Assessing Field Prunus Genotypes for Drought Responsive Potential by Carbon Isotope Discrimination and Promoter Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Leaf Ash Content Analysis and Carbon Isotope Discrimination Analysis
2.3. DNA Isolation
2.4. PCR Amplification
2.5. Cloning and Sequencing
2.6. In Silico Analysis of PpDhn2 and DREB2B Promoter Regions
3. Results
3.1. Relationship between Leaf Ash Content and Δ13C
3.2. Phylogenetic Analysis Based on Promoter Regions of PpDhn2 and DREB2B Genes
3.3. Drought-Related cis-Regulatory Elements Fround in PpDhn2 and DREB2 Promoters
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bartels, D.; Sunkar, R. Drought and Salt Tolerance in Plants. CRC. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Agarwal, P.; Reddy, M.K.; Sopory, S.K. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 2006, 25, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Olvera-Carrillo, Y.; Garciarrubio, A.; Campos, F.; Covarrubias, A. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 2008, 148, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Hundertmark, M.; Hincha, D.K. LEA (Late Embryogenesis Abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Roychoudhury, A. Group II late embryogenesis abundant (LEA) proteins: Structural and functional aspects in plant abiotic stress. Plant Growth Regul. 2016, 79, 1–17. [Google Scholar] [CrossRef]
- Close, T.J. Dehydrins: Emergence of a biochemical role of a family of plant dehydration proteins. Physiol. Plant. 1996, 97, 795–803. [Google Scholar] [CrossRef]
- Hara, M.; Terashima, S.; Fukaya, T.; Kuboi, T. Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta 2003, 217, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, A.; Muto, A.; Bruno, L.; Woloszynska, M.; Van Lijsebettens, M.; Bitonti, M.B.; Lijsebettens, M. Van; Bitonti, M.B. A dehydrin gene isolated from feral olive enhances drought tolerance in Arabidopsis transgenic plants. Front. Plant Sci. 2015, 6, 392. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Du, D.; An, Y.; Yang, W.; Wang, J.; Cheng, T.; Zhang, Q. Overexpression of Prunus mume Dehydrin Genes in Tobacco Enhances Tolerance to Cold and Drought. Front. Plant Sci. 2017, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Vornam, B.; Gailing, O.; Derory, J.; Plomion, C.; Kremer, A.; Finkeldey, R. Characterisation and natural variation of a dehydrin gene in Quercus petraea (Matt.) Liebl. Plant Biol. 2011, 13, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Conde, T.; Yakovlev, I.; Majada, J.P.; Aranda, I.; Johnsen, Ø. Dehydrins in maritime pine (Pinus pinaster) and their expression related to drought stress response. Tree Genet. Genomes 2012, 8, 957–973. [Google Scholar] [CrossRef]
- Artlip, T.; Wisniewski, M. Tissue-specific Expresson of a Dehydrin Gene in One-year-old ‘Rio Oso Gem’ Peach Trees. J. Am. Soc. Hortic. Sci. 1997, 122, 784–787. [Google Scholar]
