Growth and Physiological Responses of Adenophora triphylla (Thunb.) A.DC. Plug Seedlings to Day and Night Temperature Regimes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Measurement of Soluble Protein, Soluble Sugar, and Starch
2.3. Determination of Total Phenol and Flavonoid
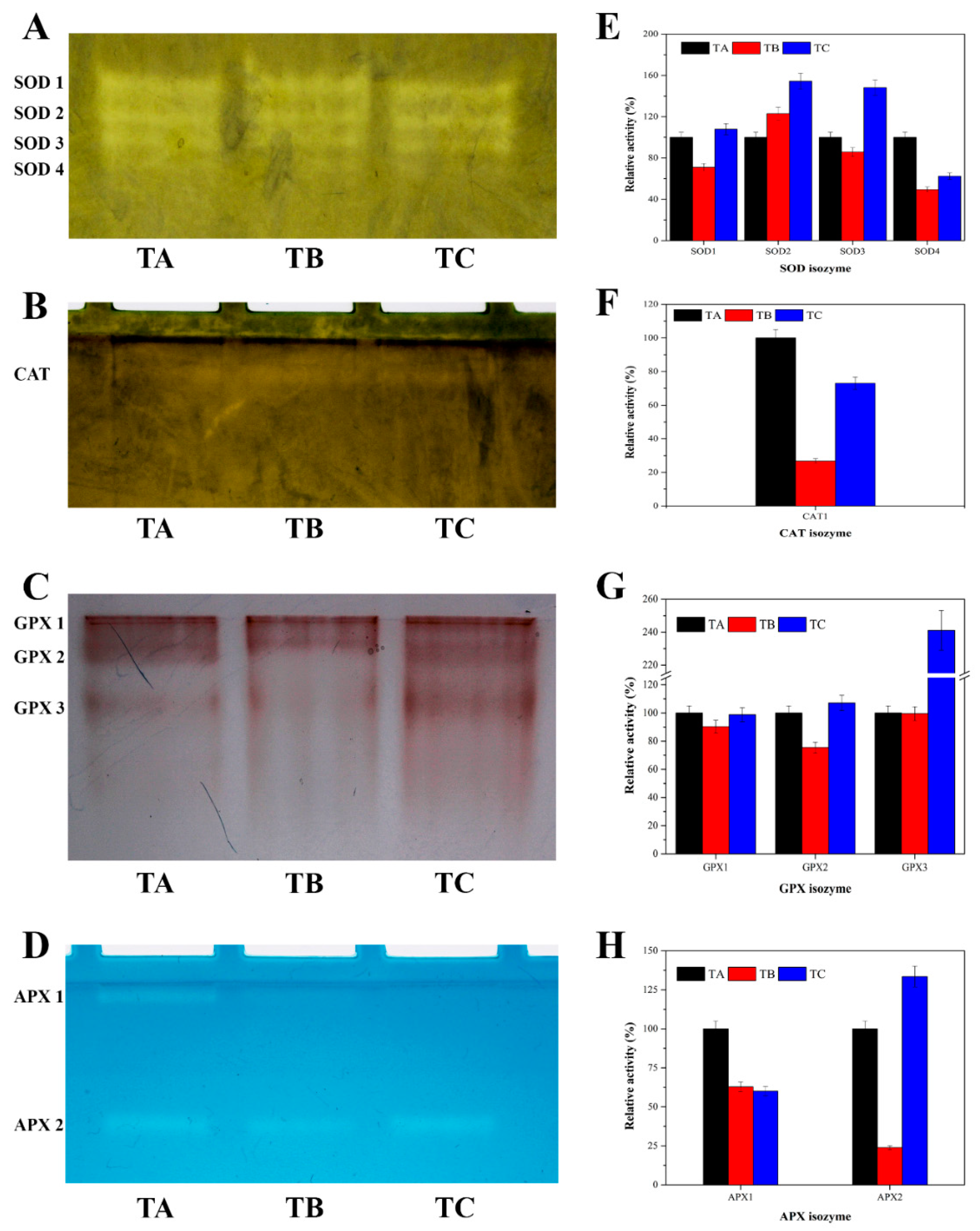
2.4. Estimation of H2O2 Content and Antioxidant Enzymes Activities
2.5. Native PAGE Profiling of Antioxidant Enzymes and Quantification of Activity
2.6. Statistical Analysis
3. Results and Discussion
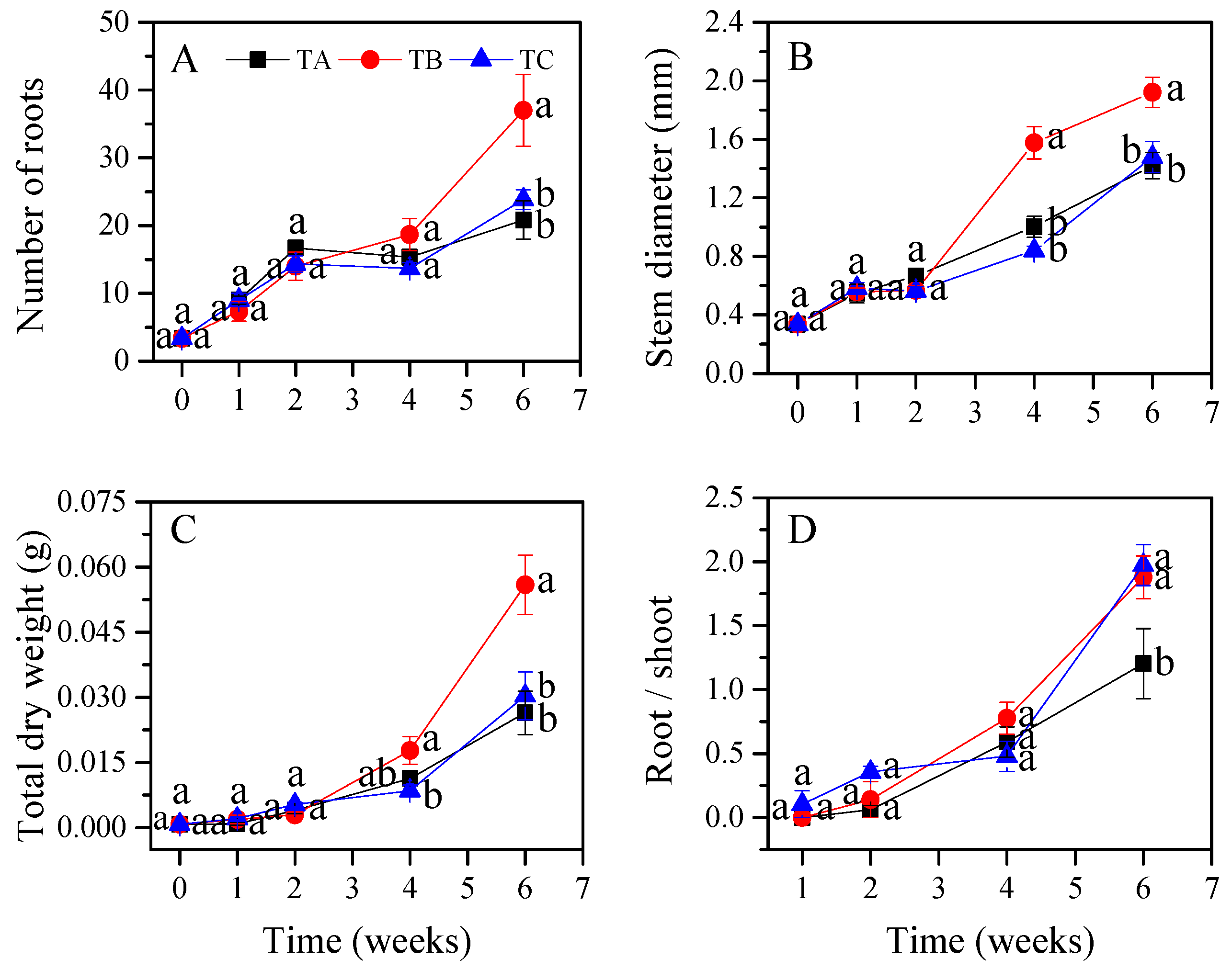
3.1. Seedling Growth Characteristics under Different Temperature Regimes
3.2. Contents of Soluble Sugar, Starch, and Soluble Protein in Seedling Grown under Various Temperature Conditions
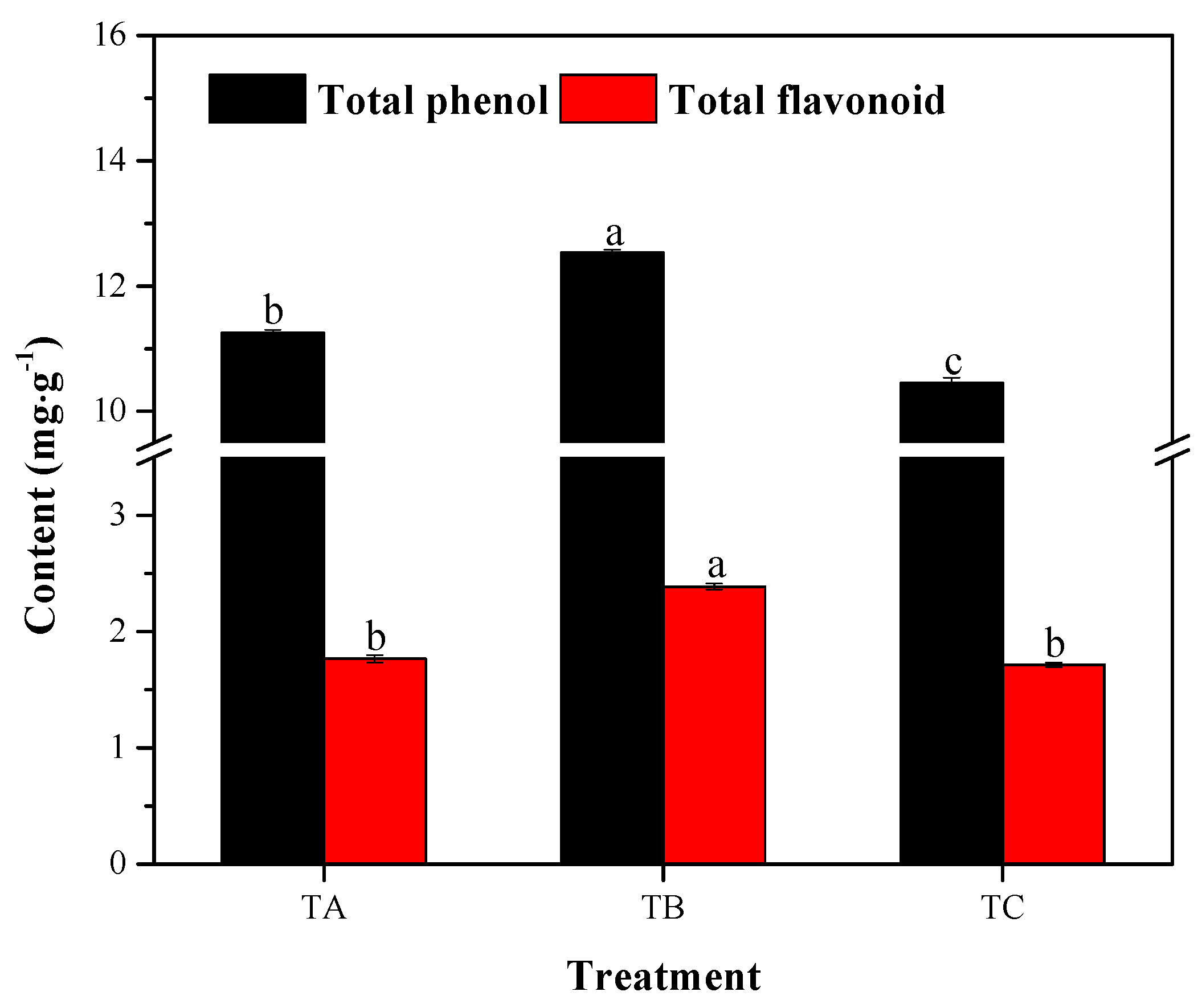
3.3. Total Phenol and Flavonoid Accumulation under Various Temperature Conditions
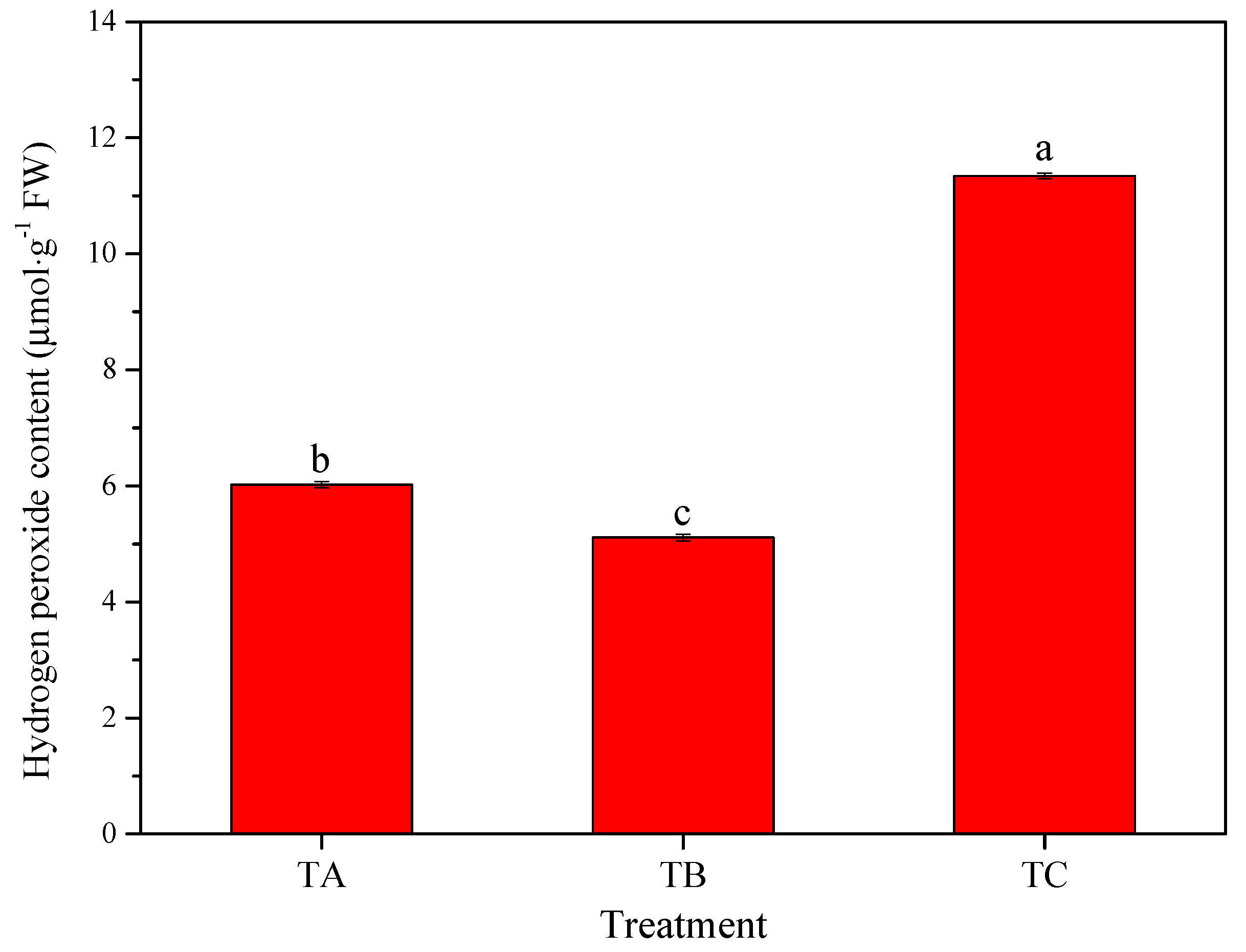
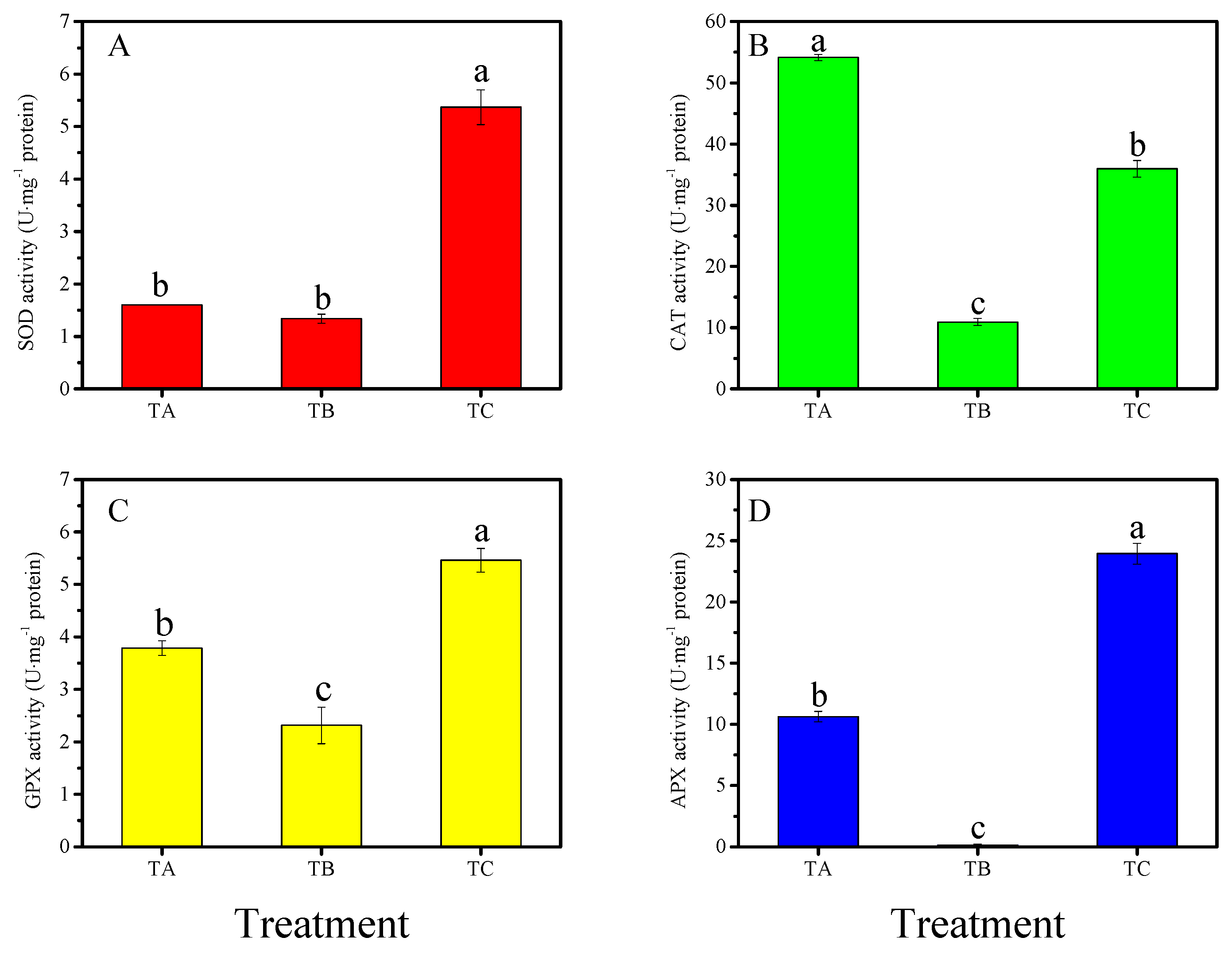
3.4. Physiological Responses of Seedlings under Various Temperature Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gorovoi, P.G.; Ponomarchuk, G.I.; Strigina, L.I. A chemotaxonomic study on russian far-eastern Campanulaceae. Phytochemistry 1971, 10, 2419–2423. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, J.Y.; Shin, S.R.; Yoon, K.Y. Comparison of antioxidant activity in wild plant (Adenophora triphylla) leaves and roots as a potential source of functional foods. Int. J. Food Sci. Nutr. 2009, 60, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lee, E.H.; Lee, T.J.; Kim, S.W.; Kim, B.H. Anti-obesity effect and action mechanism of Adenophora triphylla root ethanol extract in C57BL/6 obese mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2013, 77, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Kang, M.; Kim, Y.S. A triterpenoid saponin from Adenophora triphylla var. japonica suppresses the growth of human gastric cancer cells via regulation of apoptosis and autophagy. Tumor Biol. 2014, 35, 12021–12030. [Google Scholar]
- Ahn, E.K.; Oh, J.S. Lupenone isolated from Adenophora triphylla var. japonica extract inhibits adipogenic differentiation through the downregulation of PPARγ in 3T3-L1 cells. Phytother. Res. 2013, 27, 761–766. [Google Scholar] [PubMed]
- Konno, C.; Saito, T.; Oshima, Y.; Hikino, H.; Kabuto, C. Structure of methyl adenophorate and triphyllol, triterpenoids of Adenophora triphylla var. japonica roots. Planta Med. 1981, 42, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Yoo, H.H.; Baek, S.H.; Park, M.; Park, J.; Lee, S.; Kim, C.; Lee, K. Quality control of Adenophorae Radix. Korean J. Pharmacogenet. 2003, 34, 10–13. (In Korean) [Google Scholar]
