Understanding Activation Effects on Low-Temperature Biochar for Optimization of Herbicide Sorption
Abstract
1. Introduction
2. Materials and Methods
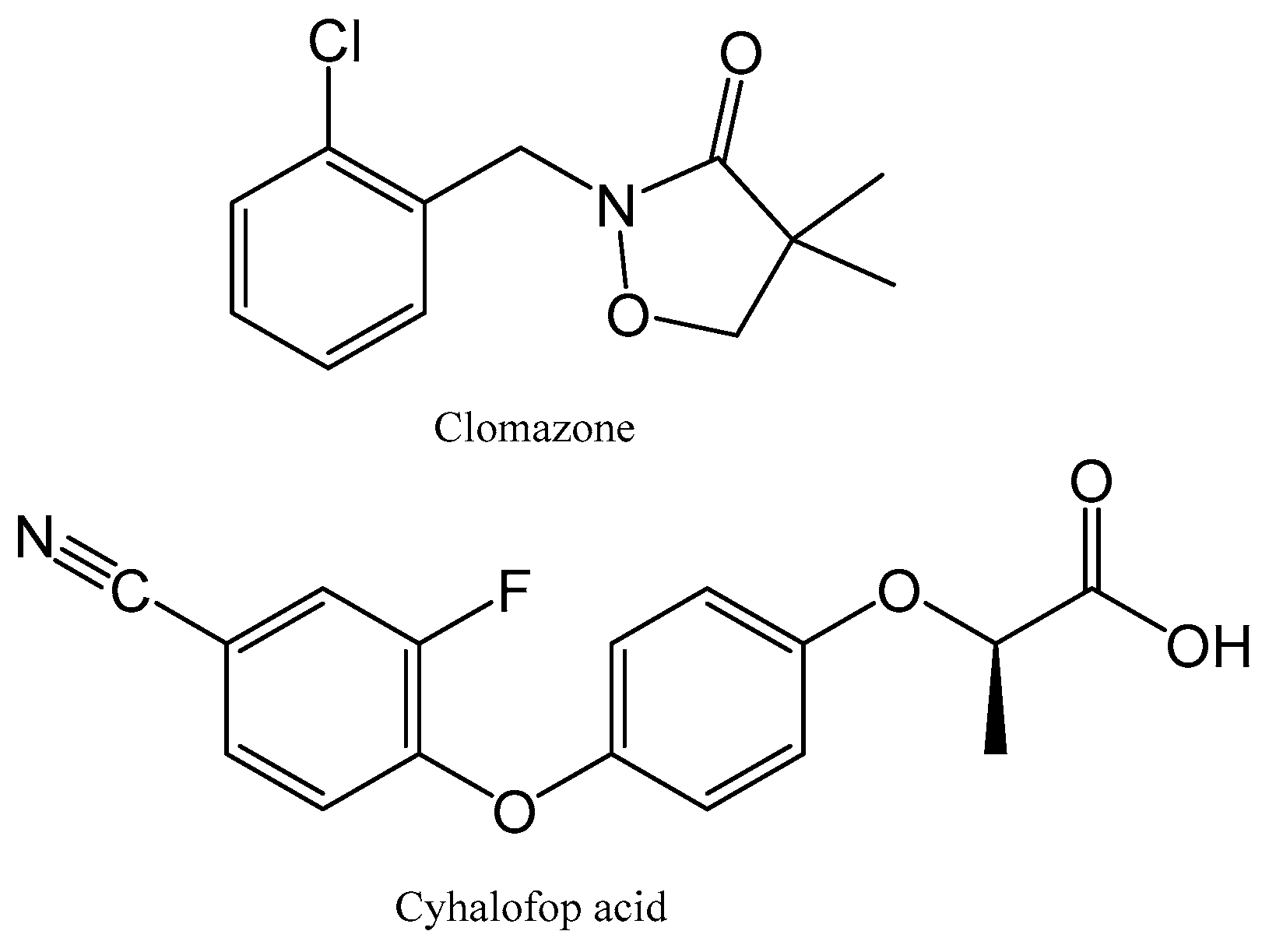
2.1. Chemicals and Biochars
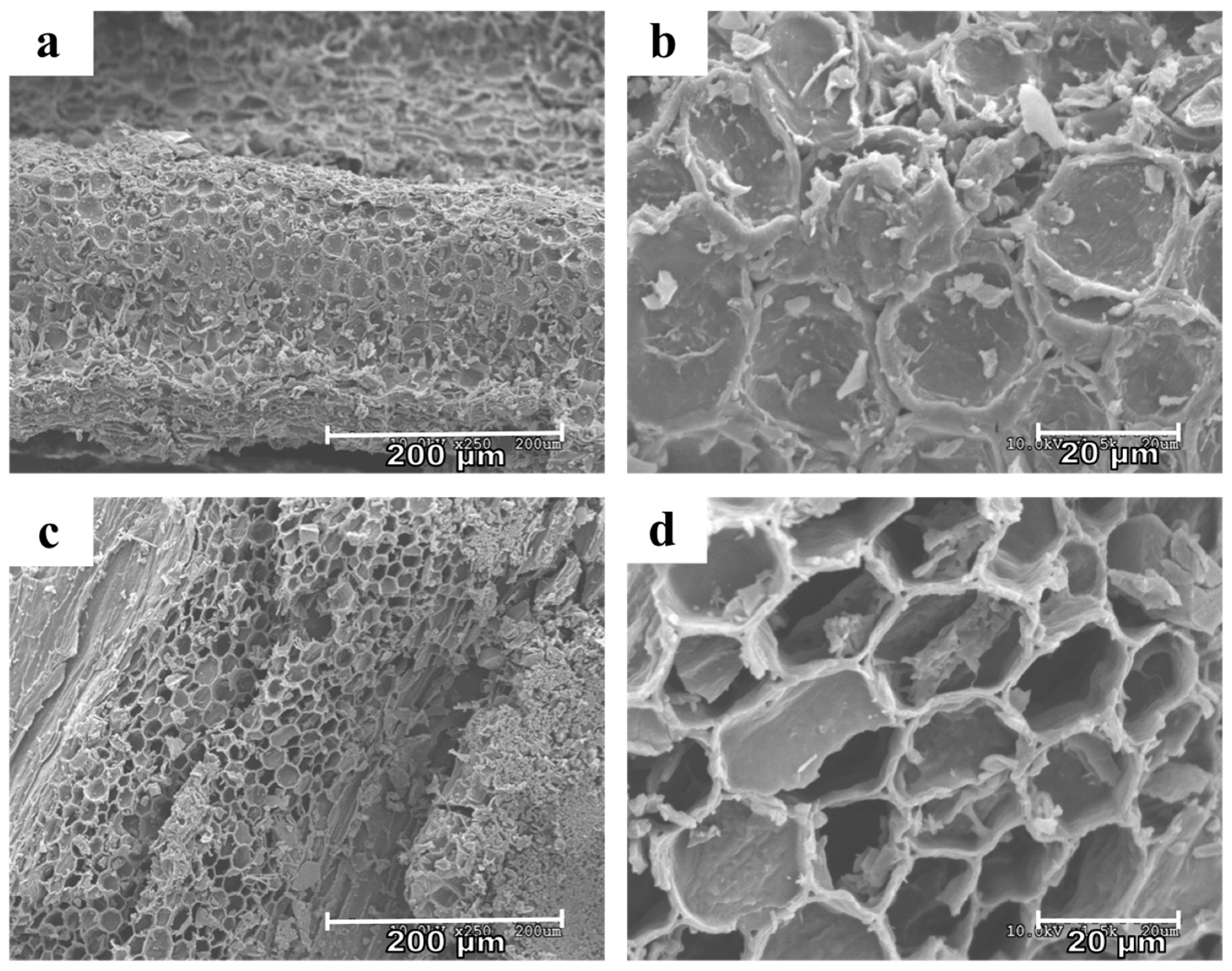
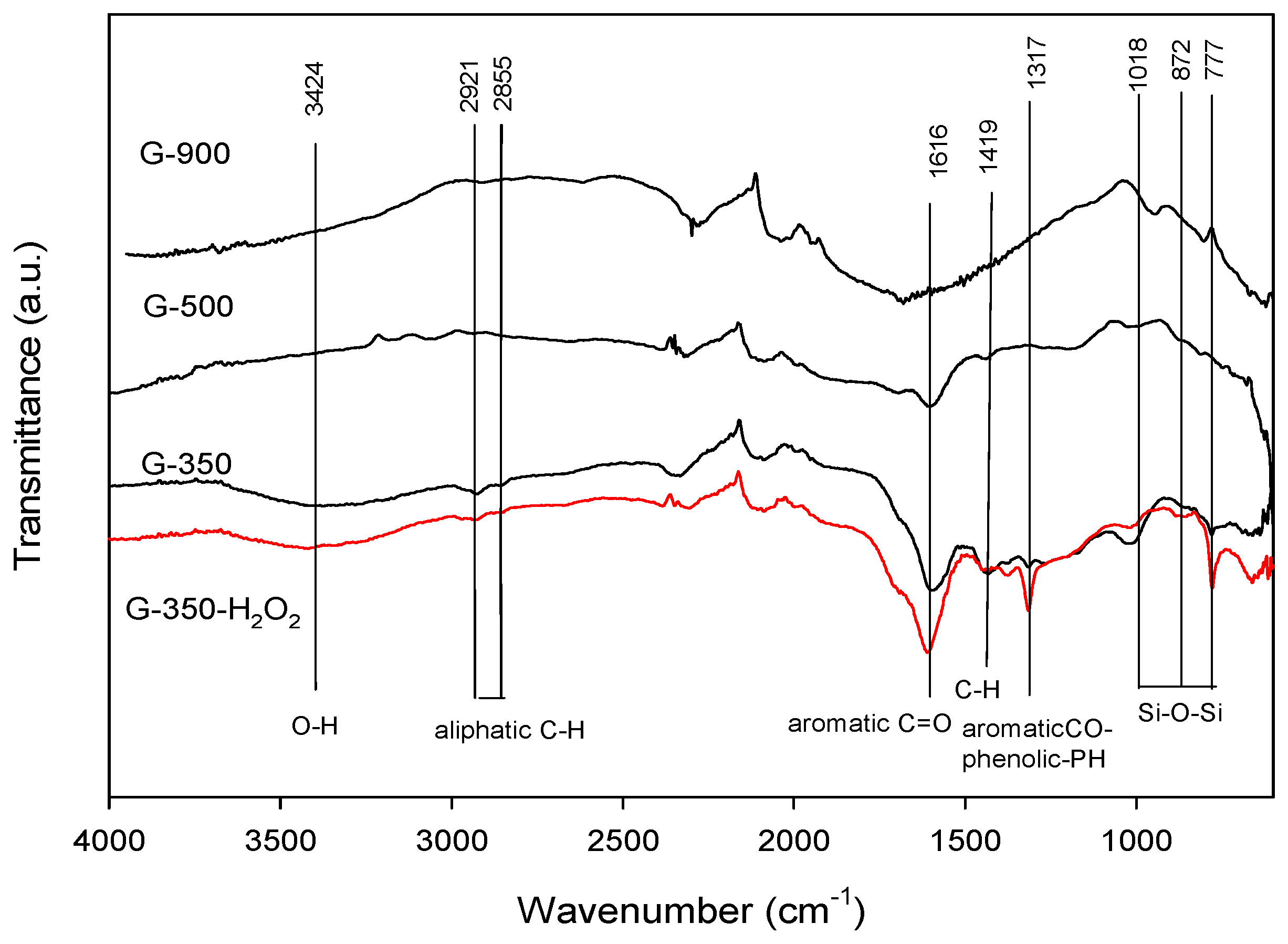
2.2. Biochar Characterization-G-350 Versus G-350 H2O2
2.3. Water Vapor and Herbicide Sorption
2.4. HPLC Analysis
2.5. Statistical Analyses
3. Results and Discussion
3.1. Biochar Activation Observations
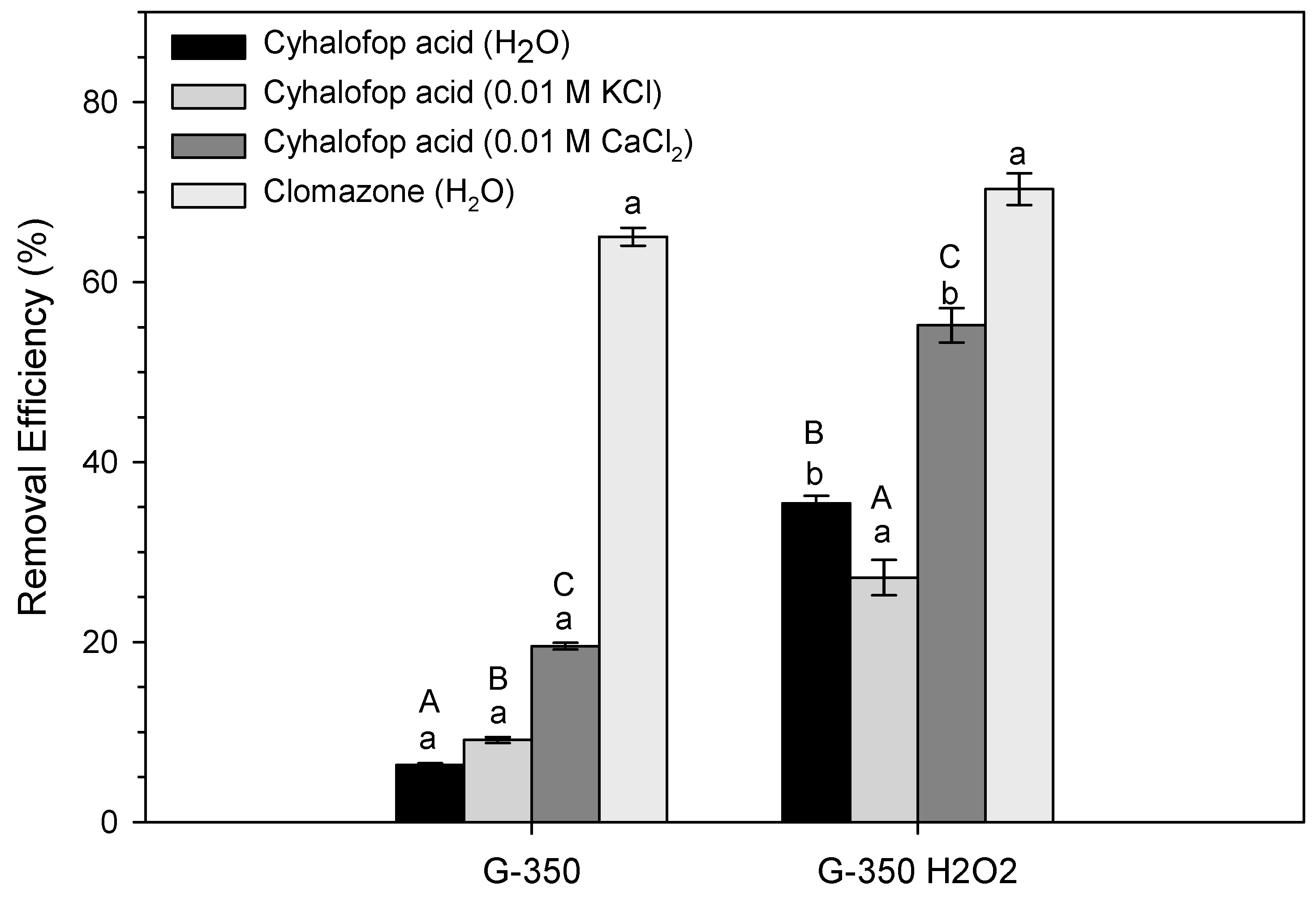
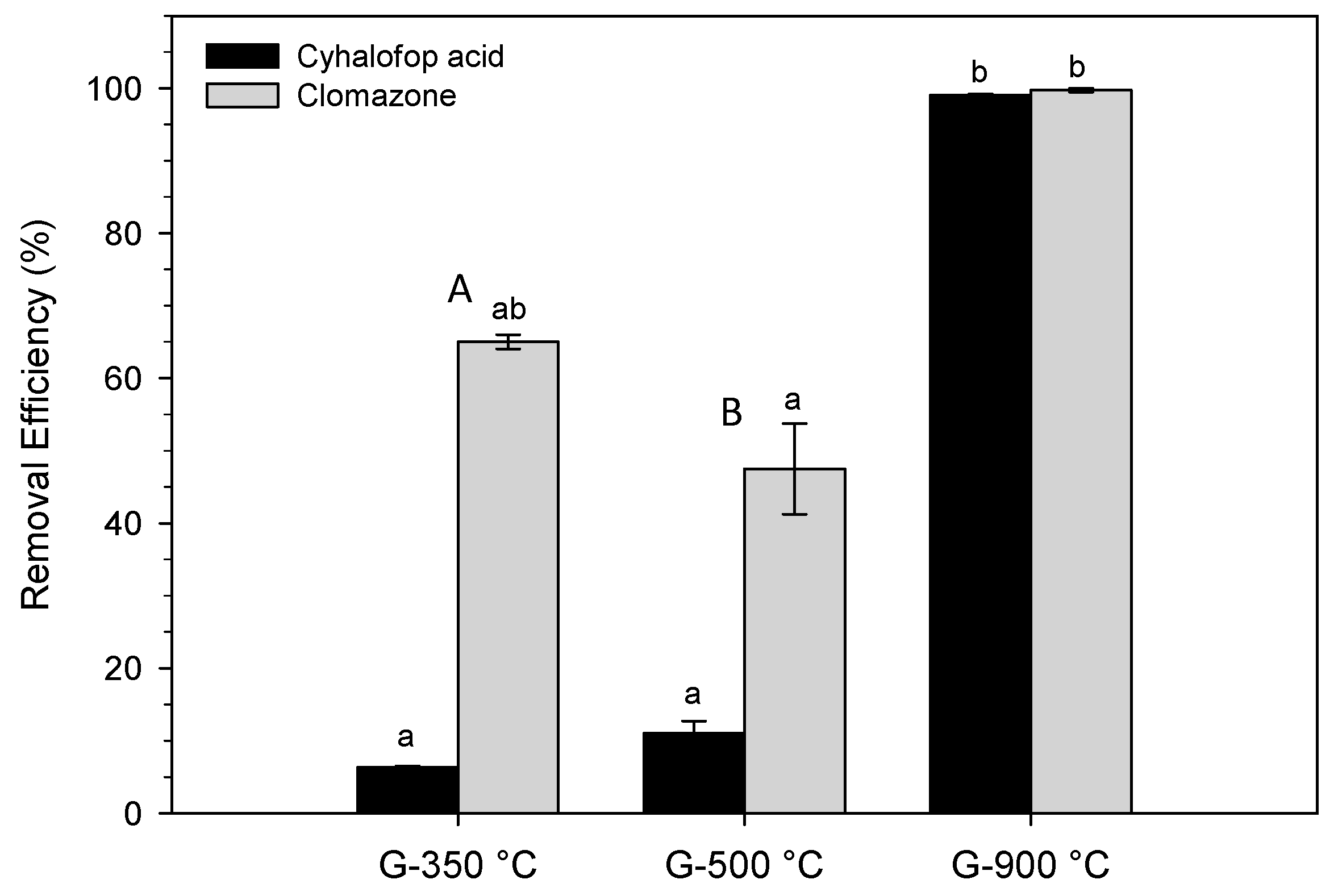
3.2. Herbicide Sorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, N.; Spokas, K.; Schmidt, H.P.; Kägi, R.; Böhler, M.A.; Bucheli, T.D. Activated carbon, biochar and charcoal: Linkages and synergies across pyrogenic carbon’s ABCs. Water 2018, 10, 1–19. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.W.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Sik, Y. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Azargohar, R.; Dalai, A.K. Steam and KOH activation of biochar: Experimental and modeling studies. Microporous Mesoporous Mater. 