Testing and Improving the WOFOST Model for Sunflower Simulation on Saline Soils of Inner Mongolia, China
Abstract
1. Introduction
2. Materials and Methods
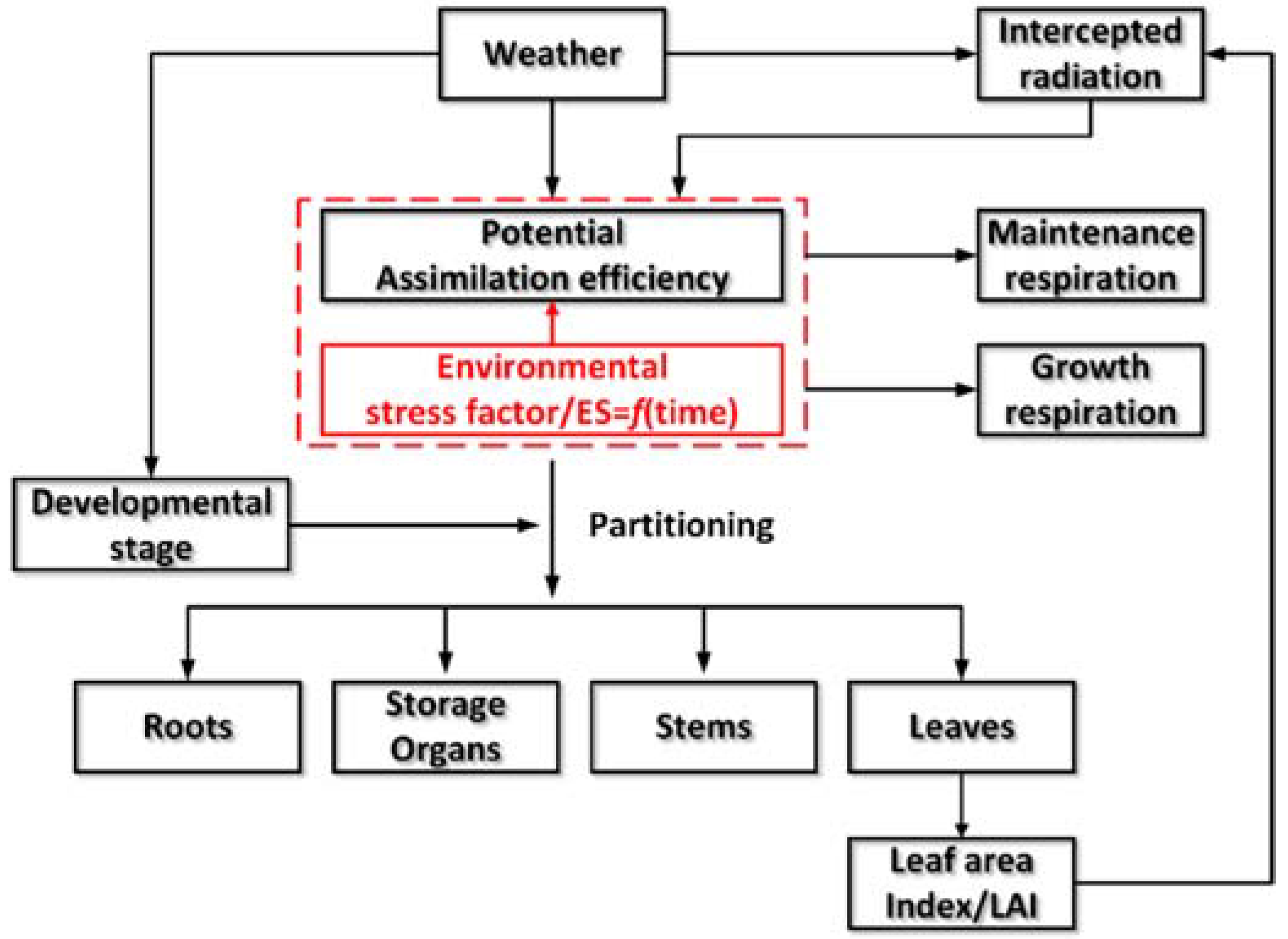
2.1. Descriptions of WOFOST and New Improvements
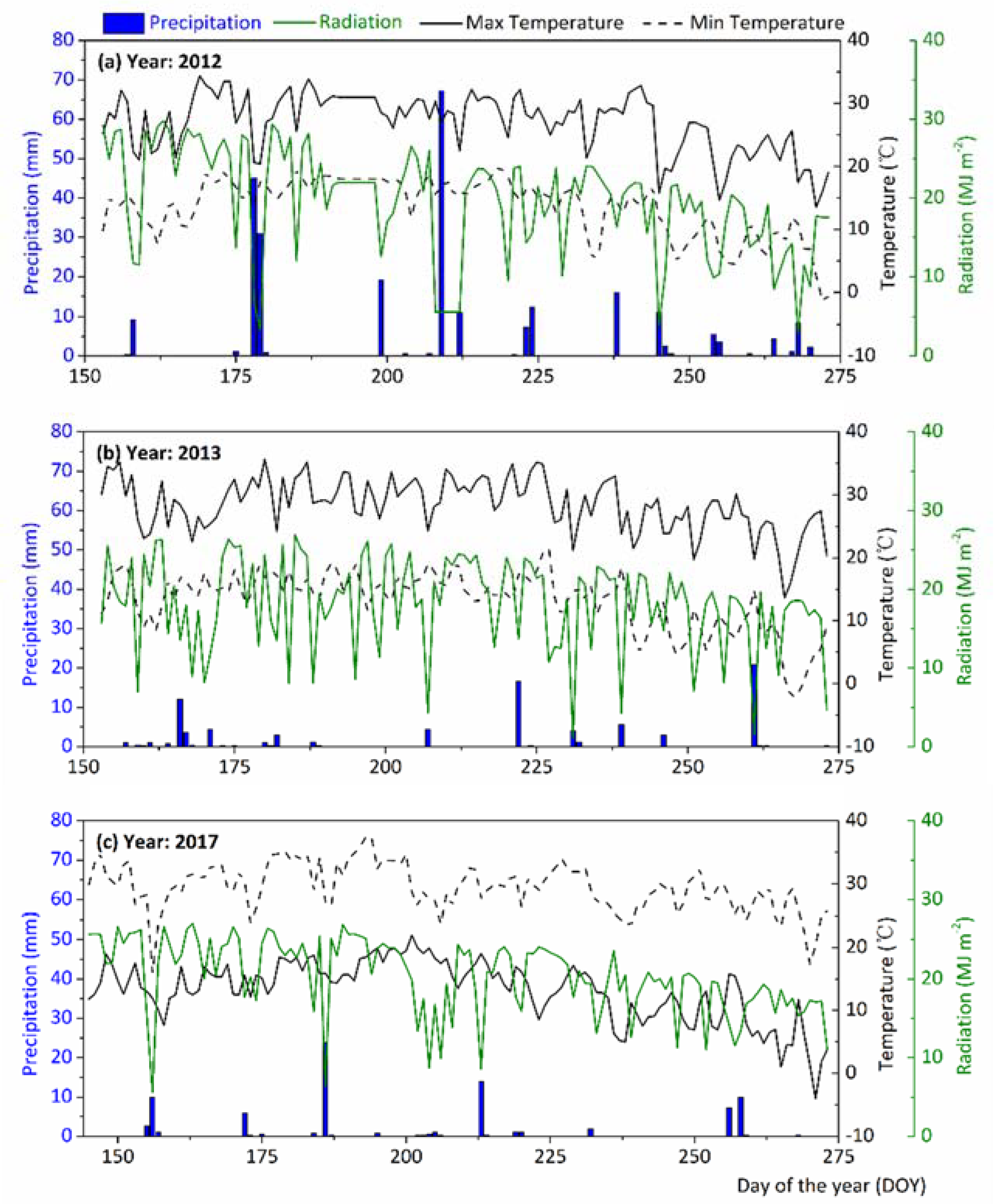
2.2. Study Site
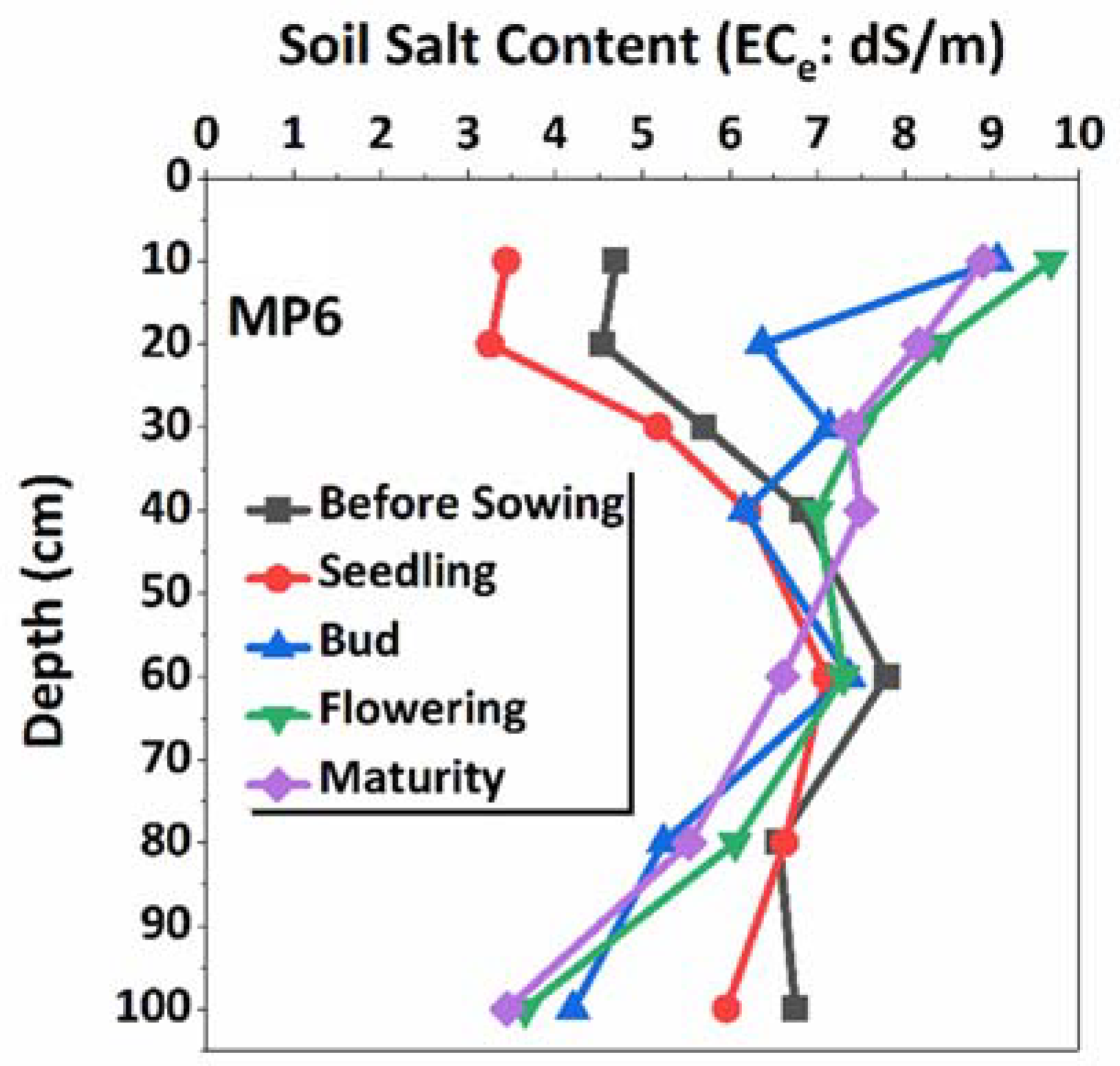
2.3. Micro-Plot Experiments
2.4. Field Experiments
2.5. Simulation Strategies
2.6. Water and Salt Stresses of the Feddes Model
2.7. Statistical Analysis
3. Results
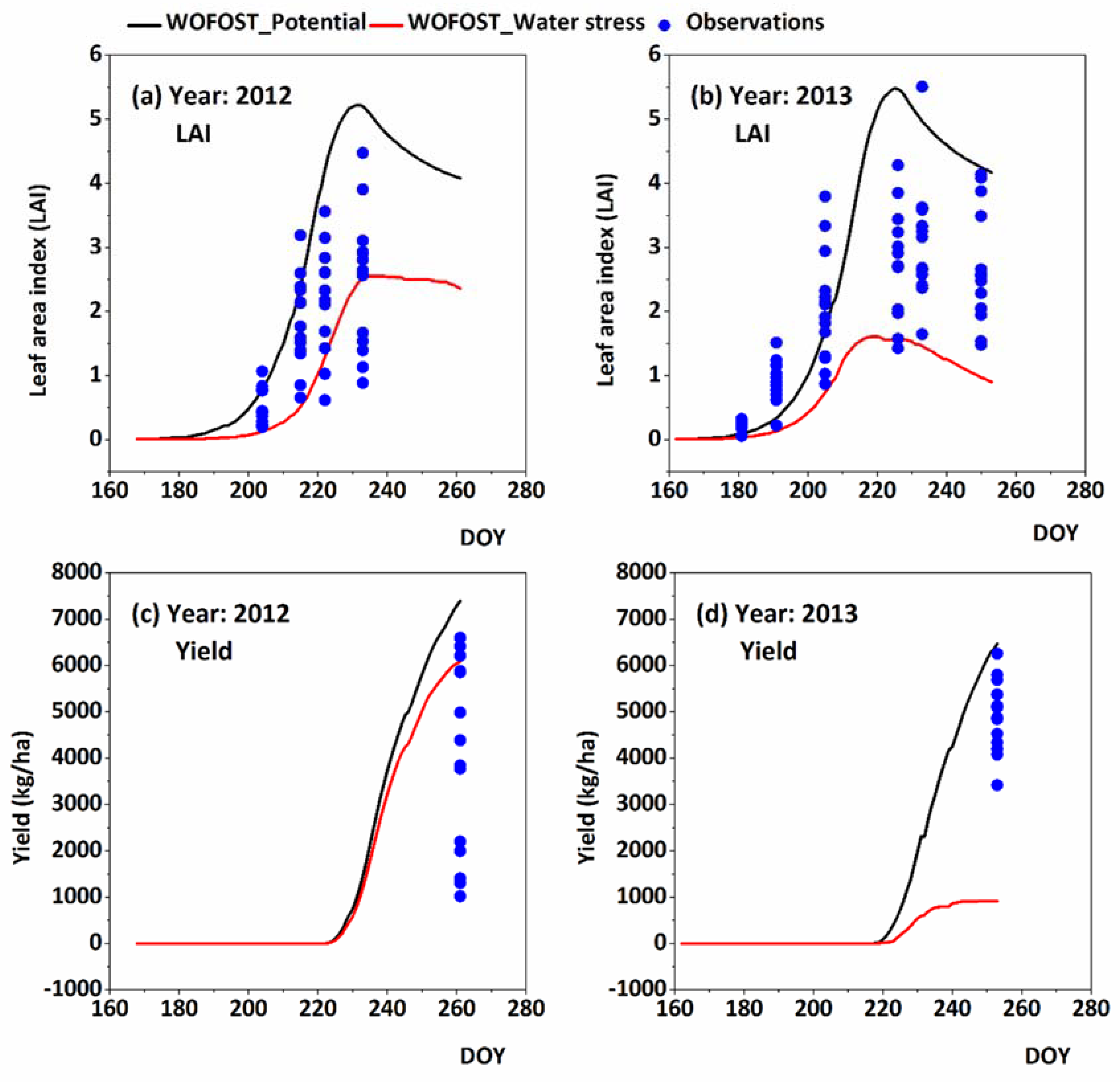
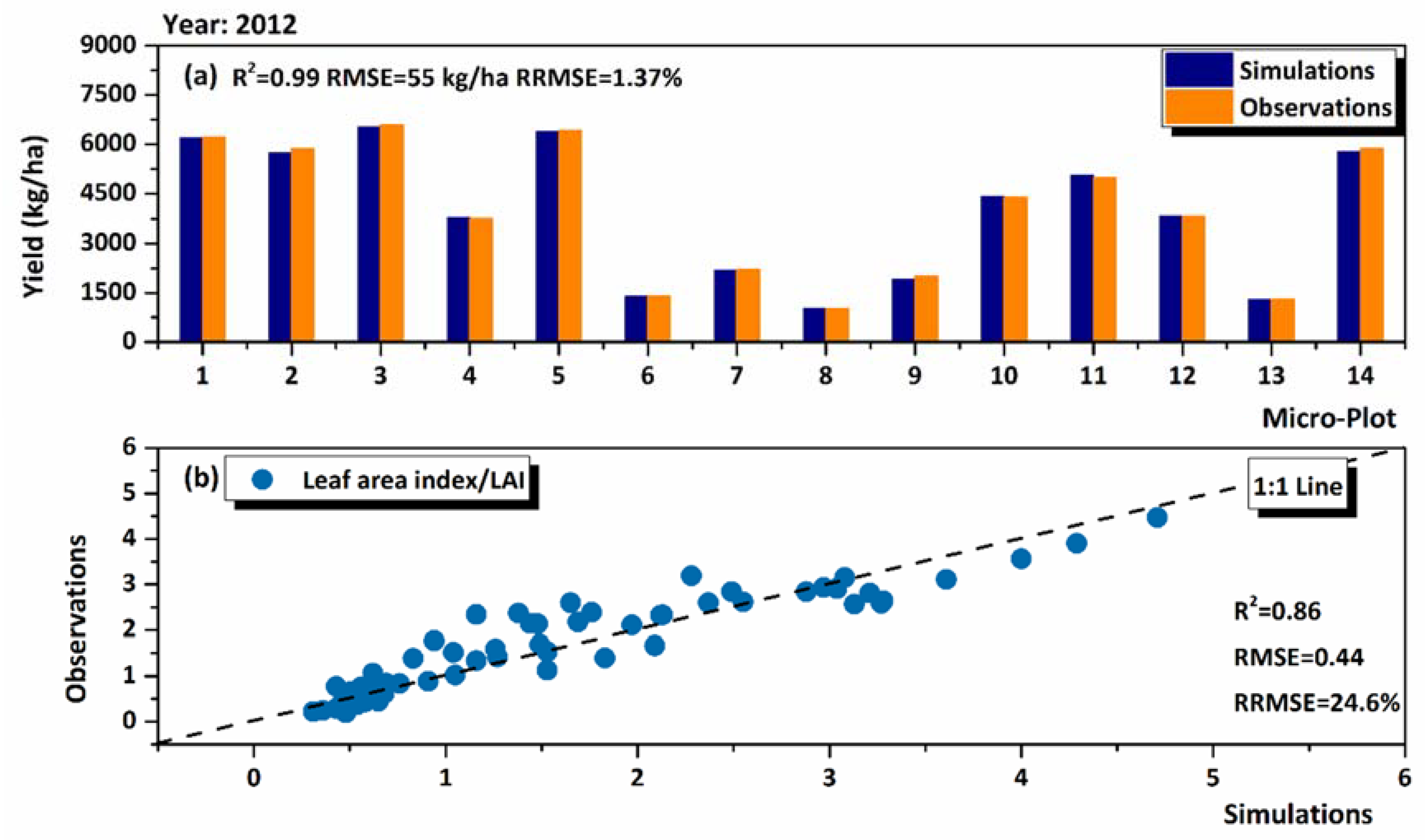
3.1. Model Performance
3.2. Stress Factors Used in the WOFOST-ES and Feddes Models
3.3. Relationship between Environmental Stress Factors and Soil Conditions
4. Discussion
4.1. Environmental Stresses in Different Growth Periods
4.2. Quantification of Stresses Associated with Soil Conditions
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Fourati, H.T.; Bouaziz, M.; Benzina, M.; Bouaziz, S. Detection of terrain indices related to soil salinity and mapping salt-affected soils using remote sensing and geostatistical techniques. Environ. Monit. Assess. 2017, 189, 177. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Soil salinization and waterlogging: A threat to environment and agricultural sustainability. Ecol. Indic. 2015, 57, 128–130. [Google Scholar] [CrossRef]
- Li, J.; Pu, L.; Han, M.; Zhu, M.; Zhang, R.; Xiang, Y. Soil salinization research in China: Advances and prospects. J. Geogr. Sci. 2014, 24, 943–960. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Almansouri, M.; Kinet, J.-M.; Lutts, S. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant Soil 2001, 231, 243–254. [Google Scholar] [CrossRef]
- Eynard, A.; Lal, R.; Wiebe, K. Crop response in salt-affected soils. J. Sustain. Agric. 2005, 27, 5–50. [Google Scholar] [CrossRef]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Gleadow, R.; Pegg, A.; Blomstedt, C.K. Resilience of cassava (Manihot esculenta Crantz) to salinity: Implications for food security in low-lying regions. J. Exp. Bot. 2016, 67, 5403–5413. [Google Scholar] [CrossRef] [PubMed]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil salinity: A threat to global food security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Du, T.; Kang, S.; Zhang, J.; Davies, W.J. Deficit irrigation and sustainable water-resource strategies in agriculture for china’s food security. J. Exp. Bot. 2015, 66, 2253–2269. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, A.; Gharechahi, J.; Nakhoda, B.; Salekdeh, G.H. Physiology and proteome responses of two contrasting rice mutants and their wild type parent under salt stress conditions at the vegetative stage. J. Plant Physiol. 2014, 171, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Kirnak, H.; Higgs, D.; Saltali, K. Supplementary calcium enhances plant growth and fruit yield in strawberry cultivars grown at high (NaCl) salinity. Sci. Hortic. 2002, 93, 65–74. [Google Scholar] [CrossRef]
- Bahrami, H.; Razmjoo, J. Effect of salinity stress (NaCl) on germination and early seedling growth of ten sesame cultivars (Sesamum indicum L.). Int. J. AgriSci. 2012, 2, 529–537. [Google Scholar]