- Bassett, C.L.; Wisniewski, M.E.; Artlip, T.S.; Richart, G.; Norelli, J.L.; Farrell, R.E. Comparative expression and transcript initiation of three peach dehydrin genes. Planta 2009, 230, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, M.E.; Bassett, C.L.; Renaut, J.; Farrell, R.; Tworkoski, T.; Artlip, T.S. Differential regulation of two dehydrin genes from peach (Prunus persica) by photoperiod, low temperature and water deficit. Tree Physiol. 2006, 26, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Bielsa, B.; Leida, C.; Rubio-Cabetas, M.J. Physiological characterization of drought stress response and expression of two transcription factors and two LEA genes in three Prunus genotypes. Sci. Hortic. (Amst.) 2016, 213, 260–269. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xiang, X.; Geng, M.; You, Q.; Huang, X. Effect of HbDHN1 and HbDHN2 Genes on Abiotic Stress Responses in Arabidopsis. Front. Plant Sci. 2017, 8, 470. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.M.; El-Bastawisy, Z.M.; El-Sayed, A.K.; Ebeed, H.T.; Nemat Alla, M.M. Roles of dehydrin genes in wheat tolerance to drought stress. J. Adv. Res. 2015, 6, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, E.; Sardaro, M.L.S.; Shariati, J.V.; Rossini, L.; Porceddu, E. Isolation, promoter analysis and expression profile of Dreb2 in response to drought stress in wheat ancestors. Gene 2014, 549, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Seki, M.; Miura, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Organization and expression of two Arabidopsis DREB2 genes encoding DRE-binding proteins involved in dehydration-and high-salinity-responsive gene expression. Plant Mol. Biol. 2000, 42, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.-K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Pou, A.; Medrano, H.; Flexas, J.; Tyerman, S.D. A putative role for TIP and PIP aquaporins in dynamics of leaf hydraulic and stomatal conductances in grapevine under water stress and re-watering. Plant Cell Environ. 2013, 36, 828–843. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Tomás, M.; Medrano, H.; Pou, A.; Escalona, J.M.; Martorell, S.; Ribas-Carbó, M.; Flexas, J. Water-use efficiency in grapevine cultivars grown under controlled conditions: Effects of water stress at the leaf and whole-plant level. Aust. J. Grape Wine Res. 2012, 18, 164–172. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- De Almeida Silva, M.; Moura dos Santos, C.; Labate, C.A.; Guidetti-Gonzalez, S.; de santana Borges, J.; Ferreira, L.C.; Oliveira De Lima, R.; Fritsche-Neto, R. Breeding for Water Use Efficiency. In Plant Breeding for Abiotic Stress Tolerance; Fritsche, R., Borém, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 87–102. ISBN 978-3-642-30552-8. [Google Scholar]
