Abstract
Soil and water pollution by metals and other toxic chemicals is difficult to measure and control, and, as such, presents an ongoing global threat to sustainable agriculture and human health. Efforts to remove contaminants by plant-mediated pathways, or “phytoremediation”, though widely studied, have failed to yield consistent, predictable removal of biological and chemical contaminants. Emerging research has revealed that one major limitation to using plants to clean up the environment is that plants are programmed to protect themselves: Like white blood cells in animals, border cells released from plant root tips carry out an extracellular trapping process to neutralize threats and prevent injury to the host. Variability in border cell trapping has been found to be correlated with variation in sensitivity of roots to aluminum, and removal of border cell results in increased Al uptake into the root tip. Studies now have implicated border cells in responses of diverse plant roots to a range of heavy metals, including arsenic, copper, cadmium, lead, mercury, iron, and zinc. A better understanding of border cell extracellular traps and their role in preventing toxin uptake may facilitate efforts to use plants as a nondestructive approach to neutralize environmental threats.
1. Root Border Cells
Most plant species synthesize cell populations that are programmed to disperse into the external environment surrounding the root tip in response to free water or abrasion (Figure 1). For many years, these so-called “sloughed root cap cell” populations were thought to be a product of tissue disintegration based on the logical presumption that cells falling from the root surface must be dead. This was despite the observation in 1919 [1] that “sloughed root cap cells” from pea and corn could remain 100% viable for months in hydroponic culture. Long-term survival of the detached cells in culture eventually was confirmed, but the presumption remained that these cells expressed the phenotypes of the whole plant with regard to pathogen recognition and response [2].
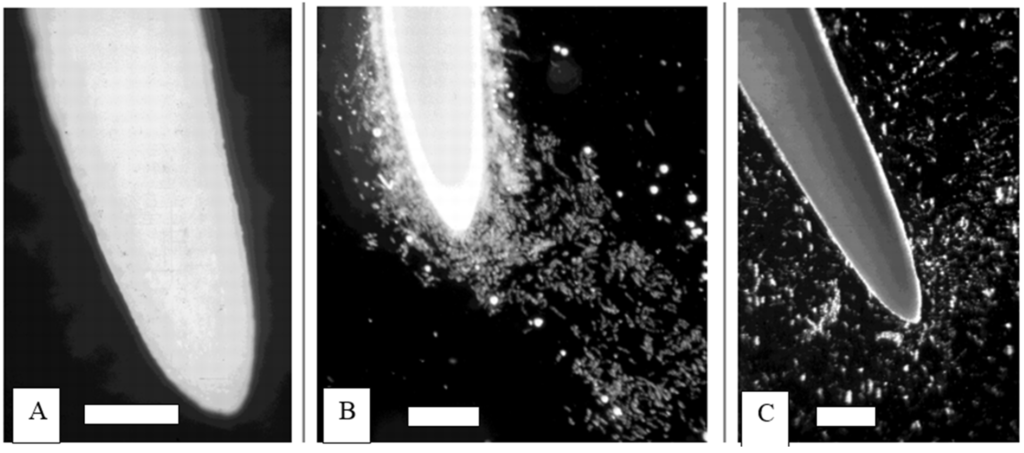
Figure 1.
Dynamics of border cell dispersal upon immersion into water. (A) When roots are maintained at >98% humidity, border cells remain tightly appressed to the surface and invisible; (B) Upon immersion of the root tip into water, the root cap mucilage absorbs water instantaneously, and cells begin to disperse within seconds; (C) Within minutes, all border cells disperse into suspension, leaving the root tip surface free of cells. Scale bars: 1 mm.
Direct tests revealed instead that protein profiles and gene expression patterns in the detached cells are markedly distinct even from progenitor root cap cells [3]. Therefore, the term “border cells” was introduced as a new alternative to “sloughed root cap” cells to emphasize that these cell populations comprise a cellular interface that does not function biochemically in the same manner as cells within the root cap [4,5]. Despite observations that border cells synthesize and export a slimy matrix that immobilizes diverse plant pathogens, the actual function of the cells remained obscure until parallels with newly described immune responses in animals were discovered [6].
2. Extracellular Traps in Animals and Plants
In 2004, a previously overlooked foundation of mammalian defense was reported for the first time: In response to stress signals, neutrophils within the blood system export a slimy matrix that immobilizes diverse pathogens [7]. These “neutrophil extracellular traps” or “NETs” are comprised of proteins including histone, actin, and enzymes involved in reactive oxygen species (ROS) pathways, together with extracellular DNA (exDNA) [8]. Pathogens such as Group A Streptococcus produce extracellular enzymes with DNase activity (exDNase) that facilitate release from NETs and allow systemic spread of the bacteria [9]. The importance of exDNase as a survival mechanism has been validated in vitro, as knockout mutations of the exDNases result in loss of pathogen virulence [10].