- Ahmad, S.; Khan, N.; Iqbal, M.; Hussain, A.; Hassan, M. Salt tolerance of cotton (Gossypium hirsutum L.). Asian J. Plant Sci. 2002, 1, 715–719. [Google Scholar]
- Salehi, M.; Arzani, A. Grain quality traits in triticale influenced by field salinity stress. Aust. J. Crop Sci. 2013, 7, 580. [Google Scholar]
- Zeng, W.; Xu, C.; Wu, J.; Huang, J. Sunflower seed yield estimation under the interaction of soil salinity and nitrogen application. Field Crop. Res. 2016, 198, 1–15. [Google Scholar] [CrossRef]
- Katerji, N.; van Hoorn, J.W.; Hamdy, A.; Mastrorilli, M. Salt tolerance classification of crops according to soil salinity and to water stress day index. Agric. Water Manag. 2000, 43, 99–109. [Google Scholar] [CrossRef]
- Zeng, W.; Xu, C.; Huang, J.; Wu, J.; Ma, T. Emergence rate, yield, and nitrogen-use efficiency of sunflowers (Helianthus annuus) vary with soil salinity and amount of nitrogen applied. Commun. Soil Sci. Plant Anal. 2015, 46, 1006–1023. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Elliott, J.; Deryng, D.; Ruane, A.C.; Müller, C.; Arneth, A.; Boote, K.J.; Folberth, C.; Glotter, M.; Khabarov, N.; et al. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA 2014, 111, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Scarrow, R. Climate change: Variance in crop yields. Nat. Plants 2017. [Google Scholar] [CrossRef] [PubMed]
- Secco, D.; Whelan, J.; Rouached, H.; Lister, R. Nutrient stress-induced chromatin changes in plants. Curr. Opin. Plant Biol. 2017, 39, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Van Diepen, C.A.; Wolf, J.; van Keulen, H.; Rappoldt, C. Wofost: A simulation model of crop production. Soil Use Manag. 1989, 5, 16–24. [Google Scholar] [CrossRef]
- McCown, R.L.; Hammer, G.L.; Hargreaves, J.N.G.; Holzworth, D.P.; Freebairn, D.M. Apsim: A novel software system for model development, model testing and simulation in agricultural systems research. Agric. Syst. 1996, 50, 255–271. [Google Scholar] [CrossRef]
- Simunek, J.; Van Genuchten, M.T.; Sejna, M. The HYDRUS-1D software package for simulating the one-dimensional movement of water, heat, and multiple solutes in variably-saturated media. Univ. Calif.-Riverside Res. Rep. 2005, 3, 1–240. [Google Scholar]
- Arnold, J.G.; Moriasi, D.N.; Gassman, P.W.; Abbaspour, K.C.; White, M.J.; Srinivasan, R.; Santhi, C.; Harmel, R.; Van Griensven, A.; Van Liew, M.W.; et al. Swat: Model use, calibration, and validation. Trans. ASABE 2012, 55, 1491–1508. [Google Scholar] [CrossRef]
- Mudgal, A.; Baffaut, C.; Anderson, S.H.; Sadler, E.J.; Thompson, A.L. APEX model assessment of variable landscapes on runoff and dissolved herbicides. Trans. ASABE 2010, 53, 1047–1058. [Google Scholar] [CrossRef]
- Keating, B.A.; Carberry, P.S.; Hammer, G.L.; Probert, M.E.; Robertson, M.J.; Holzworth, D.; Huth, N.I.; Hargreaves, J.N.G.; Meinke, H.; Hochman, Z.; et al. An overview of apsim, a model designed for farming systems simulation. Eur. J. Agron. 2003, 18, 267–288. [Google Scholar] [CrossRef]
- Stöckle, C.O.; Kemanian, A.R.; Nelson, R.L.; Adam, J.C.; Sommer, R.; Carlson, B. Cropsyst model evolution: From field to regional to global scales and from research to decision support systems. Environ. Model. Softw. 2014, 62, 361–369. [Google Scholar] [CrossRef]