2008, 110, 413–421. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.J.; Mitch, W.A.; Xu, W. Activity and reactivity of pyrogenic carbonaceous matter toward organic compounds. Environ. Sci. Technol. 2017, 51, 8893–8908. [Google Scholar] [CrossRef] [PubMed]
- Ifthikar, J.; Wang, J.; Wang, Q.; Wang, T.; Wang, H.; Khan, A.; Jawad, A.; Sun, T.; Jiao, X.; Chen, Z. Highly efficient lead distribution by magnetic sewage sludge biochar: Sorption mechanisms and bench applications. Bioresour. Technol. 2017, 238, 399–406. [Google Scholar] [CrossRef]
- Moreno-Castilla, C. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon N. Y. 2004, 42, 83–94. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Pignatello, J.J. Effects of post-pyrolysis air oxidation of biomass chars on adsorption of neutral and ionic compounds. Environ. Sci. Technol. 2016, 50, 6276–6283. [Google Scholar] [CrossRef]
- Kupryianchyk, D.; Hale, S.; Zimmerman, A.R.; Harvey, O.; Rutherford, D.; Abiven, S.; Knicker, H.; Schmidt, H.P.; Rumpel, C.; Cornelissen, G. Sorption of hydrophobic organic compounds to a diverse suite of carbonaceous materials with emphasis on biochar. Chemosphere 2016, 144, 879–887. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.J.; Jiang, H.; Chen, J.J.; Li, W.W.; Yu, H.Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef]
- Lawrinenko, M.; Laird, D.A.; Johnson, R.L.; Jing, D. Accelerated aging of biochars: Impact on anion exchange capacity. Carbon N. Y. 2016, 103, 217–227. [Google Scholar] [CrossRef]
- Mia, S.; Dijkstra, F.A.; Singh, B. Aging induced changes in biochar’s functionality and adsorption behavior for phosphate and ammonium. Environ. Sci. Technol. 2017, 51, 8359–8367. [Google Scholar] [CrossRef] [PubMed]
- Gámiz, B.; Velarde, P.; Spokas, K.A.; Celis, R.; Cox, L. Changes in sorption and bioavailability of herbicides in soil amended with fresh and aged biochar. Geoderma 2019, 337, 341–349. [Google Scholar] [CrossRef]
- Ray, P.G.; Pews, R.G.; Flake, J.; Secor, J.; Hamburg, A. Cyhalofop butyl: A new graminicide for use in rice. In Proceedings of the 10th Australian Weeds Conference and 14th Asian Pacific Weed Science Society Conference, Brisbane, Australia, 6 August–10 September 1993. [Google Scholar]
