Characterization of Chromosomal Rearrangement in New Wheat—Thinopyrum intermedium Addition Lines Carrying Thinopyrum—Specific Grain Hardness Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Fluorescence In Situ Hybridization (FISH)
2.3. 90K SNP Arrays Analysis
2.4. Molecular Marker Analysis
2.5. Cloning and Sequencing of the Pina Gene
2.6. Agronomic Traits Survey and Grain Hardness Tests
3. Results
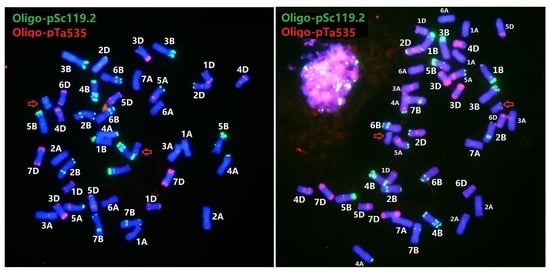
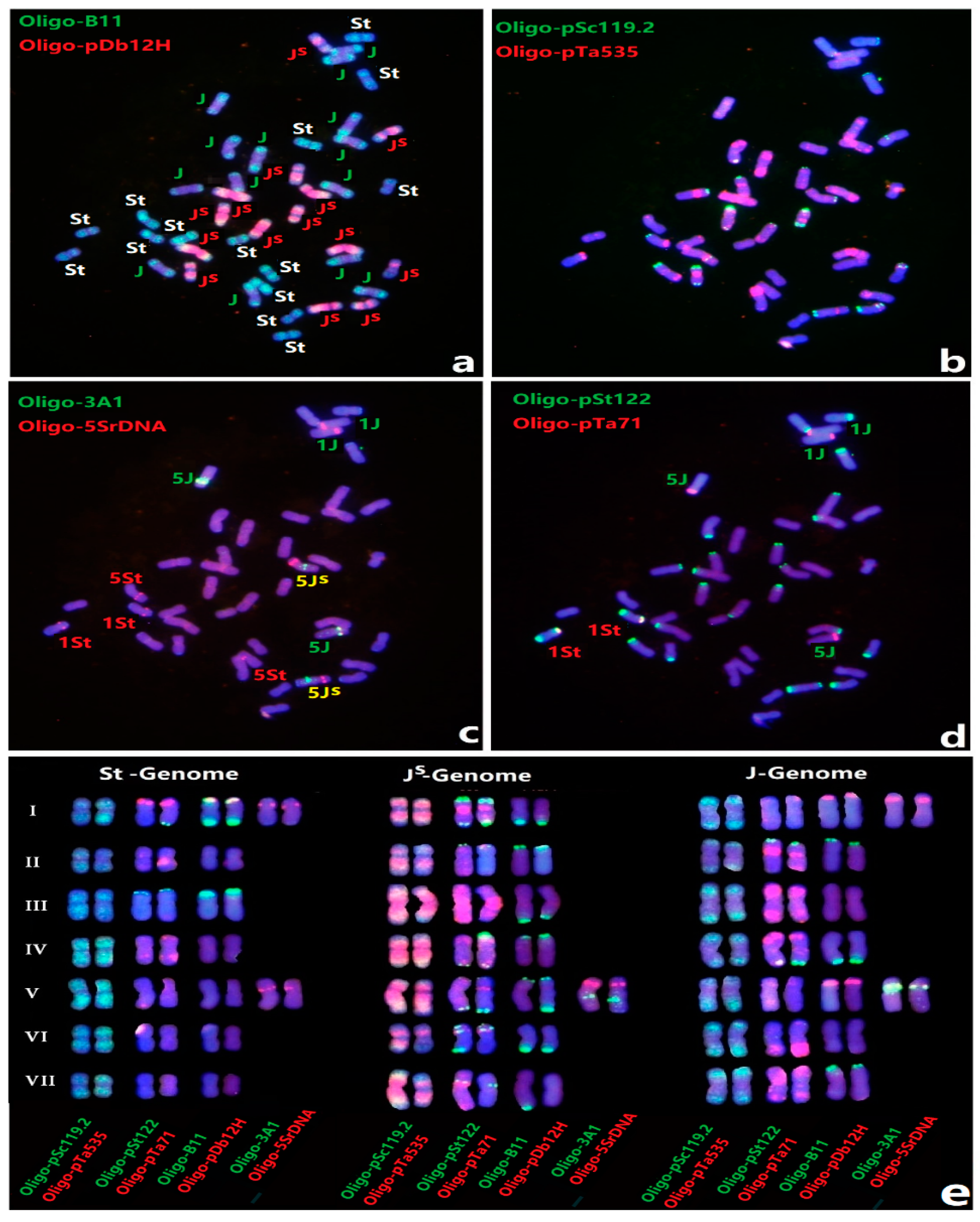
3.1. FISH of Th. Intermedium by Multiple Oligo Probes
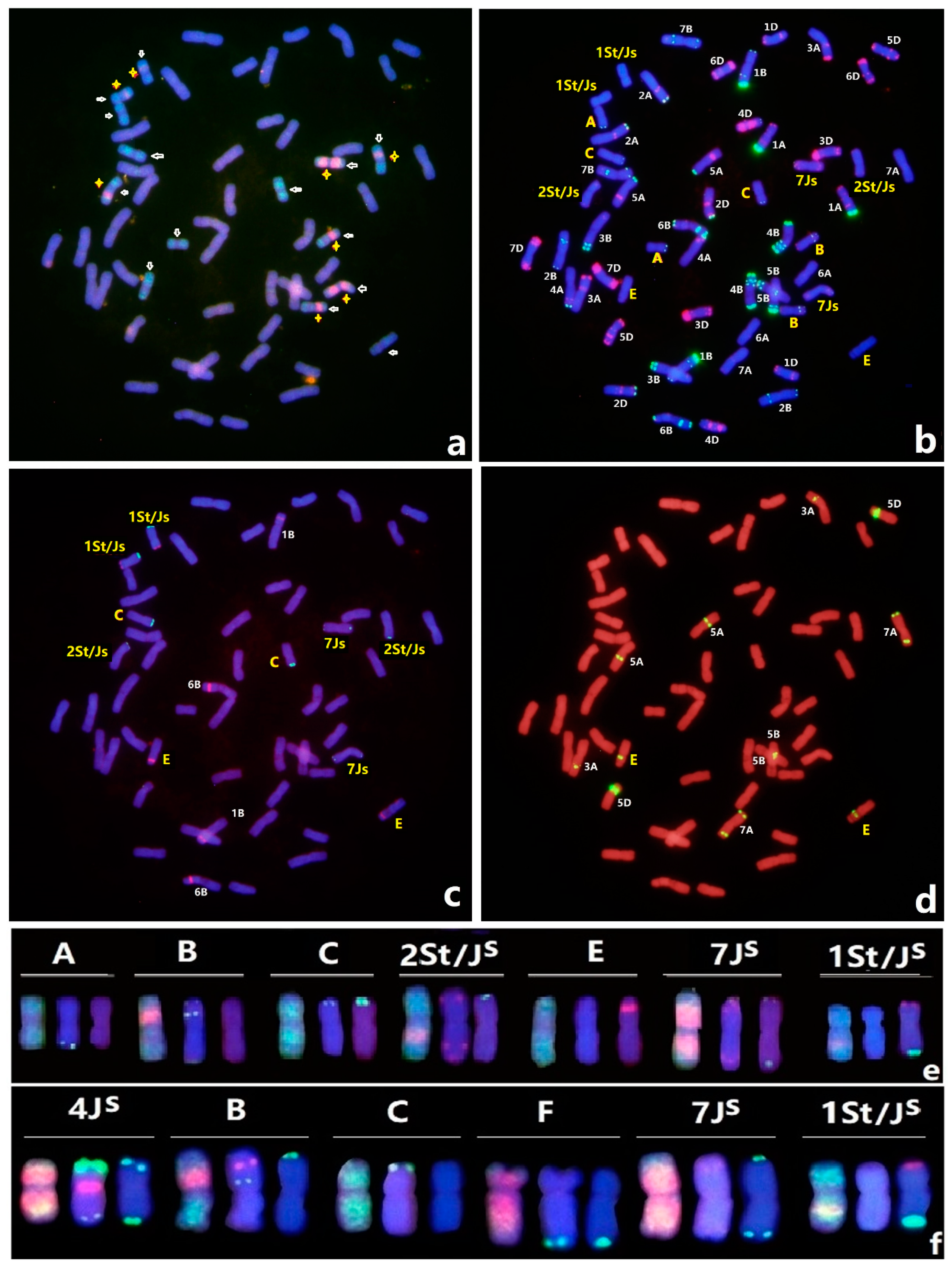
3.2. An Updated FISH Karyotype of Zhong 5 and Zhong 2
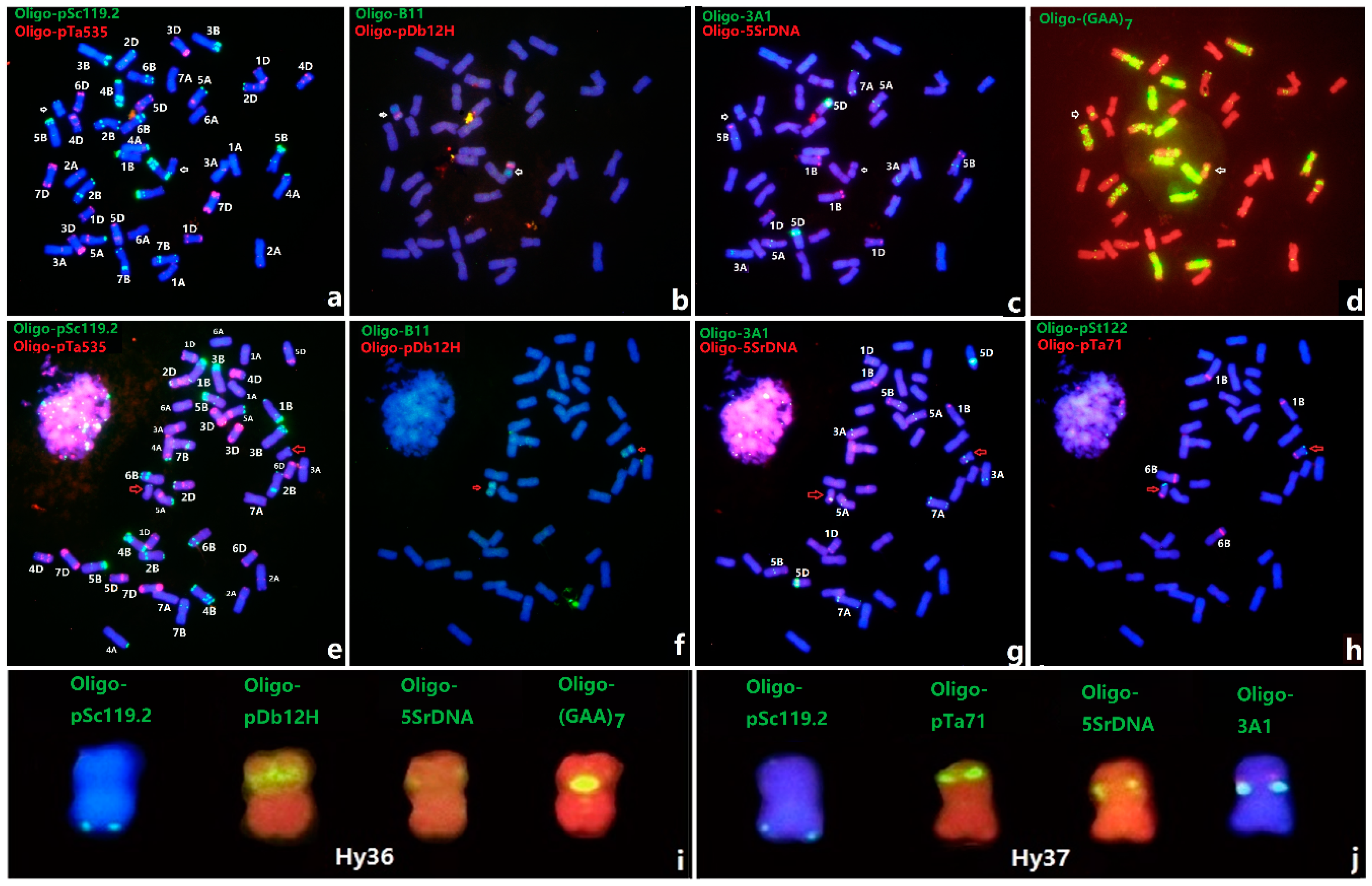
3.3. ND-FISH of Lines Hy36 and Hy37
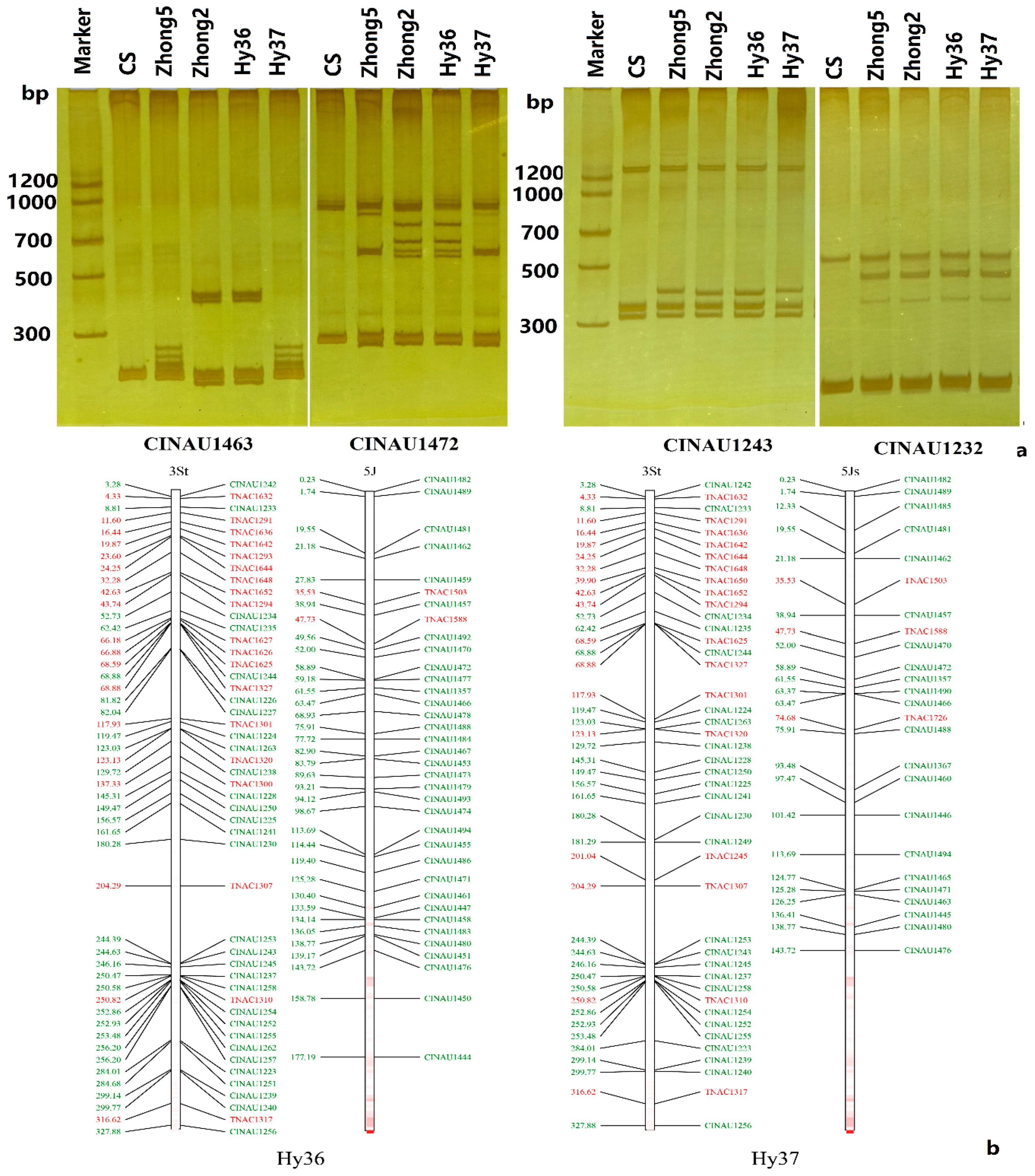
3.4. Molecular Markers Validation of Linkage Groups
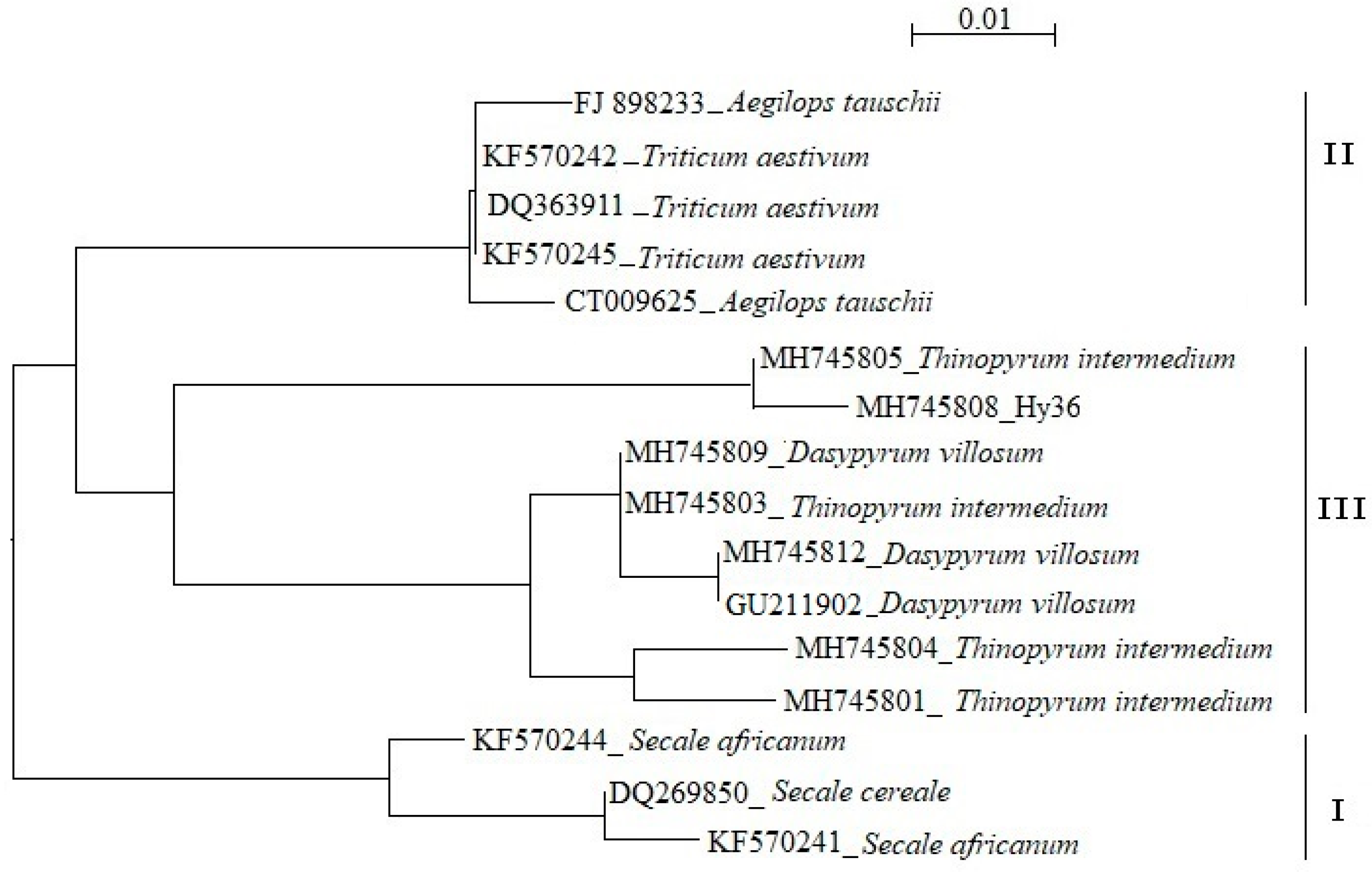
3.5. Molecular Cloning of Th. Intermedium—Specific Pina-like Genes
3.6. Agronomic Traits and Grain Quality Observation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dewey, D.R. The genomic system of classification as a guide to intergeneric hybridization in the perennial Triticeae. In Gene Manipulation in Plant Improvement; Gustafson, J.P., Ed.; Plenum: New York, NY, USA, 1984; pp. 209–279. [Google Scholar] [CrossRef]
- Chen, Q. Detection of alien chromatin introgression from Thinopyrum into wheat using S genomic DNA as a probe—A landmark approach for Thinopyrum genome research. Cytogenet. Genome Res. 2005, 109, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X. Thinopyrum ponticum and the promising source of resistance to fungal and viral diseases of wheat. J. Genet. Genom. 2009, 36, 557–565. [Google Scholar] [CrossRef]
- Cauderon, Y.; Saigne, B.; Dauge, M. The resistance to wheat rusts of Agropyron intermedium and its use in wheat improvement. In Proceedings of the fourth International Wheat Genetics Symposium, Columbia, MI, USA, 6–11 August 1973; Sears, E.R., Sears, L.M.S., Eds.; University of Missouri: Columbia, MI, USA, 1973; pp. 401–407. [Google Scholar]
