Agromorphological Traits and Mineral Content in Tomato Accessions from El Salvador, Central America
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
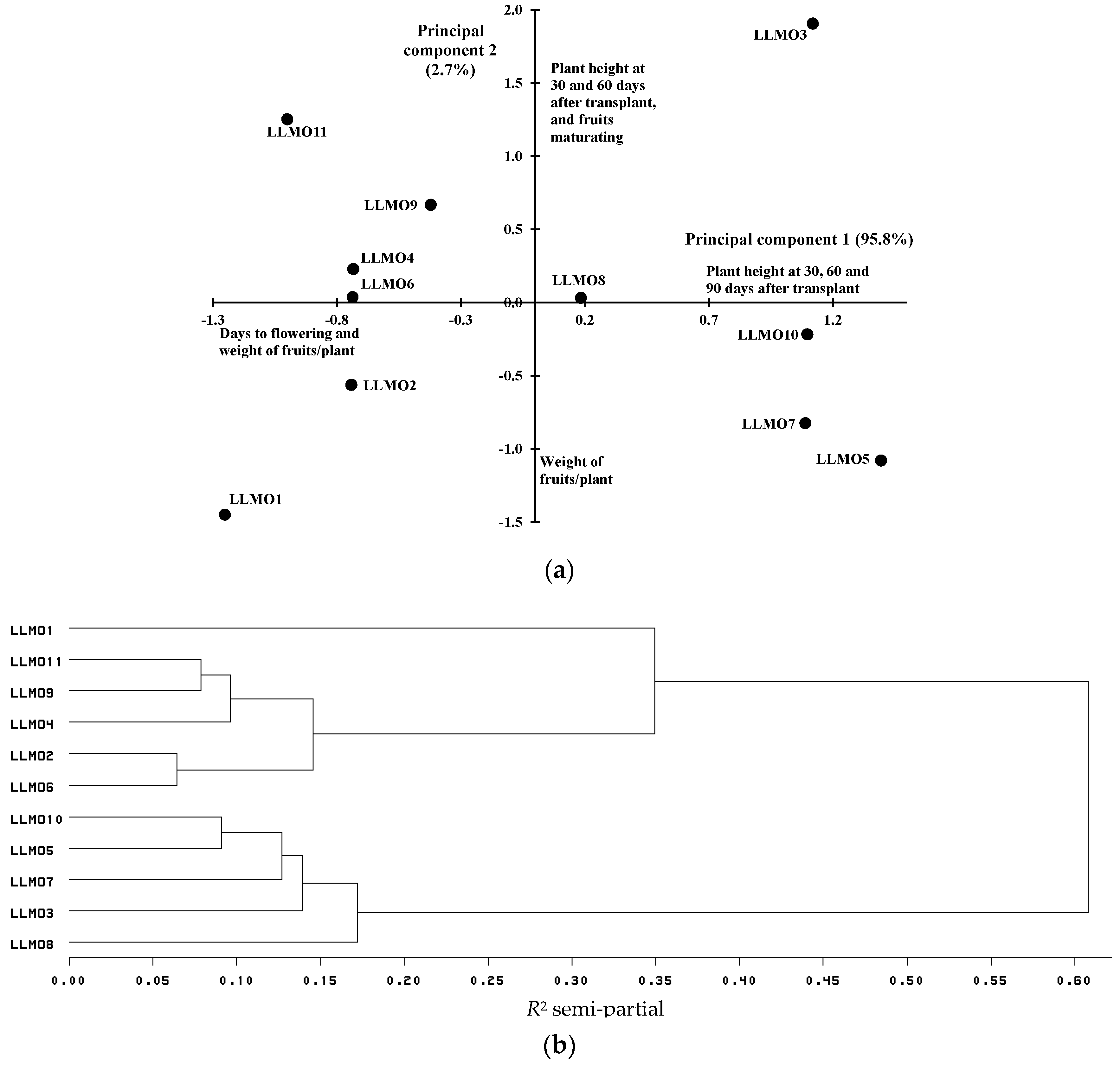
3.1. Variation in Agromorphological Traits
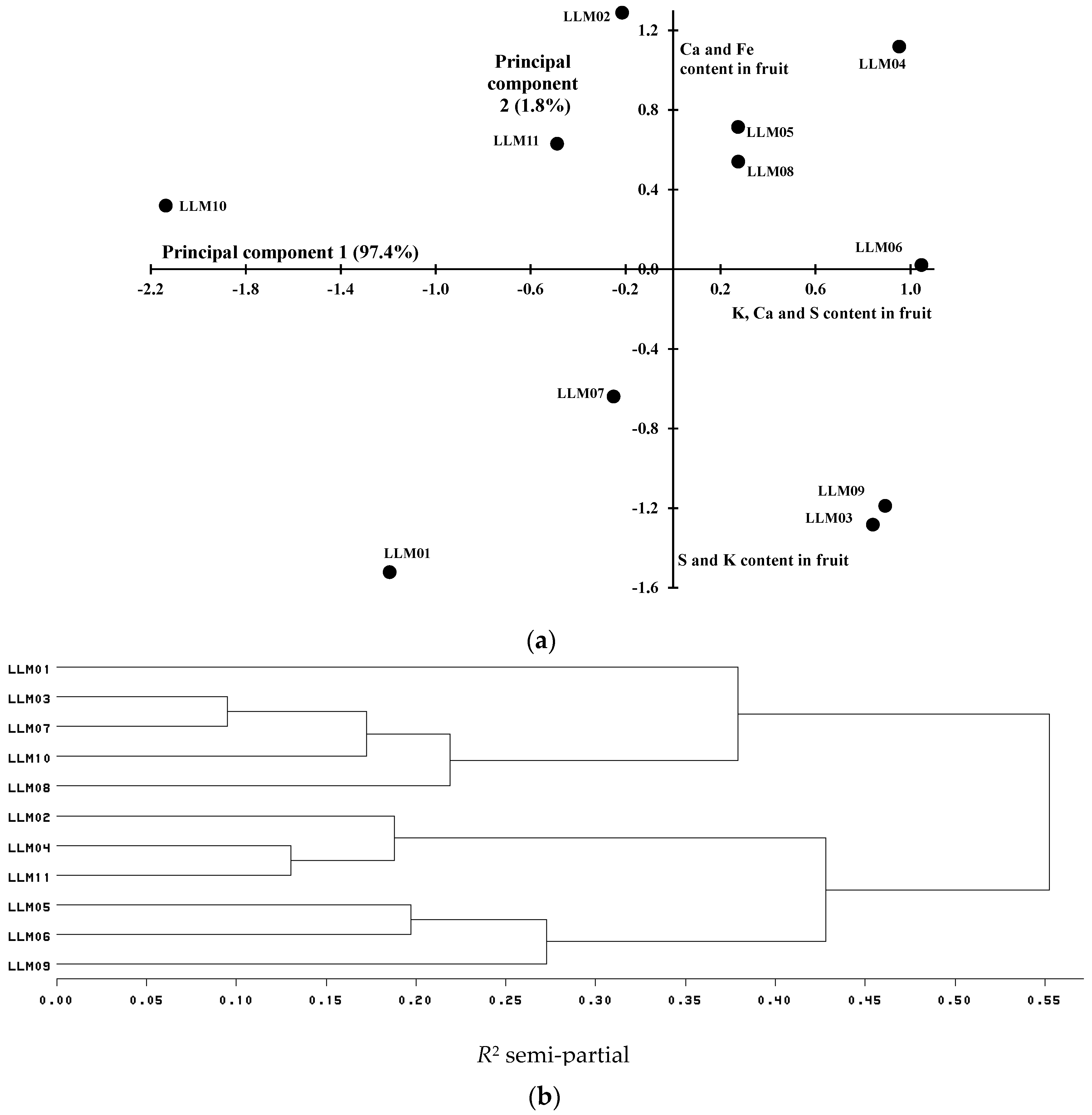
3.2. Mineral Content in Tomato Fruits
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jenkins, J.A. The origin of the cultivated tomato. Econ. Bot. 1948, 2, 379–392. [Google Scholar] [CrossRef]
- Rick, C.M. Genetic variability in tomato species. Plant Mol. Biol. Rep. 1983, 1, 81–87. [Google Scholar] [CrossRef]
- Peralta, I.E.; Spooner, D.M.; Knapp, S. Taxonomy of wild tomatoes and their relatives (Solanum sect. Lycopersicoides, sect. Juglandifolia, sect. Lycopersicon; Solanaceae). Syst. Bot. Mon. 2008, 84, 1–186. [Google Scholar]
- Grandillo, S.; Chetelat, R.; Knapp, S.; Spooner, D.; Peralta, I.; Cammreri, M.; Perez, O.; Termolino, P.; Chiusano, M.L.; Ercolano, M.R.; et al. Solanum sect. Lycopersicon. In Wild Crop Relatives: Genomic and Breeding Resources Vegetables; Kole, C., Ed.; Springer: New York, NY, USA, 2011; pp. 129–215. [Google Scholar]
- Ortiz, R.; Izquierdo, J. Yield stability differences among tomato genotypes grown in Latin America and the Caribbean. HortScience 1994, 29, 1175–1177. [Google Scholar]
- Ortiz, R.; Crossa, J.; Vargas, M.; Izquierdo, J. Studying the effect of environmental variables on the genotype x environment interaction of tomato. Euphytica 2007, 153, 119–134. [Google Scholar] [CrossRef]
- Terzopoulus, P.J.; Walters, S.A.; Bebeli, P.J. Evaluation of Greek tomato landraces populations for heterogeneity of horticultural traits. Eur. J. Hort. Sci. 2009, 74, 24–29. [Google Scholar]
- Mazzucato, A.; Ficcadenti, N.; Caioni, M.; Mosconi, P.; Piccinini, E.; Sanampudi, V.R.R.; Sestili, S.; Ferrari, V. Genetic diversity and distinctiviness in tomato (Solanum lycopersicum L.) landraces: The Italian case study of ‘A pera Abruzzese’. Sci. Hort. 2010, 125, 55–62. [Google Scholar] [CrossRef]
- Cebolla-Cornejo, J.; Roselló, S.; Nuez, F. Phenotypic and genetic diversity of Spanish tomato landraces. Sci. Hort. 2013, 162, 150–164. [Google Scholar] [CrossRef]
- Lenucci, M.S.; Cadinu, D.; Taurino, M.; Piro, G.; Dalessandro, G. Antioxidant composition in cherry and high-pigment tomato cultivars. J. Agric. Food Chem. 2006, 54, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, N.; Vallejo, F.A. Evaluating the fruit production and quality of cherry tomato (Solanum lycopersicum var cerasiforme). Rev. Fac. Nac. Agr. 2012, 65, 6593–6604. [Google Scholar]