- Farquhar, G.D.; Richards, R.A. Isotopic Composition of Plant Carbon Correlates with Water-use Efficiency of Wheat Genotypes. Aust. J. Plant Physiol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Melišová, L.; Hronková, M.; Holková, L.; Klemš, M.; Smutná, P. Use of ABA treatment for the activation of drought protective mechanisms in barley under non-stress conditions. Acta Univ. Agric. Silvic. Mendel. Brun. 2015, 63, 87–93. [Google Scholar] [CrossRef]
- Moghaddam, A.; Raza, A.; Vollmann, J.; Ardakani, M.R.; Wanek, W.; Gollner, G.; Friedel, J.K. Carbon isotope discrimination and water use efficiency relationships of alfalfa genotypes under irrigated and rain-fed organic farming. Eur. J. Agron. 2013, 50, 82–89. [Google Scholar] [CrossRef]
- Araus, J.L.; Amaro, T.; Casadesús, J.; Asbati, A.; Nachit, M.M. Relationships between ash content, carbon isotope discrimination and yield in durum wheat. Aust. J. Plant Physiol. 1998, 25, 835–842. [Google Scholar] [CrossRef]
- Zhu, L.; Liang, Z.S.; Xu, X.; Li, S.H. Relationship between Carbon Isotope Discrimination and Mineral Content in Wheat Grown under Three Different Water Regimes. J. Agron. Crop Sci. 2008, 194, 421–428. [Google Scholar] [CrossRef]
- Glenn, D.M. An analysis of ash and isotopic carbon discrimination (Δ13C) methods to evaluate water use efficiency in apple. Sci. Hortic. (Amst.) 2014, 171, 32–36. [Google Scholar] [CrossRef]
- Glenn, D.M.; Gasic, K. Influence of within year treatments and between year environmental differences on peach leaf ash and carbon isotopic discrimination responses. Sci. Hortic. (Amst.) 2015, 193, 258–260. [Google Scholar] [CrossRef]
- Glenn, D.M.; Bassett, C. Apple ∆13C Discrimination Is Related to Shoot Ash Content. HortScience 2011, 46, 213–216. [Google Scholar]
- Blum, A. Drought Resistance and Its Improvement. In Plant Breeding for Water-Limited Environments; Blum, A., Ed.; Springer: New York, NY, USA, 2011; pp. 53–152. ISBN 9781441974907. [Google Scholar]
- Masle, J.; Farquhar, G.D.; Wong, S.C. Transpiration Ratio and Plant Mineral Content Are Related Among Genotypes of a Range of Species. Aust. J. Plant Physiol. 1992, 19, 709–721. [Google Scholar] [CrossRef]
- Layne, R.E.C. Peach Rootstocks. In Rootstocks for Fruit Crops; Rom, R.C., Carlson, R.F., Eds.; Wiley: New York, NY, USA, 1987; pp. 185–216. [Google Scholar]
- Bielsa, B.; Jiwan, D.; Fernandez i Marti, A.; Dhingra, A.; Rubio-Cabetas, M.J. Detection of SNP and validation of a SFP InDel (deletion) in inverted repeat region of the Prunus species chloroplast genome. Sci. Hortic. (Amst.) 2014, 168, 108–112. [Google Scholar] [CrossRef]
- Byrne, D.H.; Raseira, M.B.; Bassi, D.; Piagnani, M.C.; Gasic, K.; Reighard, G.L.; Moreno, M.A.; Pérez, S. Peach. In Fruit Breeding; Badenes, M.L., Byrne, D.H., Eds.; Springer: Boston, MA, USA, 2012; pp. 505–569. ISBN 978-1-4419-0762-2. [Google Scholar]
- Felipe, A.J. ‘Felinem’, ‘Garnem’, and ‘Monegro’ almond × peach hybrid rootstocks. HortScience 2009, 44, 196–197. [Google Scholar]