- Hansen, S.; Abrahamsen, P.; Petersen, C.T.; Styczen, M. Daisy: Model use, calibration, and validation. Trans. ASABE 2012, 55, 1317–1333. [Google Scholar] [CrossRef]
- Del Grosso, S.J.; Mosier, A.R.; Parton, W.J.; Ojima, D.S. Daycent model analysis of past and contemporary soil N2O and net greenhouse gas flux for major crops in the USA. Soil Tillage Res. 2005, 83, 9–24. [Google Scholar] [CrossRef]
- Giltrap, D.L.; Li, C.; Saggar, S. Dndc: A process-based model of greenhouse gas fluxes from agricultural soils. Agric. Ecosyst. Environ. 2010, 136, 292–300. [Google Scholar] [CrossRef]
- Eweys, O.A.; Elwan, A.A.; Borham, T.I. Integrating wofost and noah lsm for modeling maize production and soil moisture with sensitivity analysis, in the east of the Netherlands. Field Crop. Res. 2017, 210, 147–161. [Google Scholar] [CrossRef]
- Jones, J.W.; Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Batchelor, W.D.; Hunt, L.A.; Wilkens, P.W.; Singh, U.; Gijsman, A.J.; Ritchie, J.T. The dssat cropping system model. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, X.; Huang, Q.; Huo, Z.; Huang, G. Assessment of irrigation performance and water productivity in irrigated areas of the middle Heihe River basin using a distributed agro-hydrological model. Agric. Water Manag. 2015, 147, 67–81. [Google Scholar] [CrossRef]
- Šimůnek, J.; van Genuchten, M.T.; Šejna, M. Recent developments and applications of the HYDRUS computer software packages. Vadose Zone J. 2016, 15. [Google Scholar] [CrossRef]
- Lapola, D.M.; Priess, J.A.; Bondeau, A. Modeling the land requirements and potential productivity of sugarcane and jatropha in Brazil and India using the LPJmL dynamic global vegetation model. Biomass Bioenergy 2009, 33, 1087–1095. [Google Scholar] [CrossRef]
- Nendel, C.; Berg, M.; Kersebaum, K.C.; Mirschel, W.; Specka, X.; Wegehenkel, M.; Wenkel, K.O.; Wieland, R. The MONICA model: Testing predictability for crop growth, soil moisture and nitrogen dynamics. Ecol. Model. 2011, 222, 1614–1625. [Google Scholar] [CrossRef]
- Wu, L.; McGechan, M.B.; McRoberts, N.; Baddeley, J.A.; Watson, C.A. Spacsys: Integration of a 3D root architecture component to carbon, nitrogen and water cycling—Model description. Ecol. Model. 2007, 200, 343–359. [Google Scholar] [CrossRef]
- Jing, Q.; Jégo, G.; Bélanger, G.; Chantigny, M.H.; Rochette, P. Simulation of water and nitrogen balances in a perennial forage system using the STICS model. Field Crop. Res. 2017, 201, 10–18. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, G.; Li, X.; Hu, B.X.; Wang, G. Numerical modeling of wheat irrigation using coupled HYDRUS and WOFOST models. Soil Sci. Soc. Am. J. 2012, 76, 648–662. [Google Scholar] [CrossRef]
- Li, Y.; Kinzelbach, W.; Zhou, J.; Cheng, G.; Li, X. Modelling irrigated maize with a combination of coupled-model simulation and uncertainty analysis, in the northwest of China. Hydrol. Earth Syst. Sci. 2012, 16, 1465. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.; Zhou, J.; Zhang, G.; Chen, C.; Wang, J. Assimilating remote sensing information into a coupled hydrology-crop growth model to estimate regional maize yield in arid regions. Ecol. Model. 2014, 291, 15–27. [Google Scholar] [CrossRef]