- Bressy, F.C.; Brito, G.B.; Barbosa, I.S.; Teixeira, L.S.G.; Korn, M.G.A. Determination if trace element concentrations in tomato samples at different stages of maturation by ICP OES and ICP-MS following microwave-assisted digestion. Microchem. J. 2013, 109, 145–149. [Google Scholar] [CrossRef]
- Fernández-Ruiz, V.; Olives, A.I.; Cámara, M.; Sánchez-Mata, M.C.; Esperanza-Torija, M. Mineral and trace elements content in 30 accessions of tomato fruits (Solanum lyopersicum L.) and wild relatives (Solanum pimpinellifolium L.; Solanum cheesmaniae L. Riley, and Solanum habrochaites S. Knapp & D.M. Spooner). Biol. Trace Elem. Res. 2011, 141, 329–339. [Google Scholar] [PubMed]
- Guil-Guerrero, J.L.; Rebolloso-Fuentes, M.M. Nutrient composition and antioxidant activity of eight tomato (Lycopersicon esculentum) varieties. J. Food Comp. Anal. 2008, 22, 123–129. [Google Scholar] [CrossRef]
- Hernández-Suárez, M.; Peña-Méndez, E.; Rodríguez-Galdón, B.; Rodríguez-Rodríguez, E.; Díaz-Romero, C. Influence of agronomic variables on quality of tomato fruits. Agric. Sci. 2011, 2, 424–431. [Google Scholar]
- Oyetayo, F.L.; Ibitoye, M.F. Phytochemical and nutrient/antinutrient interactions in cherry tomato (Lycopersicum esculentum) fruits. Intern. J. Adv. Biol. Res. 2012, 2, 681–684. [Google Scholar]
- Rick, C.M. Potential genetic resources in tomato species: Clues from observations in native habitats. In Genes, Enzymes and Populations; Srb, A., Ed.; Plenum Press: New York, NY, USA, 1973; pp. 255–269. [Google Scholar]
- Hoisington, D.; Khairallah, M.; Reeves, T.; Ribaut, J.-M.; Skovmand, B.; Taba, S.; Warburton, M. Plant genetic resources: What can they contribute toward increased crop productivity? Proc. Natl. Acad. Sci. USA 1999, 96, 5937–5943. [Google Scholar] [CrossRef] [PubMed]
- Terzopoulus, P.J.; Bebeli, P.J. Phenotypic diversity in Greek tomato (Solanum lycopersiucm L.) landraces. Sci. Hort. 2010, 126, 138–144. [Google Scholar] [CrossRef]
- Koutsika-Sortitiou, M.; Mylonas, I.; Tsivelikas, A.; Traka-Mavrona, E. Compensation studies on the tomato landraces ‘Tomataki Santorinis’. Sci. Hort. 2016, 198, 78–85. [Google Scholar] [CrossRef]