- Jackson, R.; Douglas, M. An aquatic risk assessment for cyhalofop-butyl: A new herbicide for control of barnyard grass in rice. In Proceedings of the Human and Environmental Exposure to Xenobiotics, Proceedings of the XI Symposium Pesticide Chemistry, Cremona, Italy, 11–15 September 1999; Volume 1999, pp. 345–354. [Google Scholar]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; De Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE 2017, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Huff, M.D.; Lee, J.W. Biochar-surface oxygenation with hydrogen peroxide. J. Environ. Manage. 2016, 165, 17–21. [Google Scholar] [CrossRef]
- McDermot, H.L.; Arnell, J.C. Charcoal sorption studies. II The sorption of water by hydrogen treated charcoals. J. Phys. Chem. 1954, 58, 492–498. [Google Scholar] [CrossRef]
- Medic, D.; Darr, M.; Shah, A.; Rahn, S. Effect of torrefaction on water vapor adsorption properties and resistance to microbial degradation of corn stover. Energy & Fuels 2012, 26, 2386–2393. [Google Scholar]
- Rockland, L.B. Saturated salt solutions for static control of relative humidity between 5o and 40C. Anal. Chem. 1960, 32, 1375–1376. [Google Scholar] [CrossRef]
- OECD (Economic Co-operation and Development). Guideline 106: Adsorption—Desorption Using a Batch Equilibrium Method; OECD Publishing: Paris, France, 2000; ISBN 9789264069602. [Google Scholar]
- Wu, J.; Wang, K.; Zhang, Y.; Zhang, H. Determination and study on dissipation and residue determination of cyhalofop-butyl and its metabolite using HPLC-MS/MS in a rice ecosystem. Environ. Monit. Assess. 2014, 186, 6959–6967. [Google Scholar] [CrossRef]
- Gámiz, B.; Velarde, P.; Spokas, K.A.; Hermosín, M.C.; Cox, L. Biochar soil additions affect herbicide fate: Importance of application timing and feedstock species. J. Agric. Food Chem. 2017, 65, 3109–3117. [Google Scholar] [CrossRef]
- Yang, J.; Pignatello, J.J.; Pan, B.; Xing, B. Degradation of p -nitrophenol by lignin and cellulose chars: H2O2 -mediated reaction and direct reaction with the char. Environ. Sci. Technol. 2017, 51, 8972–8980. [Google Scholar] [CrossRef] [PubMed]