- Asano, N.; Nishida, M.; Miyauchi, M.; Ikeda, K.; Yamamoto, M.; Kizu, H.; Kameda, Y.; Watson, A.A.; Nash, R.J.; Fleet, G.W.J. Polyhydroxylated pyrrolidine and piperidine alkaloids from Adenophora triphylla var. japonica (Campanulaceae). Phytochemistry 2000, 53, 379–382. [Google Scholar] [CrossRef]
- Choi, H.J.; Chung, M.J.; Ham, S.S. Antiobese and hypocholesterolaemic effects of an Adenophora triphylla extract in HepG2 cells and high fat diet-induced obese mice. Food Chem. 2010, 119, 437–444. [Google Scholar] [CrossRef]
- Kang, M.; Ha, I.J.; Chun, J.; Kang, S.S.; Kim, Y.S. Separation of two cytotoxic saponins from the roots of Adenophora triphylla var. japonica by high-speed counter-current chromatography. Phytochem. Anal. 2013, 24, 148–154. [Google Scholar] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Garruna-Hernandez, R.; Orellana, R.; Larque-Saavedra, A.; Canto, A. Understanding the physiological responses of a tropical crop (Capsicum chinense Jacq.) at high temperature. PLoS ONE 2014, 9, e111402. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Figueroa, T.; Lopez-Hernandez, L.; Lopez, M.G.; Hurtado, M.D.D.; Vazquez-Barrios, M.E.; Guevara-Olvera, L.; Gonzalez, R.G.G.; Rivera-Pastrana, D.M.; Torres-Robles, H.; Mercado-Silva, E.M. Conditioning garlic “seed” cloves at low temperature modifies plant growth, sugar, fructan content, and sucrose sucrose fructosyl transferase (1-SST) expression. Sci. Hortic. 2015, 189, 150–158. [Google Scholar] [CrossRef]
- Szymanska, R.; Slesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Xu, X.X.; Hu, Q.; Yang, W.N.; Jin, Y. The roles of call wall invertase inhibitor in regulating chilling tolerance in tomato. BMC Plant Biol. 2017, 17, 195. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Gao, Q.; Chen, J.X. Effects of day and night temperature difference on growth, development, yield and fruit quality of tomatoes. Chin. J. Appl. Ecol. 2015, 26, 2700–2706. (In Chinese) [Google Scholar]
- Si, Y.P.; Heins, R.D. Influence of day and night temperatures on sweet pepper seedling development. J. Am. Soc. Hortic. Sci. 1996, 121, 699–704. [Google Scholar]
- Yang, Z.Q.; Li, Y.S.; Li, P.; Zhang, F.M.; Thomas, B.W. Effect of difference between day and night temperature on tomato (Lycopersicon esculentum Mill.) root activity and low molecular weight organic acid secretion. Soil Sci. Plant Nutr. 2016, 62, 423–431. [Google Scholar] [CrossRef]