- García-Martínez, S.; Corrado, G.; Ruiz, J.J.; Rao, R. Diversity and structure of a simple of traditional Italian and Spanish tomato accessions. Genet. Resour. Crop Evol. 2013, 60, 789–798. [Google Scholar] [CrossRef]
- Galiana-Balaguer, L.; Rosello, S.; Nuez, F. Characterization and selection of balanced sources of variability for breeding tomato (Lycopersicon) internal quality. Genet. Resour. Crop Evol. 2006, 53, 907–923. [Google Scholar] [CrossRef]
- Sadashiva, A.T.; Christopher, M.G.; Krithika, T.K. Genetic enhancement of tomato crop for abiotic stress tolerance. In Climate-Resilient Horticulture: Adaptation and Mitigation Strategies; Sinh, H.P., Rao, N.K.S., Shivashankar, K.S., Eds.; Springer: New Delhi, India, 1973; pp. 113–124. [Google Scholar]
- Ashraf, A.; Foolad, M.R. Crop breeding for salt tolerance in the era of molecular markers and marker-assisted selection. Plant Breed. 2013, 132, 10–20. [Google Scholar] [CrossRef]
- Hartman, J.B.; St Clair, D.A. Combining ability for beet armyworm, Spodoptera exigua, resistance and horticultural traits of selected Lycopersicon pennellii-derived inbred backcross lines of tomato. Plant Breed. 1999, 118, 523–530. [Google Scholar] [CrossRef]
- Cervantes-Moreno, R.; Rodríguez-Pérez, J.E.; Carrillo, C.; Sahagún-Castellanos, J.; Rodríguez-Guzmán, E. Tolerancia de 26 colectas de tomates nativos de México al nematodo Meloidogyne incognita (Kofoid y White) Chitwood. Rev. Chapingo Ser. Hort. 2014, 20, 5–18. [Google Scholar] [CrossRef]
- Bauchet, G.; Cause, M. Genetic diversity in tomato (Solanum lycopersicum) and its wild relatives. In Genetic Diversity in Plants; Caliskan, M., Ed.; InTech Europe: Rijeka, Croatia, 2012; pp. 133–162. [Google Scholar]
- Hernández-Bautista, A.; Lobato-Ortiz, R.; Cruz-Izquierdo, S.; García-Zavala, J.J.; Chávez-Servia, J.L. Variación fenotípica, heterosis y heredabilidad de una cruza interespecífica de jitomate. Interciencia 2014, 39, 327–332. [Google Scholar]
- Van den Heuvel, T.; Rene, R.J.; Gremmen, B.; van woerkum, C.; van Trijp, H. Consumers’ images regarding genomics as a tomato breeding technology: “Maybe it can provide a more tasty tomato”. Euphytica 2008, 159, 207–216. [Google Scholar] [CrossRef]
- Méndez-Infante, I.; Vera-Guzmán, A.M.; Chávez-Servia, J.L.; Carrillo-Rodríguez, J.C. Quality of fruits in Mexican tomato (Lycopersicon esculentum Mill.) landraces. VITAE-Rev. Fac. Quím. Farm. 2011, 18, 26–32. [Google Scholar]