- Ma, T.; Zeng, W.; Li, Q.; Wu, J.; Huang, J. Effects of water, salt and nitrogen stress on sunflower (Helianthus annuus L.) at different growth stages. J. Soil Sci. Plant Nutr. 2016, 16, 1024–1037. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, J.; Hoffmann, M.P.; Xu, C.; Ma, T.; Huang, J. Testing the apsim sunflower model on saline soils of Inner Mongolia, China. Field Crop. Res. 2016, 192, 42–54. [Google Scholar] [CrossRef]
- Todorovic, M.; Albrizio, R.; Zivotic, L.; Saab, M.-T.A.; Stöckle, C.; Steduto, P. Assessment of aquacrop, cropsyst, and wofost models in the simulation of sunflower growth under different water regimes all rights reserved. Agron. J. 2009, 101, 509–521. [Google Scholar] [CrossRef]
- Boogaard, H.L.; van Diepen, C.A.; Rotter, R.P.; Cabrera, J.M.C.A.; van Laar, H.H. WOFOST 7.1; User’s Guide for the WOFOST 7.1 Crop Growth Simulation Model and WOFOST Control Center 1.5; SC-DLO: Wageningen, The Netherlands, 1998; pp. 927–4499. [Google Scholar]
- Zeng, W.; Xu, C.; Huang, J.; Wu, J.; Tuller, M. Predicting near-surface moisture content of saline soils from near-infrared reflectance spectra with a modified gaussian model. Soil Sci. Soc. Am. J. 2016, 80, 1496–1506. [Google Scholar] [CrossRef]
- Chen, J. Adjustment coefficient of sunflower leaf area. Chin. J. Oil Crop Sci. 1984, 1, 71–73. [Google Scholar]
- Sezen, S.; Yazar, A.; Kapur, B.; Tekin, S. Comparison of drip and sprinkler irrigation strategies on sunflower seed and oil yield and quality under mediterranean climatic conditions. Agric. Water Manag. 2011, 98, 1153–1161. [Google Scholar] [CrossRef]
- Doherty, J. Pest: A unique computer program for model-independent parameter optimisation. In Water Down Under 94: Groundwater/Surface Hydrology Common Interest Papers; Preprints of Papers, Adelaide, South Australia, 21–25 November 1994; Institution of Engineers: Barton, Australia, 1994. [Google Scholar]
- Feddes, R.A.; Kowalik, P.J.; Zaradny, H. Simulation of Field Water Use and Crop Yield; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1978. [Google Scholar]
- Ren, D.; Xu, X.; Hao, Y.; Huang, G. Modeling and assessing field irrigation water use in a canal system of hetao, upper yellow river basin: Application to maize, sunflower and watermelon. J. Hydrol. 2016, 532, 122–139. [Google Scholar] [CrossRef]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance–current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar]
- Zhao, Q.; Zhan, W.; Wu, J.; Huang, J.; Zhang, S.; Chen, A.; Gao, H. Effect of salt stress on growth and yield of sunflower. J. Irrig. Drain. 2014, 33, 32–35. [Google Scholar]
- Kopačková, V.; Ben-Dor, E.; Carmon, N.; Notesco, G. Modelling diverse soil attributes with visible to longwave infrared spectroscopy using PLSR employed by an automatic modelling engine. Remote Sens. 2017, 9, 134. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Tao, J.; Weng, Y. Soil salinity retrieval from advanced multi-spectral sensor with partial least square regression. Remote Sens. 2015, 7, 488–511. [Google Scholar] [CrossRef]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Zeng, W.; Xu, C.; Zhao, G.; Wu, J.; Huang, J. Estimation of sunflower seed yield using partial least squares regression and artificial neural network models. Pedosphere 2017, in press. [Google Scholar] [CrossRef]