- Lecouls, A.C.; Bergougnoux, V.; Rubio-Cabetas, M.J.; Bosselut, N.; Voisin, R.; Poessel, J.L.; Faurobert, M.; Bonnet, A.; Salesses, G.; Dirlewanger, E.; et al. Marker-assisted selection for the wide-spectrum resistance to root-knot nematodes conferred by the Ma gene from Myrobalan plum (Prunus cerasifera) in interspecific Prunus material. Mol. Breed. 2004, 13, 113–124. [Google Scholar] [CrossRef]
- Alimohammadi, A.; Shiran, B.; Martínez-Gómez, P.; Ebrahimie, E. Identification of water-deficit resistance genes in wild almond Prunus scoparia using cDNA-AFLP. Sci. Hortic. (Amst.) 2013, 159, 19–28. [Google Scholar] [CrossRef]
- Gradziel, T.M.; Martínez-Gómez, P.; Dicenta, F.; Kester, D.E. The Utilization of Related Prunus Species for Almond Variety Improvement. J. Am. Pomol. Soc. 2001, 55, 100–108. [Google Scholar]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Francey, R.J.; Tans, P.P.; Allison, C.E.; Enting, I.G.; White, J.W.C.; Trolier, M. Changes in oceanic and terrestrial carbon uptake since 1982. Nature 1995, 373, 326–330. [Google Scholar] [CrossRef]
- Doyle, J.; Doyle, J. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Madan, A. CAP3: A DNA sequence assembly program. Genome Res. 1999, 9, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Lee, T.Y.; Huang, H.D.; Huang, H.Y.; Pan, R.L. PlantPAN: Plant promoter analysis navigator, for identifying combinatorial cis-regulatory elements with distance constraint in plant gene groups. BMC Genom. 2008, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.K.; Wanek, W.; Clifford, S.C.; Popp, M. Contrasting adaptations to drought stress in field-grown Ziziphus mauritiana and Prunus persica trees: Water relations, osmotic adjustment and carbon isotope composition. Aust. J. Plant Phisiol. 2000, 27, 985–996. [Google Scholar] [CrossRef]
- Brendel, O.; Le Thiec, D.; Scotti-Saintagne, C.; Bodénès, C.; Kremer, A.; Guehl, J.-M. Quantitative trait loci controlling water use efficiency and related traits in Quercus robur L. Tree Genet. Genomes 2008, 4, 263–278. [Google Scholar] [CrossRef]
- Gradziel, T.M. Almond (Prunus dulcis) Breeding. In Breeding Plantation Tree Crops: Temperate Species; Jain, S.M., Prriyadarshan, P.M., Eds.; Springer: New York, NY, USA, 2009; pp. 1–32. ISBN 978-0-387-71202-4. [Google Scholar]
- Kester, D.E.; Gradziel, T.M. Almonds (Prunus). In Fruit Breeding; Moore, J.N., Janick, J., Eds.; Wiley & Sons: New York, NY, USA, 1996; pp. 1–97.(Prunus). In Fruit Breeding; Moore, J.N., Janick, J., Eds.; Wiley & Sons: New York, NY, USA, 1996; pp. 1–97. [Google Scholar]
- Wang, Y.-L. Peach Growing and Germoplasm in China. Acta Hortic. 1985, 173, 51–55. [Google Scholar]