- Luna-Guevara, M.L.; Delgado-Alvarado, A. Importancia, contribución y estabilidad de antioxidantes en frutos y productos de tomate (Solanum lycopersicum L.). Av. Investig. Agrop. 2014, 18, 51–66. [Google Scholar]
- Gundersen, V.; McCall, D.; Bechmann, I.E. Comparison of major and trace element concentrations in Danish greenhouse tomatoes (Lycopersicum esculentum cv. Aromata F1) cultivated in different substrates. J. Agric. Food Chem. 2001, 49, 3808–3815. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Suárez, M.; Rodríguez-Rodríguez, E.M.; Díaz-Romero, C. Mineral and trace element concentrations in cultivars of tomatoes. Food Chem. 2007, 104, 489–499. [Google Scholar] [CrossRef]
- Kelly, S.D.; Bateman, A.S. Comparison of mineral concentrations in commercially grown organic and conventional crops-tomatoes (Lycopersicon esculentum) and lettuces (Lactuca sativa). Food Chem. 2010, 119, 738–745. [Google Scholar] [CrossRef]
- Aghili, F.; Khoshgoftarmnesh, A.H.; Afyuni, M.; Mobil, M. Mineral and ascorbic acid concentrations of greenhouse- and field-grown vegetables: Implications for human health. Int. J. Veg. Sci. 2012, 18, 64–77. [Google Scholar] [CrossRef]
- Borgognone, D.; Colla, G.; Rouphael, Y.; Cardarelli, M.; Rea, E.; Schwarz, D. Effect of nitrogen form and nutrient solution pH on growth and mineral composition of self-grafted and grafted tomatoes. Sci. Hortic. 2013, 149, 61–69. [Google Scholar] [CrossRef]
- Corrado, G.; Piffanelli, P.; Caramante, M.; Coppola, M.; Rao, R. SNP genotyping reveals genetic diversity between cultivated landraces and contemporary varieties of tomato. BMB Genom. 2013, 14, 835. [Google Scholar] [CrossRef] [PubMed]
- Cebolla-Cornejo, J.; Roselló, S.; Valcárcel, M.; Serrano, E.; Beltrán, J.; Nuez, F. Evaluation of genotypes and environments effects on tatste aroma flavor components of Spanish fresh tomato varieties. J. Agric. Food Chem. 2011, 59, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- IPGRI; AVRDC; CATIE. Descriptors for Capsicum (Capsicum spp.); International Plant Genetic Resources Institute: Rome, Italy; Asian Vegetable Research and Development Center: Taipei, Taiwan; Centro Agronómico Tropical de Investigación y Enseñanza: Turrialba, Costa Rica, 1995; pp. 1–50. [Google Scholar]
- Carrillo, J.C.; Chávez, J.L. Caracterización agromorfológica de muestras de tomate de Oaxaca. Rev. Fitotec. Mex. 2010, 33, 1–6. [Google Scholar]