- Zeng, W.; Huang, J.; Xu, C.; Ma, T.; Wu, J. Hyperspectral reflectance models for soil salt content by filtering methods and waveband selection. Ecol. Chem. Eng. S 2016, 23, 117–130. [Google Scholar] [CrossRef]
- Vega, C.R.C.; Andrade, F.H.; Sadras, V.O. Reproductive partitioning and seed set efficiency in soybean, sunflower and maize. Field Crop. Res. 2001, 72, 163–175. [Google Scholar] [CrossRef]
- Ma, T.; Zeng, W.; Li, Q.; Yang, X.; Wu, J.; Huang, J. Shoot and root biomass allocation of sunflower varying with soil salinity and nitrogen applications. Agron. J. 2017, 109, 2545–2555. [Google Scholar] [CrossRef]
- Quartacci, M.F.; Navari-Izzo, F. Water stress and free radical mediated changes in sunflower seedlings. J. Plant Physiol. 1992, 139, 621–625. [Google Scholar] [CrossRef]
- Habibi, G. Physiological, photochemical and ionic responses of sunflower seedlings to exogenous selenium supply under salt stress. Acta Physiol. Plant. 2017, 39, 213. [Google Scholar] [CrossRef]
- Jabeen, N.; Ahmad, R. Growth response and nitrogen metabolism of sunflower (Helianthus annuus L.) to vermicompost and biogas slurry under salinity stress. J. Plant Nutr. 2017, 40, 104–114. [Google Scholar] [CrossRef]
- Pereira, S.I.; Moreira, H.; Argyras, K.; Castro, P.M.; Marques, A.P. Promotion of sunflower growth under saline water irrigation by the inoculation of beneficial microorganisms. Appl. Soil Ecol. 2016, 105, 36–47. [Google Scholar] [CrossRef]
- Zeng, W.; Xu, C.; Wu, J.; Huang, J.; Zhao, Q.; Wu, M. Impacts of salinity and nitrogen on the photosynthetic rate and growth of sunflowers (Helianthus annuus L.). Pedosphere 2014, 24, 635–644. [Google Scholar] [CrossRef]
- Cabelguenne, M.; Debaeke, P.; Bouniols, A. Epicphase, a version of the epic model simulating the effects of water and nitrogen stress on biomass and yield, taking account of developmental stages: Validation on maize, sunflower, sorghum, soybean and winter wheat. Agric. Syst. 1999, 60, 175–196. [Google Scholar] [CrossRef]
- Silva, S.; Ferreira Cardoso, J.A.; Oliveira, H.; do Nascimento, R.; Guimaraes, R.F.B.; Leão, A.B. Growth and biomass of sunflower under different nitrogen levels and available water in the soil of a semi-arid region. Aust. J. Crop Sci. 2017, 11, 32. [Google Scholar] [CrossRef]
- Gallego, S.M.; Benavides, M.P.; Tomaro, M.L. Effect of heavy metal ion excess on sunflower leaves: Evidence for involvement of oxidative stress. Plant Sci. 1996, 121, 151–159. [Google Scholar] [CrossRef]
- Murillo, J.; Maranon, T.; Cabrera, F.; López, R. Accumulation of heavy metals in sunflower and sorghum plants affected by the guadiamar spill. Sci. Total Environ. 1999, 242, 281–292. [Google Scholar] [CrossRef]
- Cai, G.; Vanderborght, J.; Couvreur, V.; Mboh, C.M.; Vereecken, H. Parameterization of root water uptake models considering dynamic root distributions and water uptake compensation. Vadose Zone J. 2017. [Google Scholar] [CrossRef]