- Chen, Q.; Conner, R.L.; Laroche, A.; Ji, W.; Armstrong, K.C.; Fedak, G. Genomic in situ hybridization analysis of Thinopyrum chromatin in a wheat—Th. intermedium partial amphiploid and six derived chromosome addition lines. Genome 1999, 42, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Li, G.R.; Chang, Z.J.; Zhou, J.P.; Ren, Z.L. Characterization of a partial amphiploid between Triticum aestivum cv. Chinese Spring and Thinopyrum intermedium ssp. trichophorum. Euphytica 2006, 149, 11–17. [Google Scholar] [CrossRef]
- Bao, Y.G.; Wu, X.; Zhang, C.; Li, X.F.; He, F.; Qi, X.L.; Wang, H.G. Chromosomal constitutions and reactions to powdery mildew and stripe rust of four novel wheat—Thinopyrum intermedium partial amphiploids. J. Genet. Genom. 2014, 12, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, Y.; Qi, J.; Wang, H.; Wang, R.C.C.; Bao, Y.; Li, X. Identification of chromosomes in Thinopyrum intermedium and wheat Th. intermedium amphiploids based on multiplex oligonucleotide probes. Genome 2018, 61, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Larkin, P.J.; Banks, P.M.; Lagudah, E.S.; Apple, R.; Chen, X.; Xin, Z.Y.; Ohm, H.W.; McIntosh, R.A. Disomic Thinopyrum intermedium addition lines in wheat with barley yellow dwarf virus resistance and with rust resistances. Genome 1995, 38, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Liu, B.; Fedak, G.; Liu, Z. Genomic constitution and variation in five partial amphiploids of wheat-Thinopyrum intermedium as revealed by GISH, multicolor GISH and seed storage protein analysis. Theor. Appl. Genet. 2004, 109, 1070–1076. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H. Characterization of three novel wheat-Thinopyrum intermedium addition lines with novel storage protein subunits and resistance to both powdery mildew and stripe rust. J. Genet. Genom. 2016, 43, 45–48. [Google Scholar] [CrossRef]
- Li, J.; Lang, T.; Li, B.; Yu, Z.; Wang, H.; Li, G.; Yang, E.; Yang, Z. Introduction of Thinopyrum intermedium ssp. trichophorum chromosomes to wheat by trigeneric hybridization involving Triticum, Secale and Thinopyrum genera. Planta 2017, 245, 1121–1135. [Google Scholar] [CrossRef]