The discovery of NETs in animals finally provided insight into why plants invest so much energy in producing thousands of healthy cells destined to disperse from root tips into the soil: A parallel extracellular trapping process operates in plants [6]. In response to pathogens and other stress signals, viable border cells rapidly synthesize and export an extracellular complex comprised of DNA together with >100 proteins including histone, actin and ROS enzymes [11,12]. When root tips are treated with DNase I, resistance to pathogen invasion is abolished [6,12]. As in animal pathogens such as Group A Streptococcus, knockout mutations of exDNase in the bacterial plant pathogen, Ralstonia solanacaerum, result in reduced virulence and loss of ability of the pathogen to move systemically through the plant [13].
Like the defense pathway-inducing signals from pathogens, metals including lead, copper, mercury, silver and cadmium also activate ROS pathways in mammalian cells [14,15]. A recent survey of human neutrophils now has implicated NETs in the systemic localization patterns, or trapping, of metals within human blood [16]. Given the remarkable parallels between exDNA-based immune responses in animals and plants, this observation may help to explain a series of studies, summarized below, suggesting that root border cells also play a role in trapping and localization of metals.
3. Border Cell Trapping of Aluminum
Aluminum toxicity is a limiting factor in crop production in acid soils, which facilitate solubilization of the metal [17]. Genotypic variation in plant sensitivity has been well documented, but mechanisms for resistance remain under investigation [18,19]. Roots are an important target for Al-induced damage, and inhibition of root growth occurs rapidly in response to exposure of the root tip to aluminum [17,20]. The hypothesis that border cells play a role in avoidance of Al uptake was tested directly using roots of pea (Pisum sativum L.) and snapbean (Phaseolus vulgaris L.) from the Fabaceae family [21,22]. Seedling roots with and without border cells were immersed into liquid containing Al [22]. Even though border cells disperse from the root tip within minutes upon immersion into liquid (Figure 1), there was an obvious increase in Al staining within the root whose border cells were gone at the time of immersion (Figure 2B) compared with those whose border cells were present (Figure 2C).
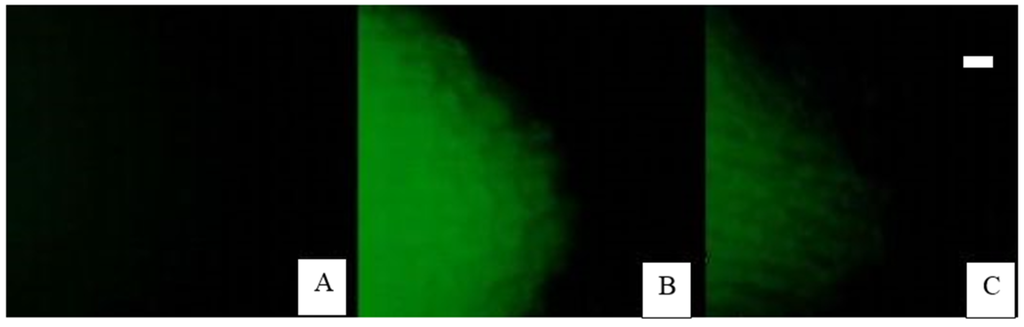
Figure 2.
Border cell inhibition of aluminum uptake into the root cap detected by lumogallion staining. (A) Control roots incubated for 30 min at pH 5.2, in the absence of Al reveal no fluorescence; (B) Intense staining occurs in root tips whose border cells were dispersed prior to immersion of the root into 200 μM Al for 30 min; (C) Reduced uptake of aluminum into root tips whose border cells were present on the root cap periphery at the time the roots were immersed into 200 μM Al for 30 min [22]. Scale bar: 30 microns.
Border cells from an Al-sensitive snapbean cultivar incubated with Al in a simple salt solution were killed more rapidly than cells from a resistant cultivar, suggesting that whole-plant tolerance mechanisms are expressed in the border cell populations [21]. Of particular interest was the finding that individual cells from the resistant cultivar produced larger mucilage layers (now called “extracellular traps”) [11] in response to Al than cells from the sensitive cultivar (Figure 3). The mechanisms underlying Al-border cell interactions remain to be defined. However, Al is known to complex with DNA, so the discovery that DNA is an integral component of border cell extracellular traps may yield new hypotheses to be explored [23].