- Carrillo-Rodríguez, J.C.; López-Mendoza, H.; Chávez-Servia, J.L.; Rodríguez-Guzmán, E.; Sánchez-Peña, P.; Lobato-Ortiz, R. Phenotypic divergences on growth and productivity of wild and semidomesticated cherry tomato grown under greenhouse conditions. Acta Hortic. 2012, 947, 375–380. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemist: Washington, DC, USA, 1990; Volume 2, pp. 27–42. [Google Scholar]
- Peralta, I.E.; Spooner, D.M. History, origin and early cultivation of tomato (Solanaceae). In Genetic Improvement of Solanaceous Crops, Vol. 2. Tomato; Razdan, M.K., Mattoo, A.K., Eds.; Science Publishers: Enfield, CT, USA, 2007; pp. 1–27. [Google Scholar]
- Chetelat, R.T. Revised list of wild species stocks. Tomato Gen. Coop. Rep. 2004, 54, 52–76. [Google Scholar]
- Chetelat, R.T. Revised list of miscellaneous stocks. Tomato Gen. Coop. Rep. 2006, 56, 37–59. [Google Scholar]
- Álvarez-Hernández, J.C.; Cortez-Madrigal, H.; García-Ruiz, I. Exploración y caracterización de poblaciones silvestres de jitomate (Solanaceae) en tres regiones de Michoacán, México. Polibotánica 2009, 28, 139–159. [Google Scholar]
- Medina, C.I.; Lobo, M. Variabilidad morfológica en el tomate pajarito (Lycopersicum esculentum var. cerasiforme), precursor del tomate cultivado. Rev. Corpoica 2001, 3, 39–50. [Google Scholar]
- Costa, E.; da Silva, F.F.; Duarte, E.; Barbosa, D.; Zoz, T.; Zuffo, A.M. Cherry tomato production on different organic substrates under protected environment conditions. Aust. J. Crop Sci. 2018, 12, 87–92. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortifying crops with essential mineral elements. Trend Plant Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, L.; Paolotti, L.; Cortina, C.; Boggia, A. Conservation of landrace: The key role of the value for agrobiodiversity conservation. An application on ancient tomatoes varieties. Agric. Agric. Sci. Procedia 2016, 8, 307–316. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets-iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
| Population ID | Fruit Type | Region of Origin from El Salvador (Municipality, Department) | Latitude (N) | Longitude (W) | Altitude (m.a.s.l.) |
|---|---|---|---|---|---|
| LLM01 | Medium size | La Libertad, La Libertad | 13°29′18″ | 89°19′14″ | 637 |
| LLM02 | Cherry | San José Villanueva, La Libertad | 13°34′00″ | 89°16′00″ | 540 |
| LLM03 | Cherry | San José Villanueva, La Libertad | 13°34′00″ | 89°16′00″ | 540 |