- Kishii, M.; Wang, R.R.C.; Tsujimoto, H. GISH analysis revealed new aspect of genomic constitution of Thinopyrum intermedium. Czech. J. Genet. Plan. 2005, 41, 91–95. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Z.J.; Jia, J.Q.; Li, G.R.; Zhou, J.P.; Ren, Z.L. Genomic distribution of a long terminal repeat (LTR) Sabrina-like retrotransposon in Triticeae species. Cereal. Res. Commun. 2009, 37, 363–372. [Google Scholar] [CrossRef]
- Mahelka, V.; Kopecky, D.; Baum, B.R. Contrasting patterns of evolution of 45S and 5S rDNA families uncover new aspects in the genome constitution of the agronomically important grass Thinopyrum intermedium (Triticeae). Mol. Biol. Evol. 2013, 30, 2065–2086. [Google Scholar] [CrossRef] [PubMed]
- Mahelka, V.; Kopecky, D.; Pastova, L. On the genome constitution and evolution of intermediate wheatgrass (Thinopyrum intermedium, Poaceae, Triticeae). BMC Evol. Biol. 2011, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.F. Puroindolines: The molecular genetic basis of wheat grain hardness. Plant Mol. Biol. 2002, 48, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; Mukai, Y.; Leroy, P.; Charef, B.; Appels, R.; Rahman, S. The Ha locus of wheat: Identification of a polymorphic region for tracing grain hardness in crosses. Genome 1999, 42, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, M.; Takata, K.; Terasawa, Y.; Ikeda, T.M. Chromosome 5H of Hordeum species involved in reduction in grain hardness in wheat genetic background. Theor. Appl. Genet. 2011, 123, 1013–1018. [Google Scholar] [CrossRef]
- Escobar, J.S.; Scornavacca, C.; Cenci, A.; Guilhaumon, C.; Santoni, S.; Douzery, E.J.; Ranwez, V.; Glémin, S.; David, J. Multigenic phylogeny and analysis of tree incongruences in Triticeae (Poaceae). BMC Evol. Biol. 2011, 11, 181. [Google Scholar] [CrossRef]
- Li, G.; Gao, D.; La, S.; Wang, H.; Li, J.; He, W.; Yang, E.; Yang, Z. Characterization of wheat-Secale africanum chromosome 5Ra derivatives carrying Secale specific genes for grain hardness. Planta 2016, 243, 1203–1212. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Cao, Y.P.; Wang, X.E.; Feng, Y.G.; Chen, P.D. Development and characterization of a Triticum aestivum – H. villosa T5VS·5DL translocation line with soft grain texture. J. Cereal. Sci. 2010, 51, 220–225. [Google Scholar] [CrossRef]