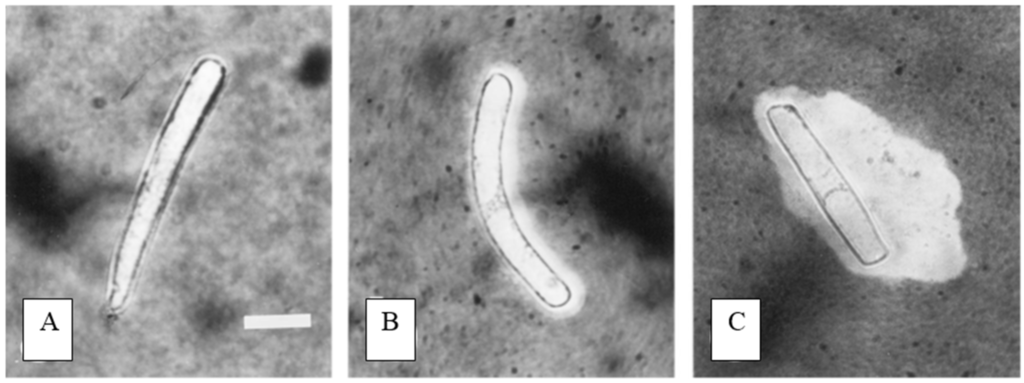
Figure 3.
Dosage dependent induction of extracellular trap formation in border cells in response to aluminum. Extracellular trap formation was visualized using India ink, which does not penetrate the trap. (A) Border cells from snapbean border cells in water have little or no visible extracellular trap. Within 1 h of immersion in 50 micromoles aluminum (B) or 100 micromoles (C), increased trap formation is evident. Trap dimensions at the higher level was significantly greater (p = 0.0001) than the lower level. [Figure reproduced with permission from reference 21]. Scale bar: 20 microns.
4. Border Cell Trapping of Other Soil Contaminants
Dynamic interactions in response to copper, cadmium, boron, lead, mercury, iron, and arsenic as well as aluminum have now been described for border cells of cereals, legumes, cotton, coyotillo and fern (Table 1) [21,22,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. Efforts to define underlying mechanisms are in early stages of discovery, but results suggest that signals controlling border cell production and trapping responses in the field may yield new approaches to plant protection [48,49,50,51].

Table 1.
Border cells and metals: publications from 2001–2015.
| Date | Metal | Plant | Reference |
|---|---|---|---|
| 2001 | aluminum | snapbean | [21] |
| 2003 | aluminum | pea | [22] |
| 2003 | aluminum | wheat | [24] |
| 2003 | copper | Silene | [25] |
| 2003 | cadmium | coyotillo | [26] |
| 2004 | aluminum | barley | [27] |
| 2005 | aluminum | barley | [28] |
| 2006 | aluminum | pea | [29] |
| 2007 | aluminum+boron | pea | [30] |
| 2008 | aluminum | cowpea | [31] |
| 2008 | iron | rice | [32] |
| 2008 | lead, mercury | mung bean | [33] |
| 2009 | aluminum | pea | [34] |
| 2011 | aluminum | rice | [35] |
| 2011 | aluminum | soybean | [36] |
| 2011 | copper, nickel, zinc | cowpea | [37] |
| 2011 | iron | rice | [38] |
| 2012 | iron | rice | [39] |
| 2012 | arsenic | cowpea | [40] |
| 2012 | iron, aluminum | rice | [41] |
| 2012 | aluminum | oats | [42] |
| 2012 | arsenic | fern | [43] |
| 2013 | boron, aluminum | pea | [44] |
| 2013 | aluminum | soybean | [45] |
| 2013 | copper | cotton | [46] |
| 2014 | cadmium | fern | [47] |
5. Border Cell Number vs. Arsenic Uptake into Edible Plants
Two studies with arsenic (Table 1), in cowpea (Vigna unguiculata) and fern (Pteris vittata) [40,43], are of particular interest in view of a recent in vivo study of arsenic taken from the environment into plants under diverse growth conditions [52]. A significant inverse correlation was found between number of border cells produced by the species of interest and uptake of arsenic into the plant (Figure 4). Thus, for example, members of the Brassica family do not produce populations of viable dispersed border cells, whereas legumes produce several thousand per day [53,54]. It will be of interest to explore the possibility that there is a direct relationship between the production and viability of border cells and the sensitivity of plants to toxins in the soil.