- Lousada, C.M.; Yang, M.; Nilsson, K.; Jonsson, M. Catalytic decomposition of hydrogen peroxide on transition metal and lanthanide oxides. J. Mol. Catal. A Chem. 2013, 379, 178–184. [Google Scholar] [CrossRef]
- Fry, H.S.; Milstead, K.L. The action of hydrogen peroxide upon simple carbon compounds. III. Glycolic acid. J. Am. Chem. Soc. 1935, 57, 2269–2272. [Google Scholar] [CrossRef]
- Gellerstedt, G.; Pettersson, I. Chemical aspects of hydrogen peroxide bleaching-Part II the bleaching of kraft pulps. J. Wood Chem. Technol. 1982, 23, 231–250. [Google Scholar] [CrossRef]
- Robinson, W.O. The determination of organic matter in soils by means of hydrogen peroxide. J. Agric. Res. 1927, 34, 339–356. [Google Scholar]
- Li, F.; Shen, K.; Long, X.; Wen, J.; Xie, X.; Zeng, X.; Liang, Y.; Wei, Y.; Lin, Z.; Huang, W.; et al. Preparation and characterization of biochars from Eichornia crassipes for cadmium removal in aqueous solutions. PLoS ONE 2016, 11, e0148132. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Trigo, C.; Cox, L.; Spokas, K. Influence of pyrolysis temperature and hardwood species on resulting biochar properties and their effect on azimsulfuron sorption as compared to other sorbents. Sci. Total Environ. 2016, 566–567, 1454–1464. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Rhoads, C.A.; Senftle, J.T.; Coleman, M.M.; Davis, A.; Painter, P.C. Further studies of coal oxidation. Fuel 1983, 62, 1387–1392. [Google Scholar] [CrossRef]
- Li, X.; Shen, Q.; Zhang, D.; Mei, X.; Ran, W.; Xu, Y.; Yu, G. Functional groups determine biochar properties (pH and EC) as studied by two-dimensional 13C NMR correlation spectroscopy. PLoS ONE 2013, 8, e65949. [Google Scholar] [CrossRef]
- Kinney, T.J.; Masiello, C.A.; Dugan, B.; Hockaday, W.C.; Dean, M.R.; Zygourakis, K.; Barnes, R.T. Hydrologic properties of biochars produced at different temperatures. Biomass and Bioenergy 2012, 41, 34–43. [Google Scholar] [CrossRef]
- PPDB Pesticides Properties Database. Available online: http://sitem.herts.ac.uk/aeru/bpdb/index.htm (accessed on 25 September 2019).