- Cao, Y.; Luo, Q.; Tian, Y.; Meng, F. Physiological and proteomic analyses of the drought stress response in Amygdalus Mira (Koehne) Yü et Lu roots. BMC Plant Biol. 2017, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lata, C.; Prasad, M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Yamaguchi-Shinozaki, K.; Takeshi, U.; Iwasaki, T.; Hosokawa, D.; Shinozaki, K. Role of Arabidopsis MYC and MYB Homologs in Drought-and Abscisic Acid-Regulated Gene Expression. Plant Cell 1997, 9, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, A.; Paul, S.; Basu, S. Cross-talk between abscisic acid-dependent and abscisic acid-independent pathways during abiotic stress. Plant Cell Rep. 2013, 32, 985–1006. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.-S.P.; Nakashima, K.; Sakuma, Y.; Simpson, S.D.; Fujita, Y.; Maruyama, K.; Fujita, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 2004, 16, 2481–2498. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.Y.; Pence, H.E.; Jin, J.B.; Miura, K.; Gosney, M.J.; Hasegawa, P.M.; Mickelbart, M.V. The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell 2010, 22, 4128–4141. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.T.; Bell, K.D.; Ou, J.H. Characterization of the hepatitis B virus EnhI enhancer and X promoter complex. J. Virol. 1991, 65, 6686–6692. [Google Scholar] [PubMed]
- Lin, C.-Y.; Chen, Y.-H.; Lee, H.-C.; Tsai, H.-J. Novel cis-element in intron 1 represses somite expression of zebrafish myf-5. Gene 2004, 334, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Lis, M.; Walther, D. The orientation of transcription factor binding site motifs in gene promoter regions: Does it matter? BMC Genom. 2016, 17, 185. [Google Scholar] [CrossRef]
- Zolotarov, Y.; Strömvik, M. De novo regulatory motif discovery identifies significant motifs in promoters of five classes of plant dehydrin genes. PLoS ONE 2015, 10, e0129016. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bañuelos, M.; Gardea, A.; Winzerling, J.; Vazquez-Moreno, L. Characterization of a Midwinter-Expressed Dehydrin (DHN) Gene from Apple Trees (Malus domestica). Plant Mol. Biol. Rep. 2009, 27, 476. [Google Scholar] [CrossRef]
- Larkindale, J.; Vierling, E. Core Genome Responses Involved in Acclimation to High Temperature. Plant Physiol. 2008, 146, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.A.; White, A.J.; Vural, S.; Hughes, M.A. Identification of promoter elements in a low-temperature-responsive gene (blt4.9) from barley (Hordeum vulgare L.). Plant Mol. Biol. 1998, 38, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Sazegari, S.; Niazi, A.; Ahmadi, S.F. A study on the regulatory network with promoter analysis for Arabidopsis DREB-genes. Bioinformation 2015, 11, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Sivamani, E.; Bahieldin, A.; Wraith, J.M.; Al-Niemi, T.; Dyer, W.E.; Ho, T.H.D.; Qu, R. Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Sci. 2000, 155, 1–9. [Google Scholar] [CrossRef]