- Ribeiro, P.R.; Zanotti, R.F.; Deflers, C.; Fernandez, L.G.; de Castro, R.D.; Ligterink, W.; Hilhorst, H.W.M. Effect of temperature on biomass allocation in seedlings of two contrasting genotypes of the oilseed crop Ricinus communis. J. Plant Physiol. 2015, 185, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Sakai, H.; Yagi, K.; Hasegawa, T. Interactions of elevated CO2 and night temperature on rice growth and yield. Agric. For. Meteorol. 2009, 149, 51–58. [Google Scholar] [CrossRef]
- Ryu, S.; Han, H.H.; Jeong, J.H.; Kwon, Y.; Han, J.H.; Do, G.R.; Choi, I.M.; Lee, H.J. Night temperatures affect fruit coloration and expressions of anthocyanin biosynthetic genes in ‘Hongro’ apple fruit skins. Eur. J. Hortic. Sci. 2017, 82, 232–238. [Google Scholar] [CrossRef]
- Gaiotti, F.; Pastore, C.; Filippetti, I.; Lovat, L.; Belfiore, N.; Tomasi, D. Low night temperature at veraison enhances the accumulation of anthocyanins in corvina grapes (Vitis vinifera L.). Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, F.W.; Yang, P.; Li, J.; Yan, G.J.; Hu, L.Y. Responses of canola (Brassica napus L.) cultivars under contrasting temperature regimes during early seedling growth stage as revealed by multiple physiological criteria. Acta Physiol. Plant. 2015, 37, 7. [Google Scholar] [CrossRef]
- Antoniou, C.; Savvides, A.; Christou, A.; Fotopoulos, V. Unravelling chemical priming machinery in plants: The role of reactive oxygen-nitrogen-sulfur species in abiotic stress tolerance enhancement. Curr. Opin. Plant Biol. 2016, 33, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Jia, H.L.; Wang, J.; Cao, Q.H.; Wen, Z.C. Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+ antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma 2014, 251, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.G.; Tian, Q.Y.; Zhang, W.H. Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol. 2007, 144, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Jeong, B.R. Proteomic analysis of salt-stress responsive proteins in roots of tomato (Lycopersicon esculentum L.) plants towards silicon efficiency. Plant Growth Regul. 2015, 77, 133–146. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.S.; Sairam, R.K.; Kushwaha, S.R.; Singh, T.P. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006, 171, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.O.; Heo, B.G.; Gorinstein, S.; Chon, S.U. Positive effects of temperature and growth conditions on enzymatic and antioxidant status in lettuce plants. Plant Sci. 2011, 181, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.X.; Sun, C.X.; Shao, H.B.; Lei, X.T. Effects of low temperature and drought on the physiological and growth changes in oil palm seedlings. Afr. J. Biotechnol. 2011, 10, 2630–2637. [Google Scholar]
- Gao, J.J.; Li, T.; Yu, X.C. Gene expression and activities of sod in cucumber seedlings were related with concentrations of Mn2+, Cu2+, or Zn2+ under low temperature stress. Agric. Sci. China 2009, 8, 678–684. [Google Scholar] [CrossRef]