| LLM04 | Cherry | San Juan Opico, La Libertad | 13°52′60″ | 89°21′00″ | 522 |
| LLM05 | Cherry | San Salvador, San Salvador | 13°41′24″ | 89°11′24″ | 649 |
| LLM06 | Cherry | San Pablo Tacachico, La Libertad | 13°58′60″ | 89°19′60″ | 307 |
| LLM07 | Cherry | Ciudad Victoria, Cabañas | 13°57′00″ | 88°37′60″ | 868 |
| LLM08 | Medium size | San Pablo Tacachico, La Libertad | 13°58′60″ | 89°19′60″ | 307 |
| LLM09 | Cherry | Ciudad Victoria, Cabañas | 13°57′00″ | 88°37′60″ | 868 |
| LLM10 | Cherry | Ciudad Victoria, Cabañas | 13°57′00″ | 88°37′60″ | 868 |
| LLM11 | Cherry | Ciudad Arce, La Libertad | 13°49′60″ | 89°25′60″ | 525 |
| Variables Evaluated | Sources of Variations | CV (%) | |||
|---|---|---|---|---|---|
| Repetitions | Accessions | Plant (Acc.) 2 | Error | ||
| Days to start of flowering 1 | 118.91 ** | 43.76 ** | - | 4.23 | 8.7 |
| Days to start of fructifying 1 | 4.45 * | 10.62 ** | - | 1.25 | 3.1 |
| Days to start of fruit maturating 1 | 471.55 ** | 29.49 ** | - | 2.82 | 2.9 |
| Plant height at 30 dat 1 | 88.86 ** | 59.91 ** | 7.07 NS | 4.82 | 9.0 |
| Plant height at 60 dat 1 | 749.72 ** | 332.94 ** | 42.29 NS | 61.44 | 13.0 |
| Plant height at 90 dat 1 | 18.40 ** | 22.15 ** | 2.49 NS | 2.12 | 13.1 |
| Number of flowers per cluster | 0.49 NS | 2.67 ** | 0.61 NS | 0.48 | 8.7 |
| Number of fruits per cluster | 0.26 NS | 1.82 ** | 0.61 NS | 0.52 | 9.2 |
| Number of fruits per plant | 54.91 * | 46.79 ** | 11.96 NS | 12.36 | 9.0 |
| Average weight of fruits per cluster | 0.006 NS | 11.214 ** | 0.230 NS | 0.43 | 11.2 |
| Average weight of fruits per plant | 0.03 NS | 56.05 ** | 1.15 NS | 2.16 | 11.2 |
| Average weight per fruit | 0.006 NS | 11.214 ** | 0.230 NS | 0.43 | 11.2 |
| Fruit length | 0.12 ** | 1.01 ** | - | 0.02 | 7.6 |
| Fruit width | 0.28 ** | 1.36 ** | - | 0.04 | 10.0 |
| Pob. ID | Days from Transplant to Start of | Plant Height (cm) at | ||||
|---|---|---|---|---|---|---|
| Flowering | Fructifying | Maturating | 30 dat | 60 dat | 90 dat | |
| LLMO1 | 22.0 cd 1 | 38.7 a | 54.0 e | 19.7 e | 51.2 c | 82.3 c |
| LLMO2 | 25.7 ab | 35.0 e | 60.0 a | 22.3 cde | 55.2 bc | 100.6 bc |
| LLMO3 | 22.7 bcd | 35.7 cde | 57.3 bcd | 26.3 ab | 74.6 a | 167.1 a |
| LLMO4 | 23.7 bc | 35.3 de | 58.0 abc | 25.1 abc | 59.8 bc | 100.6 bc |
| LLMO5 | 23.7 bc | 37.7 ab | 59.0 ab | 25.3 abc | 56.2 bc | 178.1 a |
| LLMO6 | 28.7 a | 36.3 bcde | 59.3 ab | 23.9 bcd | 58.7 bc | 100.6 bc |