- Albasha, R.; Mailhol, J.C.; Cheviron, B. Compensatory uptake functions in empirical macroscopic root water uptake models–experimental and numerical analysis. Agric. Water Manag. 2015, 155, 22–39. [Google Scholar] [CrossRef]
- Couvreur, V.; Vanderborght, J.; Javaux, M. A simple three-dimensional macroscopic root water uptake model based on the hydraulic architecture approach. Hydrol. Earth Syst. Sci. 2012, 16, 2957–2971. [Google Scholar] [CrossRef]
- Skaggs, T.H.; van Genuchten, M.T.; Shouse, P.J.; Poss, J.A. Macroscopic approaches to root water uptake as a function of water and salinity stress. Agric. Water Manag. 2006, 86, 140–149. [Google Scholar] [CrossRef]
- Green, S.; Clothier, B. Root water uptake by kiwifruit vines following partial wetting of the root zone. Plant Soil 1995, 173, 317–328. [Google Scholar] [CrossRef]
- Zeng, W.; Xu, C.; Wu, J.; Huang, J. Soil salt leaching under different irrigation regimes: HYDRUS-1D modelling and analysis. J. Arid Land 2014, 6, 44–58. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Jia, H. Analysis of spatial variation of soil salinization using a hydrochemical and stable isotopic method in a semiarid irrigated basin, Hetao plain, Inner Mongolia, north China. Environ. Process. 2016, 3, 723–733. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Wang, J.; Pang, H.; Li, Y. Buried straw layer plus plastic mulching reduces soil salinity and increases sunflower yield in saline soils. Soil Tillage Res. 2016, 155, 363–370. [Google Scholar] [CrossRef]
- Yang, H.; Hu, J.; Long, X.; Liu, Z.; Rengel, Z. Salinity altered root distribution and increased diversity of bacterial communities in the rhizosphere soil of Jerusalem artichoke. Sci. Rep. 2016, 6, 20687. [Google Scholar] [CrossRef] [PubMed]
- Di Caterina, R.; Giuliani, M.; Rotunno, T.; De Caro, A.; Flagella, Z. Influence of salt stress on seed yield and oil quality of two sunflower hybrids. Ann. Appl. Biol. 2007, 151, 145–154. [Google Scholar] [CrossRef]
- Kong, D.; Shi, H.; Wei, Z.; Chen, Y.; Zhang, L.; Huo, Z.; Li, Y.; Wang, C. Effect on physiological properties of sunflower under water-saving irrigation in the arid areas. J. Irrig. Drain. 2004, 23, 44–46. [Google Scholar]
- Chartzoulakis, K.; Klapaki, G. Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci. Hortic. 2000, 86, 247–260. [Google Scholar] [CrossRef]
- Maas, E.; Poss, J.; Hoffman, G. Salinity sensitivity of sorghum at three growth stages. Irrig. Sci. 1986, 7, 1–11. [Google Scholar] [CrossRef]
- Gornott, C.; Wechsung, F. Statistical regression models for assessing climate impacts on crop yields: A validation study for winter wheat and silage maize in Germany. Agric. For. Meteorol. 2016, 217, 89–100. [Google Scholar] [CrossRef]
- Wang, P.; Pozdniakov, S.P. A statistical approach to estimating evapotranspiration from diurnal groundwater level fluctuations. Water Resour. Res. 2014, 50, 2276–2292. [Google Scholar] [CrossRef]
- Jones, H.G. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Paleari, L.; Movedi, E.; Confalonieri, R. Trait-based model development to support breeding programs. A case study for salt tolerance and rice. Sci. Rep. 2017, 7, 4352. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, L.; Ben-Gal, A.; Jansson, P.-E.; Shani, U. Modelling transpiration and growth in salinity-stressed tomato under different climatic conditions. Ecol. Model. 2006, 190, 15–40. [Google Scholar] [CrossRef]