- Meena, M.; Zehra, A.; Dubey, M.K.; Aamir, M.; Gupta, V.K.; Upadhyay, R.S. Comparative evaluation of biochemical changes in tomato (Lycopersicon esculentum Mill.) infected by Alternaria alternata and its toxic metabolites (TeA, AOH, and AME). Front. Plant Sci. 2016, 7, 1408. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Nahakpam, S. Heat exposure alters the expression of SOD, POD, APX and CAT isozymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars. Plant Physiol. Biochem. 2012, 57, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Dewir, Y.H.; Chakrabarty, D.; Ali, M.B.; Hahn, E.J.; Paek, K.Y. Lipid peroxidation and antioxidant enzyme activities of Euphorbia milii hyperhydric shoots. Environ. Exp. Bot. 2006, 58, 93–99. [Google Scholar] [CrossRef]
- Covian, R.; Chess, D.; Balaban, R.S. Continuous monitoring of enzymatic activity within native electrophoresis gels: Application to mitochondrial oxidative phosphorylation complexes. Anal. Biochem. 2012, 431, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Wittig, I.; Braun, H.P.; Schägger, H. Blue native PAGE. Nat. Protoc. 2006, 1, 418. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wang, L.P.; Yang, Y.J.; Sun, J.; Guo, S.R. Exogenous spermidine alleviates the oxidative damage in cucumber seedlings subjected to high temperatures. J. Am. Soc. Hortic. Sci. 2012, 137, 11–19. [Google Scholar]
- Wang, Y.Q.; Li, L.; Cui, W.T.; Xu, S.; Shen, W.B.; Wang, R. Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil 2012, 351, 107–119. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 2001, 49, 4977–4982. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Jia, X.; Wang, W.K.; Liu, T.; Huang, S.P.; Yang, M.Y. Growth under elevated air temperature alters secondary metabolites in Robinia pseudoacacia L. Seedlings in Cd- and Pb-contaminated soils. Sci. Total Environ. 2016, 565, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Hura, K.; Hura, T.; Rapacz, M.; Plazek, A. Effects of low-temperature hardening on the biochemical response of winter oilseed rape seedlings inoculated with the spores of Leptosphaeria maculans. Biologia 2015, 70, 1011–1018. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Park, Y.G.; Jeong, B.R. In vitro propagation, phytochemical analysis, and evaluation of free radical scavenging property of Scrophularia kakudensis Franch tissue extracts. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.S.; Navanesan, S.; Sinniah, S.K.; Wahab, N.A.; Sim, K.S. Phenolic content, antioxidant effect and cytotoxic activity of leea indica leaves. BMC Complement. Altern. Med. 2012, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- López, S.; Maroto, J.V.; San Bautista, A.; Pascual, B.; Alagarda, J. Differences in carbohydrate content of waiting-bed strawberry plants during development in the nursery. Sci. Hortic. 2002, 94, 53–62. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Park, Y.G.; Jeong, B.R. Chemical elicitor-induced modulation of antioxidant metabolism and enhancement of secondary metabolite accumulation in cell suspension cultures of Scrophularia kakudensis Franch. Int. J. Mol. Sci. 2016, 17, 399. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, A.; Soundararajan, P.; Muneer, S.; Ko, C.H.; Jeong, B.R. Silicon mitigates salinity stress by regulating the physiology, antioxidant enzyme activities, and protein expression in Capsicum annuum ‘bugwang’. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Manivannan, A.; Cho, Y.S.; Jeong, B.R. Exogenous supplementation of silicon improved the recovery of hyperhydric shoots in Dianthus caryophyllus L. by stabilizing the physiology and protein expression. Front. Plant Sci. 2017, 8, 738. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, D.; Park, S.Y.; Ali, M.B.; Shin, K.S.; Paek, K.Y. Hyperhydricity in apple: Ultrastuctural and physiological aspects. Tree Physiol. 2006, 26, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, A.; Jana, S.; Soundararajan, P.; Ko, C.H.; Jeong, B.R. Antioxidant enzymes metabolism and cellular differentiation during the developmental stages of somatic embryogenesis in ‘Torilis japonica’ (Houtt.) DC. Plant Omics 2015, 8, 461. [Google Scholar]
- Gallo-Oller, G.; Ordoñez, R.; Dotor, J. A new background subtraction method for western blot densitometry band quantification through image analysis software. J. Immunol. Methods 2018, 457, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sønsteby, A.; Solhaug, K.A.; Heide, O.M. Functional growth analysis of ‘Sonata’ strawberry plants grown under controlled temperature and daylength conditions. Sci. Hortic. 2016, 211, 26–33. [Google Scholar] [CrossRef]
- Arena, M.E.; Radice, S. Shoot growth and development of Berberis buxifolia Lam. in Tierra del Fuego (Patagonia). Sci. Hortic. 2014, 165, 5–12. [Google Scholar] [CrossRef]
- Cookson, S.J.; Hevin, C.; Donnart, M.; Ollat, N. Grapevine rootstock effects on scion biomass are not associated with large modifications of primary shoot growth under nonlimiting conditions in the first year of growth. Funct. Plant Biol. 2012, 39, 650–660. [Google Scholar] [CrossRef]
