Med. 2000, 29, 1177–1185. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Gopi, R.; Sankar, B.; Manivannan, P.; Kishorekumar, A.; Sridharan, R.; Panneerselvam, R. Studies on germination, seedling vigour, lipid peroxidation and proline metabolism in Catharanthus roseus seedlings under salt stress. South Afr. J. Bot. 2007, 73, 190–195. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Dimassi-Theriou, K.; Therios, I.; Koukourikou-Petridou, M. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium × Prunus cerasus). Plant Physiol. Biochem. 2012, 61, 162–168. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Chen, J.; Sharma, A.; Tao, S.; Zheng, B.; Landi, M.; Yuan, H.; Yan, D. Melatonin Stimulates Activities and Expression Level of Antioxidant Enzymes and Preserves Functionality of Photosynthetic Apparatus in Hickory Plants (Carya cathayensis Sarg.) under PEG-Promoted Drought. Agronomy 2019, 9, 702. https://doi.org/10.3390/agronomy9110702
Wang J, Chen J, Sharma A, Tao S, Zheng B, Landi M, Yuan H, Yan D. Melatonin Stimulates Activities and Expression Level of Antioxidant Enzymes and Preserves Functionality of Photosynthetic Apparatus in Hickory Plants (Carya cathayensis Sarg.) under PEG-Promoted Drought. Agronomy. 2019; 9(11):702. https://doi.org/10.3390/agronomy9110702
Chicago/Turabian StyleWang, Junfeng, Juanjuan Chen, Anket Sharma, Shenchen Tao, Bingsong Zheng, Marco Landi, Huwei Yuan, and Daoliang Yan. 2019. "Melatonin Stimulates Activities and Expression Level of Antioxidant Enzymes and Preserves Functionality of Photosynthetic Apparatus in Hickory Plants (Carya cathayensis Sarg.) under PEG-Promoted Drought" Agronomy 9, no. 11: 702. https://doi.org/10.3390/agronomy9110702
APA StyleWang, J., Chen, J., Sharma, A., Tao, S., Zheng, B., Landi, M., Yuan, H., & Yan, D. (2019). Melatonin Stimulates Activities and Expression Level of Antioxidant Enzymes and Preserves Functionality of Photosynthetic Apparatus in Hickory Plants (Carya cathayensis Sarg.) under PEG-Promoted Drought. Agronomy, 9(11), 702. https://doi.org/10.3390/agronomy9110702