- Ferrer-Alegre, F.; Stockle, C.O. A model for assessing crop response to salinity. Irrigation Sci. 1999, 19, 15–23. [Google Scholar] [CrossRef]






| Name | Version | Water | Salt | Nitrogen | Temperature | CO2 | Reference |
|---|---|---|---|---|---|---|---|
| APEX | Version: 0604 | √ | √ | √ | √ | √ | [28] |
| APSIM | Version: 2 | √ | × | √ | √ | √ | [29] |
| CropSyst | Version: 4 | √ | √ | √ | √ | √ | [30] |
| DAISY | Version: 2012 | √ | × | √ | √ | √ | [31] |
| DayCent | Version: 4.5 | √ | × | √ | √ | √ | [32] |
| DNDC | Version: 8.3p | √ | × | √ | √ | × | [33] |
| WOFOST | Version: 7.1.7 | √ | × | √ | × | √ | [34] |
| DSSAT | Version: 3.5 | √ | × | √ | √ | √ | [35] |
| EPIC | Version: 0810 | √ | √ | √ | √ | √ | [36] |
| HYDRUS | Version: 4.17 | √ | √ | √ | √ | √ | [37] |
| LPJmL | — | √ | × | √ | √ | √ | [38] |
| MONICA | Version 2.0 | √ | × | √ | √ | √ | [39] |
| SPACSYS | — | √ | × | √ | √ | √ | [40] |
| STICS | Version: 8.3 | √ | × | √ | √ | √ | [41] |
| SWAT | Version: 2009 | √ | √ | √ | × | √ | [27] |
| Growth Stages | Year: 2012 | Year: 2013 | Year: 2017 | |||
|---|---|---|---|---|---|---|
| Date | DOY | Date | DOY | Date | DOY | |
| Sowing | 7 June | 159 | 4 June | 155 | 27 May | 147 |
| Bud | 3 July | 195 | 21 July | 202 | 11 July | 192 |
| Anthesis | 15 August | 228 | 7 August | 220 | 28 July | 209 |
| Maturity | 14 September | 258 | 6 September | 249 | 5 September | 248 |
| Harvest | 24 September | 268 | 16 September | 259 | 15 September | 258 |
| Experiment | Depth (cm) | BD (g/cm3) | Sand (%) | Silt (%) | Clay (%) | OM (g/kg) | Soil Texture |
|---|---|---|---|---|---|---|---|
| Micro-plot experiments in 2012 and 2013 | 0–10 | 1.35 | 12.58 | 54.94 | 32.48 | 11.6 | Silty Clay Loam |
| 10–20 | 1.35 | 11.79 | 52.81 | 35.40 | 10.63 | Silty Clay Loam | |
| 20–30 | 1.44 | 12.02 | 52.32 | 35.66 | 8.21 | Silty Clay Loam | |
| 30–40 | 1.44 | 12.27 | 53.77 | 33.96 | 6.94 | Silty Clay Loam | |
| 40–60 | 1.44 | 8.91 | 69.77 | 21.32 | 6.94 | Silt Loam | |
| 60–80 | 1.51 | 12.48 | 70.57 | 16.95 | 6.94 | Silt Loam | |
| 80–100 | 1.51 | 16.69 | 69.59 | 13.72 | 6.94 | Silt Loam | |
| Field experiments in 2017 | 0–10 | 1.35 | 10.28 | 71.67 | 18.05 | 14.64 | Silt Loam |
| 10–20 | 1.38 | 7.85 | 72.29 | 19.86 | 15.14 | Silt Loam | |
| 20–30 | 1.44 | 6.85 | 78.88 | 14.28 | 10.74 | Silt | |
| 30–40 | 1.55 | 20.2 | 61.34 | 18.46 | 10.6 | Silt Loam | |
| 40–60 | 1.61 | 16.51 | 77.82 | 5.67 | 12.86 | Silt Loam | |
| 60–80 | 1.59 | 10.36 | 66.31 | 23.33 | 11.47 | Silt Loam | |
| 80–100 | 1.52 | 11.89 | 33.05 | 55.06 | 10.24 | Clay |
| Depth (cm) | Moisture in 2012 (cm3/cm3) | Moisture in 2013 (cm3/cm3) | Moisture in 2017 (cm3/cm3) | ||||||
| Mean (SD) | Max | Min | Mean (SD) | Max | Min | Mean (SD) | Max | Min | |
| 0–10 | 0.310 (0.052) | 0.404 | 0.073 | 0.276 (0.042) | 0.348 | 0.152 | 0.218 (0.039) | 0.360 | 0.116 |
| 10–20 | 0.310 (0.049) | 0.438 | 0.189 | 0.282 (0.045) | 0.369 | 0.190 | 0.243 (0.037) | 0.404 | 0.126 |