- Sun, S.C. The approach and methods of breeding new varieties and new species from Agrotriticum hybrids. Acta Agron. Sin. 1981, 7, 51–58. (In Chinese) [Google Scholar]
- Han, F.P.; Lamb, J.C.; Birchler, J.A. High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc. Natl. Acad. Sci. USA 2006, 103, 3238–3243. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yang, Z.; Fu, S. Oligonucleotides replacing the roles of repetitive sequences pAs1.; pSc119.2.; pTa-535.; pTa71.; CCS1.; and pAWRC.1 for FISH analysis. J. Appl. Genet. 2014, 55, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, H.; Lang, T.; Li, J.; La, S.; Yang, E.; Yang, Z. New molecular markers and cytogenetic probes enable chromosome identification of wheat-Thinopyrum intermedium introgression lines for improving grain quality. Planta 2016, 244, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, W.L.; Dyer, T.A. Sequence organization of the repeating units in the nucleus of wheat which contain 5S rRNA genes. Nucleic Acids Res. 1980, 8, 4851–4865. [Google Scholar] [CrossRef]
- Yang, Z.J.; Liu, C.; Feng, J.; Li, G.R.; Zhou, J.P.; Deng, K.J.; Ren, Z.L. Studies on genomic relationship and specific marker of Dasypyrum breviaristatum in Triticeae. Hereditas 2006, 143, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Tang, C.G.; Zhao, J.; Zheng, Q.; Li, B.; Hao, C.Y.; Li, Z.S.; Zhang, X.Y. Isolation of Thinopyrum ponticum genome specific repetitive sequences and their application for effective detection of alien segments in wheat. Sci. Agric. Sin. 2016, 49, 3683–3693. [Google Scholar] [CrossRef]
- Fu, S.; Chen, L.; Wang, Y.; Li, M.; Yang, Z.; Qiu, L.; Yan, B.; Ren, Z.; Tang, Z. Oligonucleotide probes for ND-FISH analysis to identify rye and wheat chromosomes. Sci. Rep. 2015, 5, 10552. [Google Scholar] [CrossRef]
- Wang, S.; Wong, D.; Forrest, K.; Allen, A.; Chao, S.; Huang, B.E.; Maccaferri, M.; Salvi, S.; Milner, S.G.; Cattivelli, L.; et al. Characterization of polyploid wheat genomic diversity using the high-density 90.;000 SNP array. Plant Biotechnol. J. 2014, 12, 787–796. [Google Scholar] [CrossRef]