| LLMO7 | 23.7 bc | 37.3 abc | 56.0 cde | 20.7 de | 58.6 bc | 167.0 a |
| LLMO8 | 20.3 d | 36.0 bcde | 56.3 cde | 24.0 bcd | 61.1 bc | 133.9 ab |
| LLMO9 | 25.3 b | 37.0 abcd | 58.0 abc | 26.3 ab | 63.1 abc | 111.7 bc |
| LLMO10 | 22.7 bcd | 37.0 abcd | 55.3 de | 28.1 a | 60.3 bc | 167.0 a |
| LLMO11 | 22.7 bcd | 36.3 bcde | 57.7 abcd | 26.1 ab | 65.5 ab | 90.6 bc |
| Pop. ID | Flowers Per Cluster | Fruits Per Cluster | Fruits Per Plant | Fruit Weight Per Cluster (g) | Fruit Weight Per Plant (g) | Average Weight Per Fruit (g) | Fruit Length (cm) | Fruit Width (cm) |
|---|---|---|---|---|---|---|---|---|
| LLMO1 | 8.8 a 1 | 7.9 abc | 39.4 ab | 82.9 a | 414.7 a | 10.5 a | 2.9 a | 3.2 a |
| LLMO2 | 7.7 bc | 7.7 abc | 38.6 ab | 25.9 c | 129.7 c | 3.4 b | 1.8 c | 2.2 b |
| LLMO3 | 8.1 ab | 8.1 ab | 40.7 a | 35.0 bc | 175.2 bc | 4.3 b | 1.9 c | 2.0 bcd |
| LLMO4 | 7.0 c | 7.0 c | 35.1 b | 26.4 c | 132.0 c | 3.8 b | 1.9 c | 1.9 cd |
| LLMO5 | 8.4 ab | 8.4 a | 41.9 a | 39.5 b | 197.5 b | 4.7 b | 1.9 c | 2.0 bcd |
| LLMO6 | 7.7 bc | 7.7 abc | 38.3 ab | 25.6 c | 127.8 c | 3.3 b | 1.7 c | 1.8 d |
| LLMO7 | 7.9 abc | 7.9 abc | 39.6 ab | 34.6 bc | 172.9 bc | 4.4 b | 1.9 c | 1.9 cd |
| LLMO8 | 7.0 c | 7.0 c | 34.9 b | 29.4 bc | 147.1 bc | 4.2 b | 2.2 b | 2.2 b |
| LLMO9 | 8.2 ab | 8.2 ab | 40.8 a | 33.6 bc | 167.9 bc | 4.1 b | 1.8 c | 2.0 bcd |
| LLMO10 | 7.9 abc | 7.9 abc | 39.3 ab | 29.7 bc | 148.5 bc | 3.8 b | 1.8 c | 1.9 cd |
| LLMO11 | 8.1 ab | 8.1 ab | 41.1 a | 31.3 bc | 156.6 bc | 3.8 b | 1.9 c | 1.8 d |
| Mineral Content in Fruit | Sources of Variation | CV (%) | ||
|---|---|---|---|---|
| Repetition | Populations | Error | ||
| Ca | 548.7 ** | 165.6 ** | 20.7 | 24.3 |
| K | 23,477.7 ** | 7787.0 * | 3177.2 | 17.5 |
| Mg | 102.0 ** | 46.0 ** | 12.6 | 22.0 |
| Mn | 0.020 ** | 0.008 ** | 0.003 | 17.2 |
| P | 756.9 ** | 563.4 ** | 116.3 | 21.8 |
| S as SO4 | 13.6 NS | 80.1 ** | 8.8 | 21.4 |
| Na | 0.02 NS | 1.25 ** | 0.31 | 18.4 |
| Cu | 0.086 ** | 0.010NS | 0.01 | 22.2 |
| Fe | 0.046 * | 0.068 ** | 0.014 | 17.4 |
| Zn | 0.107 ** | 0.044 ** | 0.016 | 21.1 |
| Pop. ID/Macro-Elements | Ca | K | Mg | P | S | Na |
| mg/100 g of Dry Weight | ||||||
| LLM01 | 9.2 d 1 | 281.7 ab | 11.3 c | 32.8 c | 32.4 c | 12.8 a |
| LLM02 | 23.4 ab | 311.4 ab | 20.4 a | 57.5 ab | 35.7 bc | 7.2 abc |
| LLM03 | 15.1 cd | 345.2 ab | 13.4 bc | 47.1 abc | 41.5 bc | 11.1 abc |
| LLM04 | 25.5 a | 348.0 a | 18.3 ab | 62.1 a | 37.2 bc | 6.4 c |