| 20–30 | 0.337 (0.051) | 0.434 | 0.209 | 0.305 (0.050) | 0.389 | 0.113 | 0.263 (0.045) | 0.393 | 0.153 |
| 30–40 | 0.340 (0.051) | 0.426 | 0.200 | 0.319 (0.059) | 0.557 | 0.134 | 0.300 (0.048) | 0.459 | 0.098 |
| 40–60 | 0.374 (0.056) | 0.592 | 0.204 | 0.353 (0.056) | 0.429 | 0.152 | 0.347 (0.043) | 0.488 | 0.153 |
| 60–80 | 0.411 (0.055) | 0.496 | 0.192 | 0.381 (0.072) | 0.474 | 0.113 | 0.370 (0.044) | 0.493 | 0.157 |
| 80–100 | 0.427 (0.044) | 0.503 | 0.269 | 0.390 (0.067) | 0.488 | 0.137 | 0.362 (0.041) | 0.506 | 0.170 |
| Depth (cm) | Salt in 2012 (ECe, dS/m) | Salt in 2013 (ECe, dS/m) | Salt in 2017 (ECe, dS/m) | ||||||
| Mean (SD) | Max | Min | Mean (SD) | Max | Min | Mean (SD) | Max | Min | |
| 0–10 | 9.570 (8.611) | 54.982 | 2.102 | 7.419 (3.797) | 18.130 | 2.427 | 4.442 (3.431) | 15.466 | 1.295 |
| 10–20 | 7.849 (4.827) | 23.680 | 2.294 | 6.641 (3.240) | 14.748 | 1.783 | 4.655 (3.158) | 14.112 | 1.332 |
| 20–30 | 7.608 (4.051) | 20.942 | 1.250 | 6.245 (2.978) | 14.016 | 1.613 | 5.05 (3.389) | 14.445 | 1.225 |
| 30–40 | 7.873 (5.681) | 42.128 | 1.024 | 6.007 (3.531) | 22.126 | 1.169 | 5.095 (3.308) | 14.445 | 1.295 |
| 40–60 | 6.276 (3.780) | 14.948 | 1.709 | 5.425 (2.510) | 12.136 | 1.431 | 4.988 (2.993) | 14.001 | 1.243 |
| 60–80 | 5.175 (3.251) | 15.318 | 1.469 | 4.827 (2.820) | 13.549 | 1.345 | 5.174 (2.813) | 13.483 | 1.058 |
| 80–100 | 4.532 (2.708) | 11.389 | 1.228 | 4.170 (2.483) | 11.936 | 1.228 | 4.819 (2.197) | 10.419 | 1.125 |
| Growth Parameter | Unit | Value | Description | |
|---|---|---|---|---|
| LD5009 | JK601 | |||
| TSUM1 | °C·d−1 | 1250 | 1150 | Temperature sum from emergence to anthesis |
| TSUM2 | °C·d−1 | 530 | 730 | Temperature sum from anthesis to maturity |
| RGRLAI | d−1 | 0.0294 | 0.0294 | Maximum relative increase in leaf area index (LAI) |
| SLA-0 | — | 0.035 | 0.035 | Specific leaf in developmental stage 0 |
| SLA-1 | — | 0.025 | 0.025 | Specific leaf in developmental stage 1 |
| SLA-2 | — | 0.025 | 0.025 | Specific leaf in developmental stage 2 |
| EFF | kg·ha−1·h−1·j−2·m2·s | 0.85 | 0.62 | Light use efficiency for a single leaf |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zeng, W.; Ma, T.; Lei, G.; Zha, Y.; Fang, Y.; Wu, J.; Huang, J. Testing and Improving the WOFOST Model for Sunflower Simulation on Saline Soils of Inner Mongolia, China. Agronomy 2018, 8, 172. https://doi.org/10.3390/agronomy8090172
Zhu J, Zeng W, Ma T, Lei G, Zha Y, Fang Y, Wu J, Huang J. Testing and Improving the WOFOST Model for Sunflower Simulation on Saline Soils of Inner Mongolia, China. Agronomy. 2018; 8(9):172. https://doi.org/10.3390/agronomy8090172
Chicago/Turabian StyleZhu, Jiangxu, Wenzhi Zeng, Tao Ma, Guoqing Lei, Yuanyuan Zha, Yuanhao Fang, Jingwei Wu, and Jiesheng Huang. 2018. "Testing and Improving the WOFOST Model for Sunflower Simulation on Saline Soils of Inner Mongolia, China" Agronomy 8, no. 9: 172. https://doi.org/10.3390/agronomy8090172
APA StyleZhu, J., Zeng, W., Ma, T., Lei, G., Zha, Y., Fang, Y., Wu, J., & Huang, J. (2018). Testing and Improving the WOFOST Model for Sunflower Simulation on Saline Soils of Inner Mongolia, China. Agronomy, 8(9), 172. https://doi.org/10.3390/agronomy8090172