- Lin, S.Y.; Liao, Y.Y.; Roan, S.F.; Chen, I.Z.; Chen, P.A. Growth of noni fruits (Morinda citrifolia L.) and accumulation of phenolic compounds during fruit development. Sci. Hortic. 2014, 178, 168–174. [Google Scholar] [CrossRef]
- Jing, P.; Wang, D.; Zhu, C.; Chen, J. Plant physiological, morphological and yield-related responses to night temperature changes across different species and plant functional types. Front. Plant Sci. 2016, 7, 1774. [Google Scholar] [CrossRef] [PubMed]
- Loka, D.A.; Oosterhuis, D.M. Increased night temperatures during cotton’s early reproductive stage affect leaf physiology and flower bud carbohydrate content decreasing flower bud retention. J. Agron. Crop Sci. 2016, 202, 518–529. [Google Scholar] [CrossRef]
- Lesjak, J.; Calderini, D.F. Increased night temperature negatively affects grain yield, biomass and grain number in Chilean quinoa. Front. Plant Sci. 2017, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.K. Effect of day/night temperature difference on chlorophyII content, photosynthesis and fluorescence parameters of tomato at fruit stage. Photosynthetica 2016, 54, 475–477. [Google Scholar] [CrossRef]
- Criddle, R.S.; Smith, B.N.; Hansen, L.D. A respiration based description of plant growth rate responses to temperature. Planta 1997, 201, 441–445. [Google Scholar] [CrossRef]
- Hansen, L.D.; Thomas, N.R.; Arnholdt-Schmitt, B. Temperature responses of substrate carbon conversion efficiencies and growth rates of plant tissues. Physiol. Plant. 2009, 137, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Steponkus, P.L. Modification of the intracellular sugar content alters the incidence of freeze-induced membrane lesions of protoplasts isolated from Arabidopsis thaliana leaves. Plant Cell Environ. 2003, 26, 1083–1096. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Yang, M.T.; Li, X.; Zhou, Z.Q.; Wang, X.J.; Bai, J.G. Exogenous sucrose increases chilling tolerance in cucumber seedlings by modulating antioxidant enzyme activity and regulating proline and soluble sugar contents. Sci. Hortic. 2014, 179, 67–77. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars-metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, X.; Yao, X.; Zhu, Z.; Xu, S.; Zha, D. Exogenous 6-benzylaminopurine confers tolerance to low temperature by amelioration of oxidative damage in eggplant (Solanum melongena L.) seedlings. Rev. Bras. Bot. 2016, 39, 409–416. [Google Scholar] [CrossRef]