- Khan, M.S. The Role of DREB Transcription Factors in Abiotic Stress Tolerance of Plants. Biotechnol. Biotechnol. Equip. 2011, 25, 2433–2442. [Google Scholar] [CrossRef]
- Gupta, K.; Jha, B.; Agarwal, P.K. A Dehydration-Responsive Element Binding (DREB) Transcription Factor from the Succulent Halophyte Salicornia brachiata Enhances Abiotic Stress Tolerance in Transgenic Tobacco. Mar. Biotechnol. 2014, 16, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Ban, Q.; Liu, G.; Wang, Y. A DREB gene from Limonium bicolor mediates molecular and physiological responses to copper stress in transgenic tobacco. J. Plant Physiol. 2011, 168, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Junttila, O.; Ernstsen, A.; Heino, P.; Palva, E.T. Photoperiodic control of growth, cold acclimation and dormancy development in silver birch (Betula pendula) ecotypes. Physiol. Plant. 2003, 117, 206–212. [Google Scholar] [CrossRef]
- Yang, Y.; He, M.; Zhu, Z.; Li, S.; Xu, Y.; Zhang, C.; Singer, S.D.; Wang, Y. Identification of the dehydrin gene family from grapevine species and analysis of their responsiveness to various forms of abiotic and biotic stress. BMC Plant Biol. 2012, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Gasic, K.; Wang, Z.; Chen, X. Genetic Diversity and Genetic Structure in Natural Populations of Prunus davidiana Germplasm by SSR Markers. J. Agric. Sci. 2011, 3, 113–125. [Google Scholar] [CrossRef]
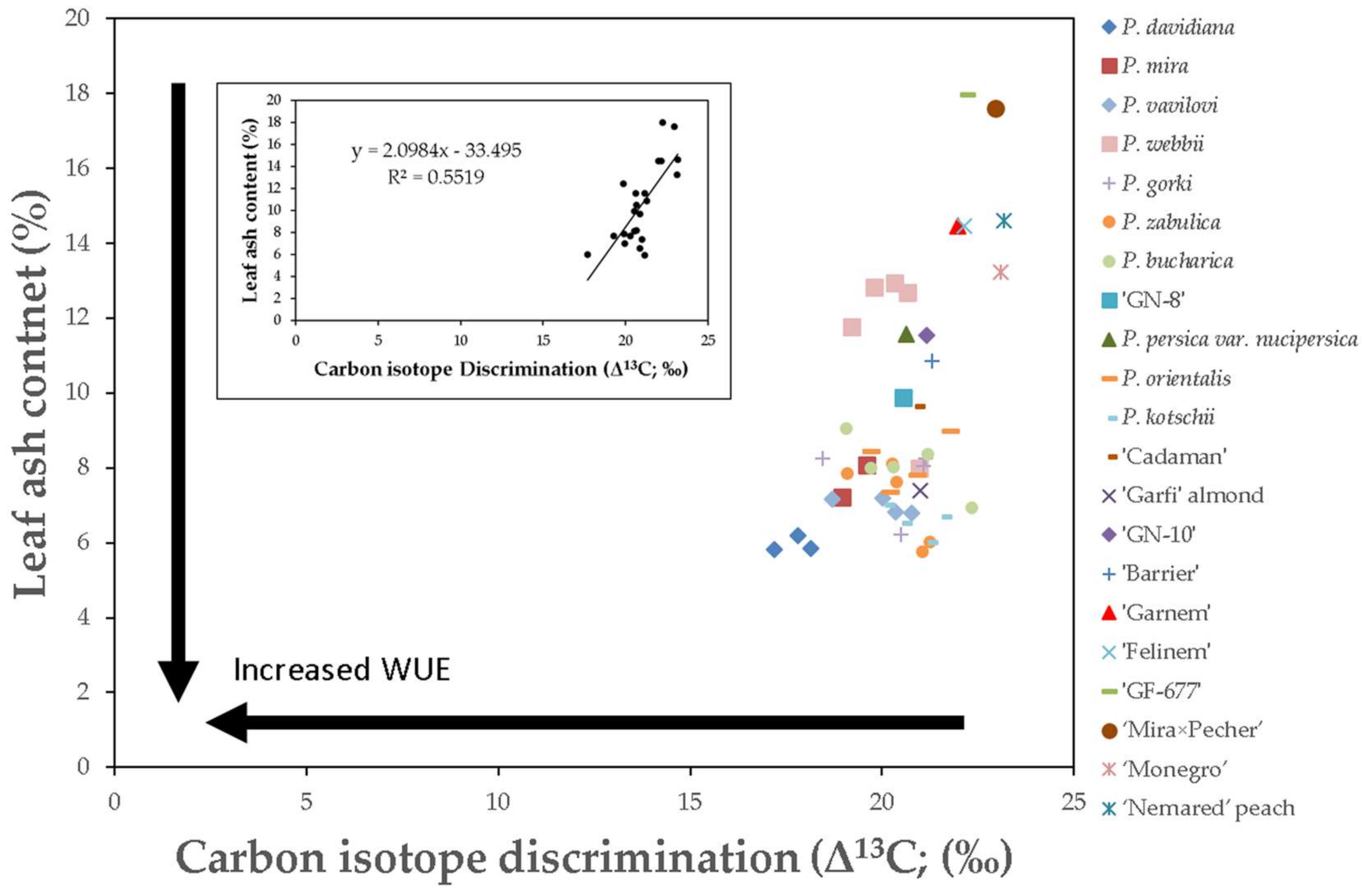
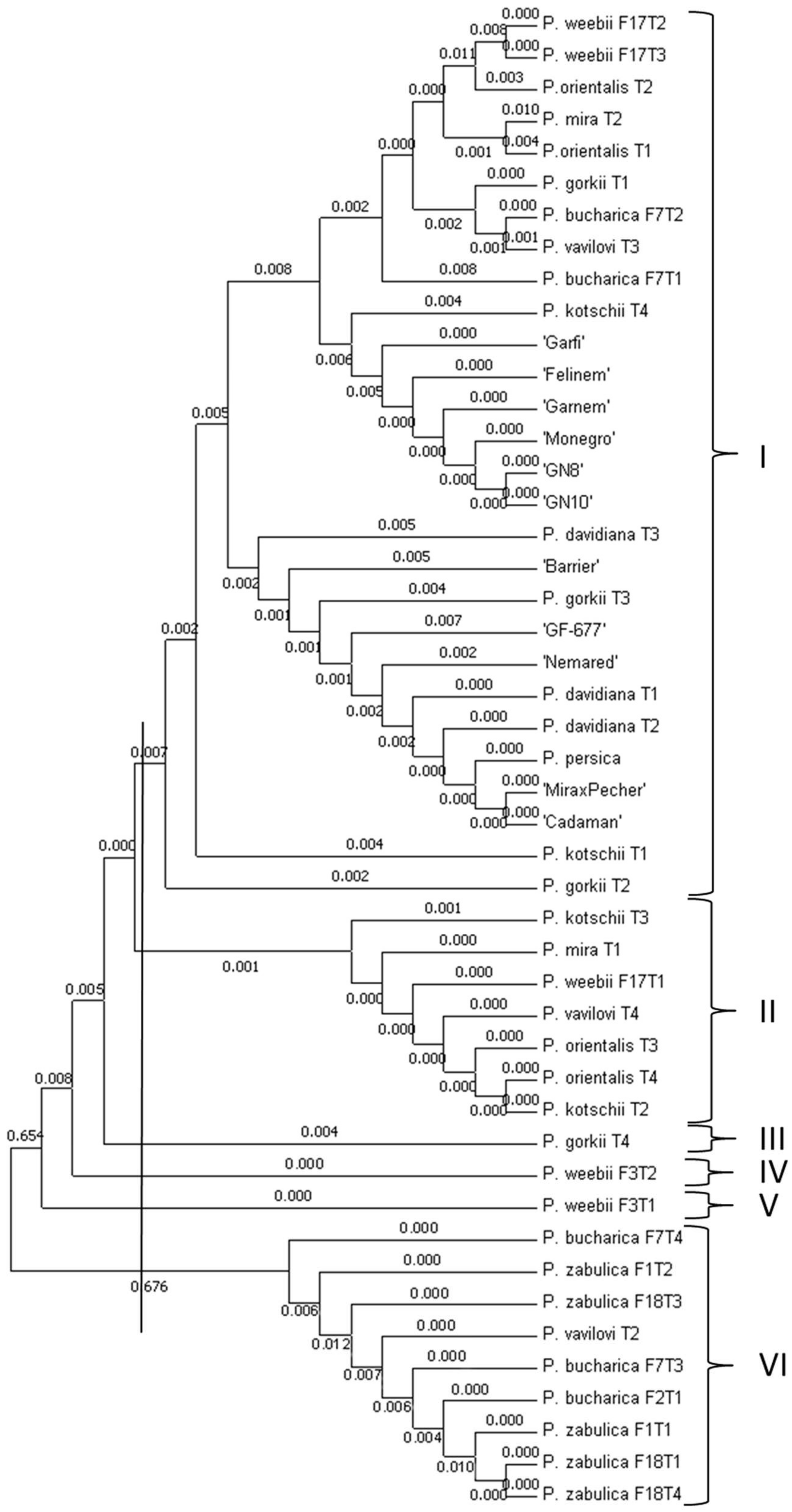
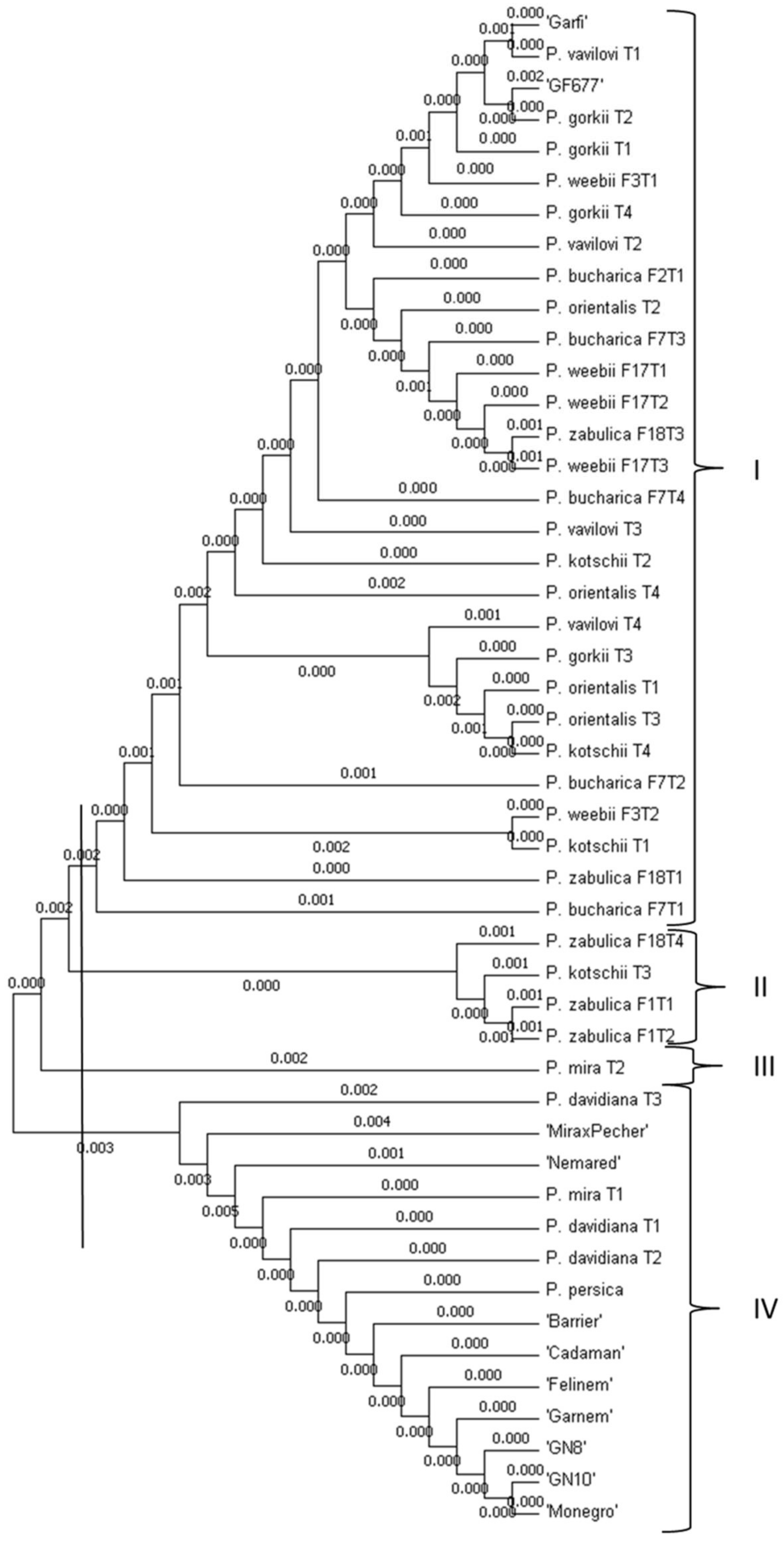

| Genotypes | Δ13C (‰) | Ash (%) |
|---|---|---|
| P. davidiana T1 | 17.798 | 6.190 |
| P. davidiana T2 | 18.157 | 5.850 |
| P. davidiana T3 | 17.186 | 5.830 |
| P. mira T1 | 19.601 | 8.070 |
| P. mira T2 | 18.944 | 7.210 |
| P. vavilovi T1 | 20.011 | 7.180 |
| P. vavilovi T2 | 20.785 | 6.790 |
| P. vavilovi T3 | 18.712 | 7.170 |
| P. vavilovi T4 | 20.349 | 6.830 |
| P. webbii F17 T1 | 19.202 | 11.760 |
| P. webbii F17 T2 | 19.785 | 12.840 |
| P. webbii F17 T3 | 20.662 | 12.680 |
| P. webbii F3 T1 | 20.321 | 12.950 |
| P. webbii F3 T2 | 20.970 | 8.000 |
| P. gorki T1 | 20.484 | 6.210 |
| P. gorki T2 | 21.139 | 8.240 |
| P. gorki T3 | 21.077 | 8.050 |
| P. gorki T4 | 18.441 | 8.260 |
| P. zabulica F1 T1 | 21.056 | 5.750 |
| P. zabulica F1 T2 | 21.241 | 6.030 |
| P. zabulica F18 T1 | 20.383 | 7.630 |
| P. zabulica F18 T3 | 19.104 | 7.850 |
| P. zabulica F18 T4 | 20.257 | 8.120 |
| P. bucharica F2 T1 | 19.053 | 9.060 |
| P. bucharica F7 T1 | 19.710 | 7.990 |
| P. bucharica F7 T2 | 21.200 | 8.370 |
| P. bucharica F7 T3 | 20.286 | 8.030 |
| P. bucharica F7 T4 | 22.344 | 6.930 |
| ‘GN-8’ | 20.548 | 9.890 |
| P. persica var. nucipersica | 20.627 | 11.570 |
| P. orientalis T1 | 20.926 | 7.810 |
| P. orientalis T2 | 19.714 | 8.450 |
| P. orientalis T3 | 21.789 | 8.990 |
| P. orientalis T4 | 20.216 | 7.350 |
| P. kotschii T1 | 20.112 | 7.010 |
| P. kotschii T2 | 21.596 | 6.690 |