- Sigmund, G.; Sun, H.; Hofmann, T.; Kah, M. Predicting the sorption of aromatic acids to noncarbonized and carbonized sorbents. Environ. Sci. Technol. 2016, 50, 3641–3648. [Google Scholar] [CrossRef]
- Ni, J.Z.; Pignatello, J.J.; Xing, B.S. Adsorption of aromatic carboxylate ions to black carbon (biochar) is accompanied by proton exchange with water. Environ. Sci. Technol. 2011, 45, 9240–9248. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Gray, M.; Johnson, M.G.; Dragila, M.I.; Kleber, M. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass Bioenergy 2014, 61, 196–205. [Google Scholar] [CrossRef]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.A.; Muñoz, M.A.; Faz, A. Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef]
- Jia, M.; Wang, F.; Bian, Y.; Jin, X.; Song, Y.; Kengara, F.O.; Xu, R.; Jiang, X. Effects of pH and metal ions on oxytetracycline sorption to maize-straw-derived biochar. Bioresour. Technol. 2013, 136, 87–93. [Google Scholar] [CrossRef]
- Li, X.; Gámiz, B.; Wang, Y.; Pignatello, J.J.; Xing, B. Competitive sorption used to probe strong hydrogen bonding sites for weak organic acids on carbon nanotubes. Environ. Sci. Technol. 2015, 49, 1409–1417. [Google Scholar] [CrossRef]
- Peng, B.; Chen, L.; Que, C.; Yang, K.; Deng, F.; Deng, X.; Shi, G.; Xu, G.; Wu, M. Adsorption of antibiotics on graphene and biochar in aqueous solutions induced by π-π interactions. Sci. Rep. 2016, 6, 31920. [Google Scholar] [CrossRef]
- Sun, K.; Jin, J.; Keiluweit, M.; Kleber, M.; Wang, Z.; Pan, Z.; Xing, B. Polar and aliphatic domains regulate sorption of phthalic acid esters (PAEs) to biochars. Bioresour. Technol. 2012, 118, 120–127. [Google Scholar] [CrossRef]

| Feedstock | Pyrolysis Temperature (°C) | Moisture % | Ash % | VM 1 % | C % | H % | N % | O % | SSA 2 (m2 g−1) |
|---|---|---|---|---|---|---|---|---|---|
| Grape wood | 350 | 3.5 | 10.9 | 39.5 | 66.6 | 4.0 | 1.1 | 17.5 | < 1 |
| Grape wood | 500 | 4.0 | 16.8 | 19.3 | 70.4 | 2.3 | 0.9 | 9.6 | < 1 |
| Grape wood | 900 | 1.3 | 22.2 | 6.6 | 71.6 | 0.1 | 1.0 | 4.9 | 124 |
| G-350 | G-350-H2O2 | |||||
|---|---|---|---|---|---|---|
| Herbicide | Kf | Nf | R2 | Kf | Nf | R2 |
| Cyhalofop | 13.9 (12.6–15.4) 1 | 0.92 ± 0.09 2 | 0.970 | 48.3 (44.6–52.4) | 0.75 ± 0.07 | 0.976 |
| Clomazone | 284 (264–306) | 0.81 ± 0.05 | 0.990 | 284 (265–305) | 0.74 ± 0.04 | 0.990 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gámiz, B.; Hall, K.; Spokas, K.A.; Cox, L. Understanding Activation Effects on Low-Temperature Biochar for Optimization of Herbicide Sorption. Agronomy 2019, 9, 588. https://doi.org/10.3390/agronomy9100588
Gámiz B, Hall K, Spokas KA, Cox L. Understanding Activation Effects on Low-Temperature Biochar for Optimization of Herbicide Sorption. Agronomy. 2019; 9(10):588. https://doi.org/10.3390/agronomy9100588
Chicago/Turabian StyleGámiz, Beatriz, Kathleen Hall, Kurt A. Spokas, and Lucia Cox. 2019. "Understanding Activation Effects on Low-Temperature Biochar for Optimization of Herbicide Sorption" Agronomy 9, no. 10: 588. https://doi.org/10.3390/agronomy9100588
APA StyleGámiz, B., Hall, K., Spokas, K. A., & Cox, L. (2019). Understanding Activation Effects on Low-Temperature Biochar for Optimization of Herbicide Sorption. Agronomy, 9(10), 588. https://doi.org/10.3390/agronomy9100588