- Yuan, F.; Zeng, Q.; Wu, J.; Wang, Q.; Yang, Z.; Liang, B.; Kang, Z.; Chen, X.X.; Han, D. QTL mapping and validation of adult plant resistance to stripe rust in Chinese wheat landrace Humai 15. Front. Plant Sci. 2018, 9, 968. [Google Scholar] [CrossRef]
- Ishikawa, G.; Nakamura, T.; Ashida, T.; Saito, M.; Nasuda, S.; Endo, T.; Wu, J. Localization of anchor loci representing five hundred annotated rice genes to wheat chromosomes using PLUG markers. Theor. Appl. Genet. 2009, 118, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dai, K.; Xiao, J.; Yuan, C.; Zhao, R.; Doležel, J.; Wu, Y.; Cao, A.; Chen, P.; Zhang, S.; Wang, X. Development of intron targeting (IT) markers specific for chromosome arm 4VS of Haynaldia villosa by chromosome sorting and next-generation sequencing. BMC Genom. 2017, 18, 167. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Lang, T.; La, S.; Li, B.; Yu, Z.; Chen, Q.; Li, J.; Yang, E.; Li, G.; Yang, Z. Precise identification of wheat- Thinopyrum intermedium translocation chromosomes carrying resistance to wheat stripe rust in line Z4 and its derived progenies. Genome 2018, 61, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Danilova, T.V.; Friebe, B.; Gill, B.S. Single–copy gene fluorescence in situ hybridization and genome analysis: Acc–2 loci mark evolutionary chromosomal rearrangements in wheat. Chromosoma 2012, 121, 597–611. [Google Scholar] [CrossRef]
- Lang, T.; Li, G.; Wang, H.; Yu, Z.; Chen, Q.; Yang, E.; Fu, S.; Tang, Z.; Yang, Z. Physical location of tandem repeats in the wheat genome and application for chromosome identification. Planta 2018. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Tang, Z.; Qiu, L.; Yang, Z.; Li, G.; Lang, T.; Zhu, W.; Zhang, J.; Fu, S. Developing new Oligo probes to distinguish specific chromosomal segments and the A.; B.; D genomes of wheat (Triticum aestivum L.) using ND-FISH. Front. Plant Sci. 2018, 9, 1104. [Google Scholar] [CrossRef]
- Deng, C.; Bai, L.; Fu, S.; Yin, W.; Zhang, Y.; Chen, Y.; Wang, R.R.; Zhang, X.; Han, F.; Hu, Z. Microdissection and chromosome painting of the alien chromosome in an addition line of wheat—Thinopyrum intermedium. PLoS ONE 2013, 8, e72564. [Google Scholar] [CrossRef]