| P. kotschii T3 | 20.554 | 6.520 |
| P. kotschii T4 | 21.210 | 6.030 |
| Cadaman’ | 20.880 | 9.660 |
| Garfi’ almond | 20.994 | 7.380 |
| ‘GN-10’ | 21.154 | 11.540 |
| Barrier’ | 21.292 | 10.860 |
| ‘Garnem’ | 21.979 | 14.470 |
| ‘Felinem’ | 22.146 | 14.450 |
| ‘GF-677′ | 22.235 | 17.970 |
| ‘Nemared’ peach | 23.169 | 14.600 |
| ‘Mira × Pecher’ | 22.948 | 17.600 |
| ‘Monegro’ | 23.105 | 13.240 |
| Mean | 20.530 | 9.124 |
| Standard deviation | 1.314 | 3.102 |
| CV (%) | 6.401 | 33.998 |
| Family | Element Name | Element Sequence (5→3′) | Description |
|---|---|---|---|
| PpDhn2 promoter sequence | |||
| bZIP | ABRELATERD1 | AACGT | Abscisic acid (ABA)-responsive element |
| (Motif sequence only) | ACGTATERD1 | ACGT | ABA-responsive element |
| (Motif sequence only) | ABRERATCAL | MACGYGB | ABA-responsive element |
| (Motif sequence only) | ACGTABREMOTIFA2OSEM | ACGTGKC | ABA-responsive element |
| bZIP | ASF1MOTIFCAMV | TGACG | ASF-1 binding site related to ABA signaling |
| bZIP | DPBFCOREDCDC3 | ACACNNG | Basic leucine zipper (bZIP) encoded by ABI5 |
| (Motif sequence only) | SEF4MOTIFGM7S | RTTTTTR | SEF4 binding site; ABA-responsive element |
| (Motif sequence only) | ABREDISTBBNNAPA | GCCACTTGTC | dist B (distal portion of B-box) shown similarity to ABRE/dist B ABRE mediated transactivation by ABI3 and ABI3-dependent response to ABA |
| (Motif sequence only) | ACGTTBOX | AACGTT | T-box according to the nomenclature of ACGT elements |
| CG-1; CAMTA | CAMTA3; SR1 | [ACG]CGCG[GTC] | Calmodulin-binding transcription activator 3 |
| (Motif sequence only) | CBFHV | RYCGAC | Binding site of CBF1 |
| (Motif sequence only) | DRE1COREZMRAB17 | ACCGAGA | DRE1 core |
| AP2; ERF | Dehydration-responsive element (DRE) | [AG]CCGAC | Mediates cold or dehydration-inducible transcription |
| (Motif sequence only) | DRE2COREZMRAB17 | ACCGAC | DRE2 core |
| (Motif sequence only) | DRECRTCOREAT | RCCGAC | Core motif of DRE/CRT cis-acting element |
| Dehydrin | LTRECOREATCOR15 | CCGAC | Core of low temperature responsive element (LTRE) |
| (Motif sequence only) | ERELEE4 | AWTTCAAA | Ethylene responsive element |
| HSF | Heat shock promoter element (HSE) | AGAAnnTTCT | Heat shock element |
| (Motif sequence only) | LTRE1HVBLT49 | CCGAAA | Low-temperature-responsive element (LTRE-1) |
| (Motif sequence only) | MYB2CONSENSUSAT | YAACKG | Myeloblastosis (MYB) recognition site |
| Myb | MYBCORE | CNGTTR | MYB2 TF |
| (Motif sequence only) | MYBCOREATCYCB1 | AACGG | MYB recognition site |
| (Motif sequence only) | MYBST1 | GGATA | MYB recognition site |
| (Motif sequence only) | MYB1AT | WAACCA | MYB recognition site |
| Myb/SANT; MYB | MYBGAHV | TAACAAA | Myb-like DNA-binding domain |
| (Motif sequence only) | MYBPLANT | MACCWAMC | MYB binding site |
| bHLH | EBOXBNNAPA | CANNTG | Myelocytomatosis (MYC) recognition site |
| NAC; NAM | MYCATERD1 | RCCGAC | MYC recognition sequence |