- De Sousa, D.P.F.; Braga, B.B.; Gondim, F.A.; Gomes-Filho, E.; Martins, K.; de Brito, P.O.B. Increased drought tolerance in maize plants induced by H2O2 is closely related to an enhanced enzymatic antioxidant system and higher soluble protein and organic solutes contents. Theor. Exp. Plant Physiol. 2016, 28, 297–306. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, H.; Liang, K.; Huang, G.; Pinyopusarerk, K. Improved tolerance of teak (Tectona grandis L.f.) seedlings to low-temperature stress by the combined effect of arbuscular mycorrhiza and paclobutrazol. J. Plant Growth Regul. 2012, 31, 427–435. [Google Scholar] [CrossRef]
- Shishido, Y.; Seyama, N.; Imada, S.; Hori, Y. Carbon budget in tomato plants as affected by night temperature evaluated by steady state feeding with 14CO2. Ann. Bot. 1989, 63, 357–367. [Google Scholar] [CrossRef]
- Warner, D.A.; Holaday, A.S.; Burke, J.J. Response of carbon metabolism to night temperature in cotton. Agron. J. 1995, 87, 1193–1197. [Google Scholar] [CrossRef]
- Peschel, W.; Kump, A.; Zomborszki, Z.P.; Pfosser, M.; Kainz, W.; Csupor, D. Phenylpropenoid content in high-altitude cultivated Rhodiola rosea L. provenances according to plant part, harvest season and age. Ind. Crop. Prod. 2018, 111, 446–456. [Google Scholar] [CrossRef]
- Cezarotto, V.S.; Giacomelli, S.R.; Vendruscolo, M.H.; Vestena, A.S.; Cezarotto, C.S.; da Cruz, R.C.; Maurer, L.H.; Ferreira, L.M.; Emanuelli, T.; Cruz, L. Influence of harvest season and cultivar on the variation of phenolic compounds composition and antioxidant properties in Vaccinium ashei leaves. Molecules 2017, 22, 1603. [Google Scholar] [CrossRef] [PubMed]
- Chandra, K.; Salman, A.S.; Mohd, A.; Sweety, R.; Ali, K.N. Protection against FCA induced oxidative stress induced DNA damage as a model of arthritis and in vitro anti-arthritic potential of Costus speciosus rhizome extract. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 383–389. [Google Scholar]
- Muneer, S.; Park, Y.G.; Kim, S.; Jeong, B.R. Foliar or subirrigation silicon supply mitigates high temperature stress in strawberry by maintaining photosynthetic and stress-responsive proteins. J. Plant Growth Regul. 2017, 36, 836–845. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Exogenous spermidine alleviates low temperature injury in mung bean (Vigna radiata L.) seedlings by modulating ascorbate-glutathione and glyoxalase pathway. Int. J. Mol. Sci. 2015, 16, 30117–30132. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yao, X.; Chen, J.; Zhu, Z.; Zhang, H.; Zha, D. Brassinosteroids protect photosynthesis and antioxidant system of eggplant seedlings from high-temperature stress. Acta Physiol. Plant. 2014, 36, 251–261. [Google Scholar] [CrossRef]
- Amari, K.; Diaz-Vivancos, P.; Pallas, V.; Sanchez-Pina, M.A.; Hernandez, J.A. Oxidative stress induction by Prunus necrotic ringspot virus infection in apricot seeds. Physiol. Plant 2007, 131, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.J.; Kang, Y.I.; Kang, K.H.; Jeong, B.R. Induction of thermotolerance and activation of antioxidant enzymes in H2O2 pre-applied leaves of cucumber and tomato seedlings. J. Jpn. Soc. Hortic. Sci. 2009, 78, 320–329. [Google Scholar] [CrossRef]