- Chen, Q.; Conner, R.L.; Li, H.J.; Sun, S.C.; Ahmad, F.; Laroche, A.; Graf, R.J. Molecular cytogenetic discrimination and reaction to wheat streak mosaic virus and the wheat curl mite in Zhong series of wheat—Thinopyrum intermedium partial amphiploids. Genome 2003, 46, 135–145. [Google Scholar] [CrossRef]
- Tang, S.; Li, Z.; Jia, X.; Larkin, P.J. Genomic in situ hybridization (GISH) analyses of Thinopyrum intermedium, its partial amphiploid Zhong 5, and disease-resistant derivatives in wheat. Theor. Appl. Genet. 2000, 100, 344–352. [Google Scholar] [CrossRef]
- Han, F.P.; Fedak, G.; Benabdelmouna, A.; Armstrong, K.; Ouellet, T. Characterization of six wheat × Thinopyrum intermedium derivatives by GISH.; RFLP.; and multicolor GISH. Genome 2003, 46, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.M.; Xu, S.J.; Wang, R.R.-C.; Larkin, P.J. Varying chromosome composition of 56-chromosome wheat × Thinopyrum intermedium partial amphiploids. Genome 1993, 36, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Fedak, G.; Chen, Q.; Conner, R.L.; Laroche, A.; Petroski, R.; Armstrong, K.W. Characterization of wheat-Thinopyrum partial amphiploids by meiotic analysis and genomic in situ hybridization. Genome 2000, 43, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, M.; Sepsi, A.; Tyankova, N.; Molnár-Láng, M. Molecular cytogenetic characterization of two high protein wheat-Thinopyrum intermedium partial amphiploids. J. Appl. Genet. 2011, 52, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Appels, R.; Gerlach, W.L.; Dennis, E.S.; Swift, H.; Peacock, W.J. Molecular and chromosomal organization of DNA sequences coding for the ribosomal RNAs in cereals. Chromosoma 1980, 78, 293–311. [Google Scholar] [CrossRef]
- Gerlach, W.L.; Bedbrook, J.R. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979, 7, 1869–1885. [Google Scholar] [CrossRef]
- Badaeva, E.D.; Friebe, B.; Gill, B.S. Genome differentiation in Aegilops. 2. Physical mapping of 5S and 18S–26S ribosomal RNA gene families in diploid species. Genome 1996, 39, 1150–1158. [Google Scholar] [CrossRef]