| LLM05 | 22.2 abc | 326.7 ab | 16.3 abc | 45.3 abc | 47.4 bc | 10.7 abc |
| LLM06 | 21.2 abc | 350.8 a | 17.6 ab | 59.1 ab | 65.0 a | 6.7 bc |
| LLM07 | 15.2 cd | 310.7 ab | 16.1 abc | 46.4 abc | 47.7 b | 12.7 ab |
| LLM08 | 21.4 abc | 327.0 ab | 17.6 ab | 43.8 abc | 34.5 bc | 10.7 abc |
| LLM09 | 15.6 bcd | 346.9 ab | 16.4 abc | 57.5 ab | 36.9 bc | 11.5 abc |
| LLM10 | 14.6 cd | 251.6 b | 13.4 bc | 44.0 abc | 33.9 bc | 8.7 abc |
| LLM11 | 20.0 abc | 303.1 ab | 15.2 abc | 42.4 bc | 35.9 bc | 5.8 c |
| Pod. ID/Micro-Elements | Fe | Zn | Mn | Cu | ||
| mg/100 g of Dry Weight | ||||||
| LLM01 | 0.283 c 1 | 0.178 c | 0.079 b | 0.149 a | ||
| LLM02 | 0.838 a | 0.386 abc | 0.128 ab | 0.215 a | ||
| LLM03 | 0.396 bc | 0.347 abc | 0.080 b | 0.235 a | ||
| LLM04 | 0.612 ab | 0.378 abc | 0.146 a | 0.256 a | ||
| LLM05 | 0.380 bc | 0.410 abc | 0.088 ab | 0.166 a | ||
| LLM06 | 0.603 ab | 0.439 ab | 0.115 ab | 0.179 a | ||
| LLM07 | 0.415 bc | 0.361 abc | 0.092 ab | 0.270 a | ||
| LLM08 | 0.423 bc | 0.231 bc | 0.088 ab | 0.233 a | ||
| LLM09 | 0.494 abc | 0.582 a | 0.113 ab | 0.187 a | ||
| LLM10 | 0.471 abc | 0.335 abc | 0.094 ab | 0.239 a | ||
| LLM11 | 0.582 ab | 0.359 abc | 0.105 ab | 0.246 a | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Linares-Menéndez, L.R.; Carrillo-Rodríguez, J.C.; Aquino-Bolaños, E.N. Agromorphological Traits and Mineral Content in Tomato Accessions from El Salvador, Central America. Agronomy 2018, 8, 32. https://doi.org/10.3390/agronomy8030032
Chávez-Servia JL, Vera-Guzmán AM, Linares-Menéndez LR, Carrillo-Rodríguez JC, Aquino-Bolaños EN. Agromorphological Traits and Mineral Content in Tomato Accessions from El Salvador, Central America. Agronomy. 2018; 8(3):32. https://doi.org/10.3390/agronomy8030032
Chicago/Turabian StyleChávez-Servia, José Luis, Araceli Minerva Vera-Guzmán, Lesser Roberto Linares-Menéndez, José Cruz Carrillo-Rodríguez, and Elia Nora Aquino-Bolaños. 2018. "Agromorphological Traits and Mineral Content in Tomato Accessions from El Salvador, Central America" Agronomy 8, no. 3: 32. https://doi.org/10.3390/agronomy8030032
APA StyleChávez-Servia, J. L., Vera-Guzmán, A. M., Linares-Menéndez, L. R., Carrillo-Rodríguez, J. C., & Aquino-Bolaños, E. N. (2018). Agromorphological Traits and Mineral Content in Tomato Accessions from El Salvador, Central America. Agronomy, 8(3), 32. https://doi.org/10.3390/agronomy8030032