- Zhu, T.; Deng, X.; Zhou, X.; Zhu, L.; Zou, L.; Li, P.; Zhang, D.; Lin, H. Ethylene and hydrogen peroxide are involved in brassinosteroid-induced salt tolerance in tomato. Sci. Rep. 2016, 6, 35392. [Google Scholar] [CrossRef] [PubMed]
- Frugoli, J.A.; Zhong, H.H.; Nuccio, M.L.; McCourt, P.; McPeek, M.A.; Thomas, T.L.; McClung, C.R. Catalase is encoded by a multigene family in Arabidopsis thaliana (L.) Heynh. Plant Physiol. 1996, 112, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Singh, M.; Dubey, R.S.; Venkataramanappa, V.; Datta, D. Phytochemicals and antioxidative enzymes defence mechanism on occurrence of yellow vein mosaic disease of pumpkin (Cucurbita moschata). Biotech 2013, 3, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.; Paliyath, G.; Murr, D.P. Antioxidant enzyme activities in apple varieties and resistance to superficial scald development. Food Res. Int. 2007, 40, 1012–1019. [Google Scholar] [CrossRef]
| Treatment | DT 1 | NT 2 | ADT 3 | DIF 4 |
|---|---|---|---|---|
| TA | 20 | 20 | 20.0 | 0 |
| TB | 25 | 15 | 21.7 | 10 |
| TC | 20 | 15 | 18.3 | 5 |
| Treatment | Content (mg·g−1) | ||
|---|---|---|---|
| Soluble Sugar | Starch | Soluble Protein | |
| TA | 86.1 ± 3.7 b | 69.9 ± 3.8 b | 6.9 ± 0.3 a |
| TB | 120.4 ± 13.7 a | 83.6 ± 2.2 ab | 7.0 ± 0.2 a |
| TC | 100.7 ± 3.4 ab | 92.6 ± 4.0 a | 5.2 ± 0.3 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ren, X.; Jeong, H.K.; Wei, H.; Jeong, B.R. Growth and Physiological Responses of Adenophora triphylla (Thunb.) A.DC. Plug Seedlings to Day and Night Temperature Regimes. Agronomy 2018, 8, 173. https://doi.org/10.3390/agronomy8090173
Liu Y, Ren X, Jeong HK, Wei H, Jeong BR. Growth and Physiological Responses of Adenophora triphylla (Thunb.) A.DC. Plug Seedlings to Day and Night Temperature Regimes. Agronomy. 2018; 8(9):173. https://doi.org/10.3390/agronomy8090173
Chicago/Turabian StyleLiu, Ya, Xiuxia Ren, Hai Kyoung Jeong, Hao Wei, and Byoung Ryong Jeong. 2018. "Growth and Physiological Responses of Adenophora triphylla (Thunb.) A.DC. Plug Seedlings to Day and Night Temperature Regimes" Agronomy 8, no. 9: 173. https://doi.org/10.3390/agronomy8090173
APA StyleLiu, Y., Ren, X., Jeong, H. K., Wei, H., & Jeong, B. R. (2018). Growth and Physiological Responses of Adenophora triphylla (Thunb.) A.DC. Plug Seedlings to Day and Night Temperature Regimes. Agronomy, 8(9), 173. https://doi.org/10.3390/agronomy8090173