- Mukai, Y.; Endo, T.R.; Gill, B.S. Physical mapping of the 5S rRNA multigene family in common wheat. J. Hered. 1990, 81, 290–295. [Google Scholar] [CrossRef]
- Li, D.Y.; Ru, Y.Y.; Zhang, X.Y. Chromosomal Distribution of the 18S-5.8S–26S rDNA Loci and Heterogeneity of Nuclear ITS Regions in Thinopyrum intermedium (Poaceae, Triticeae). Acta Bot. Sin. 2004, 46, 1234–1241. [Google Scholar]
- Li, G.; Lang, T.; Dai, G.; Li, D.; Li, C.; Song, X.; Yang, Z. Precise identification of two wheat-Thinopyrum intermedium substitutions reveals the compensation and rearrangement between wheat and Thinopyrum chromosomes. Mol. Breed. 2015, 35, 1. [Google Scholar] [CrossRef]
- Gautier, M.F.; Cosson, P.; Guirao, A.; Alary, R.; Joudrier, P. Puroindoline genes are highly conserved in diploid ancestor wheats and related species but absent in tetraploid Triticum species. Plant Sci. 2000, 153, 81–91. [Google Scholar] [CrossRef]
- Massa, A.N.; Morris, C.F. Molecular evolution of the Puroindoline-a, Puroindoline-b, and grain softness Protein-1 genes in the tribe Triticeae. J. Mol. Evol. 2006, 63, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, K.M.; Turner, M.; Mukai, Y.; Yamamoto, M.; Morell, M.K.; Appels, R.; Rahman, S. The organization of genes tightly linked to the Ha locus in Aegilops tauschii, the D-genome donor to wheat. Genome 2003, 46, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Gazza, L.; Galassi, E.; Ciccoritti, R.; Cacciatori, P.; Pogna, N.E. Qualitative traits of perennial wheat lines derived from different Thinopyrum species. Genet. Resour. Crop Evol. 2016, 63, 209–219. [Google Scholar] [CrossRef]
- Alfred, R.L.; Palombo, E.A.; Panozzo, J.F.; Bariana, H.; Bhave, M. Stability of puroindoline peptides and effects on wheat rust. World J. Microbiol. Biotechnol. 2013, 29, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, L.; Hao, M.; Ni, S.; Yuan, Z.; Dai, S.; Huang, L.; Wu, B.; Yan, Z.; Lan, X.; et al. Wheat breeding in the hometown of Chinese Spring. Crop J. 2018, 6, 82–90. [Google Scholar] [CrossRef]
| Agronomic and Quality Traits | CS | Hy36 | Hy37 |
|---|---|---|---|