| bHLH | MYCATRD22 | CACATG | Binding site for MYC |
| bHLH | G-box | CACNTG | MYC2 gene |
| Trihelix | GT3 box | GGTAAA | Negative regulator of water use efficiency |
| DREB2B promoter sequence | |||
| bZIP | (ABARE) HEXMOTIFTAH3H4 | ACGTCA | Abscisic acid response element (ABARE) |
| bZIP | ABRELATERD1 | AACGT | ABA-responsive element |
| LEA_5 | ABREMOTIFAOSOSEM /LEA5 | TACGTGTC | Motif A ABRE-like sequence |
| (Motif sequence only) | ABRERATCAL | MACGYGB | ABA-responsive element |
| (Motif sequence only) | ACGTABREMOTIFA2OSEM | ACGTGKC | ABA-responsive element |
| (Motif sequence only) | ACGTATERD1 | ACGT | ABA-responsive element |
| bZIP | ASF1MOTIFCAMV | TGACG | ASF-1 binding site related to ABA signaling |
| (Others) | DPBFCOREDCDC3 | ACACNNG | Novel bZIP encoded by ABI5 |
| CG-1; CAMTA | CAMTA3; SR1 | [ACG]CGCG[GTC] | Calmodulin-binding transcription activator 3 |
| (Motif sequence only) | ERELEE4 | AWTTCAAA | Ethylene responsive element |
| HSF | Heat shock promoter element (HSE) | AGAAnnTTCT | Heat shock element |
| (Motif sequence only) | LTRE1HVBLT49 | CCGAAA | Low-temperature-responsive element (LTRE-1) |
| (Motif sequence only) | MYB1AT | WAACCA | MYB recognition site |
| Myb | MYBCORE | CNGTTR | MYB2 TF |
| (Motif sequence only) | MYBCOREATCYCB1 | AACGG | Myb core |
| Myb/SANT; MYB | MYBGAHV | TAACAAA | Myb-like DNA-binding domain |
| (Motif sequence only) | MYBPLANT | MACCWAMC | MYB binding site |
| (Motif sequence only) | MYBST1 | GGATA | MYB recognition site |
| bHLH | EBOXBNNAPA | CANNTG | MYC recognition site |
| (Motif sequence only) | MYB2CONSENSUSAT | YAACKG | MYB recognition site |
| (Motif sequence only) | SEF3MOTIFGM | AACCCA | SEF3 binding site |
| (Motif sequence only) | SEF4MOTIFGM7S | RTTTTTR | SEF4 binding site |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielsa, B.; Bassett, C.; Glenn, D.M.; Rubio-Cabetas, M.J. Assessing Field Prunus Genotypes for Drought Responsive Potential by Carbon Isotope Discrimination and Promoter Analysis. Agronomy 2018, 8, 42. https://doi.org/10.3390/agronomy8040042
Bielsa B, Bassett C, Glenn DM, Rubio-Cabetas MJ. Assessing Field Prunus Genotypes for Drought Responsive Potential by Carbon Isotope Discrimination and Promoter Analysis. Agronomy. 2018; 8(4):42. https://doi.org/10.3390/agronomy8040042
Chicago/Turabian StyleBielsa, Beatriz, Carole Bassett, D. Michael Glenn, and María José Rubio-Cabetas. 2018. "Assessing Field Prunus Genotypes for Drought Responsive Potential by Carbon Isotope Discrimination and Promoter Analysis" Agronomy 8, no. 4: 42. https://doi.org/10.3390/agronomy8040042
APA StyleBielsa, B., Bassett, C., Glenn, D. M., & Rubio-Cabetas, M. J. (2018). Assessing Field Prunus Genotypes for Drought Responsive Potential by Carbon Isotope Discrimination and Promoter Analysis. Agronomy, 8(4), 42. https://doi.org/10.3390/agronomy8040042