| Plant height (cm) | 142 ± 2.65 | 118.91 ± 6.34 ** | 111.08 ± 8.99 ** |
| Tillers per plant | 28 ± 1.00 | 20.09 ± 7.44 * | 10.75 ± 7.11 ** |
| Spike length (cm) | 9.93 ± 0.12 | 10.54 ± 1.36 | 11.72 ± 0.96 * |
| Spikelet number | 26.33 ± 0.58 | 23.10 ± 1.55 | 21.83 ± 1.47 |
| Grain length (cm) | 5.93 ± 0.07 | 6.61 ± 0.11 * | 5.92 ± 0.07 |
| Grain width | 2.7 ± 0.04 | 2.92 ± 0.12 | 3.00 ± 0.06 |
| Thousand-kernel weight (g) | 25.30 ± 0.68 | 25.97 ± 0.47 | 24.87 ± 0.39 |
| Protein content (%) | 11.86 ± 0.28 | 14.80 ± 0.42 * | 14.51 ± 0.36 * |
| Test weight (g/L) | 732.10 ± 12.00 | 706.20 ± 6.08 * | 741.00 ± 10.50 |
| Wet gluten content (%) | 29.58 ± 0.85 | 36.41 ± 0.70 * | 36.25 ± 1.22 * |
| Stable time (min) | 2.5 ± 0.28 | 4.08 ± 0.30 * | 5.60 ± 0.56 * |
| Grain hardness | 55.0 ± 3.28 | 47.8 ± 2.16 * | 56.0 ± 3.11 |
| Zeleny sedimentation value (mL) | 36.8 ± 4.08 | 50.98 ± 2.08 ** | 44.47 ± 2.77 * |
| Milling yield (%) | 62.9 ± 3.25 | 50.92 ± 2.20 * | 59.10 ± 4.80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Wang, H.; Xu, Y.; Li, Y.; Lang, T.; Yang, Z.; Li, G. Characterization of Chromosomal Rearrangement in New Wheat—Thinopyrum intermedium Addition Lines Carrying Thinopyrum—Specific Grain Hardness Genes. Agronomy 2019, 9, 18. https://doi.org/10.3390/agronomy9010018
Yu Z, Wang H, Xu Y, Li Y, Lang T, Yang Z, Li G. Characterization of Chromosomal Rearrangement in New Wheat—Thinopyrum intermedium Addition Lines Carrying Thinopyrum—Specific Grain Hardness Genes. Agronomy. 2019; 9(1):18. https://doi.org/10.3390/agronomy9010018
Chicago/Turabian StyleYu, Zhihui, Hongjin Wang, Yunfang Xu, Yongshang Li, Tao Lang, Zujun Yang, and Guangrong Li. 2019. "Characterization of Chromosomal Rearrangement in New Wheat—Thinopyrum intermedium Addition Lines Carrying Thinopyrum—Specific Grain Hardness Genes" Agronomy 9, no. 1: 18. https://doi.org/10.3390/agronomy9010018
APA StyleYu, Z., Wang, H., Xu, Y., Li, Y., Lang, T., Yang, Z., & Li, G. (2019). Characterization of Chromosomal Rearrangement in New Wheat—Thinopyrum intermedium Addition Lines Carrying Thinopyrum—Specific Grain Hardness Genes. Agronomy, 9(1), 18. https://doi.org/10.3390/agronomy9010018