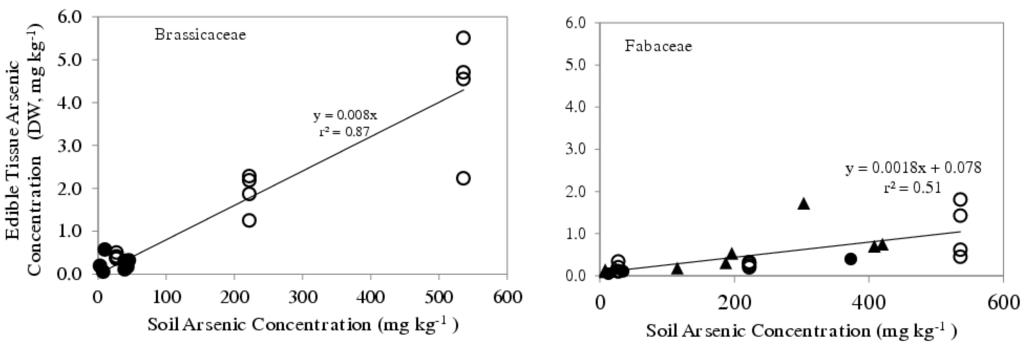
Figure 4.
Arsenic concentration in the edible portion of Brassicaceae (left, no border cell production) and Fabaceae (right, 3000–4000 border cells produced per root per day) as a function of soil arsenic concentration. Values were compiled from reference [52]. Open symbols (○) represent vegetables grown in the greenhouse, closed symbols (●) represent vegetables grown in home gardens, and the closed triangles (▲) represent values from the literature.
6. Rhizofiltration vs. Rhizoprotection
Multiple research studies have focused on phytotechnologies (detection, degradation, removal or contaminant of soil, groundwater, surface water, sediments, or air), but these studies have suffered from the inability to show consistent, predictable removal of biological and chemical contaminants [55,56]. However, emerging research on extracellular trapping by border cells of plant roots (Table 1), highlights the potential for utilization of plants in bioremediation of contaminated water and soil and may help to explain variability with divergent species. “Rhizofiltration” is a category of phytoremediation that focuses on using plant root systems to remove contaminants from soil and water [57]. Rhizofiltration has been researched as a remediation tool for nearly fifty years, but despite continued efforts, use of this approach has been hampered by unexplained variability in uptake of pathogens and metals by plants and lack of efficacy in removal of contaminants [58,59,60,61,62,63,64,65]. The discovery that border cells trap metals suggests that plants have mechanisms to prevent uptake into plant tissue, while at the same time sequestering contaminants. Because contaminant removal models rely on kinetic constants based on root uptake, this recent finding could easily account for lack of agreement between modeled and measured plant “uptake”.
Border cells naturally disperse into liquid and accumulate into a visible mass at the bottom of the vessel as new border cells are produced to replace the detached populations [1]. It will be of interest in future studies to test directly the amount of metals and other contaminants that are trapped by border cells in their role as “neutrophils” protecting the plant from danger [66,67], and to explore the use of this simple approach to remove hazardous chemicals from soil and water under diverse conditions. Considering the key role metals can play in the metabolism of microorganisms as well as plants and animals [16], such information may also yield new insights into potential relationships between metal trapping and microbial growth, development, and establishment of the rhizosphere “microbiome” [68,69]. Studies reporting variation in border cell production and properties among different species will be important tools for defining mechanisms and consequences of metal trapping [70,71,72,73,74,75].
7. Conclusions
Phytotechnologies may be used to prevent contaminant exposure and, in effect, be a tool for primary prevention in environmental public health [76]. Of particular importance will be studies to determine if the same mechanisms which have been implicated in metal trapping within roots also operate in border cell populations [77]. An improved understanding of border cell extracellular traps and their role in preventing toxin uptake may facilitate efforts to further utilize plants as a nondestructive approach to reduce environmental threats. Data thus far indicate the promise of phytotechnologies, and border cell extracellular traps may be the key to take this remediation strategy to the next level.
Acknowledgments
We thank the National Science Foundation for support of research on Extracellular DNA in Defense of Plant Cells.
Author Contributions
All authors made major contributions to concepts, context, and editorial input.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Knudson, L. Viability of detached root-cap cells. Am. J. Bot. 1919, 6, 309–310. [Google Scholar] [CrossRef]
- Hawes, M.C.; Wheeler, H. Factors affecting victorin-induced cell death: Temperature and plasmolysis. Physiol. Plant Pathol. 1982, 20, 137–144. [Google Scholar] [CrossRef]
- Brigham, L.A.; Woo, H.H.; Hawes, M.C. Differential expression of proteins and mRNAs from border cells and root tips of pea. Plant Physiol. 1995, 109, 457–463. [Google Scholar] [PubMed]
- Hawes, M.C.; Brigham, L.A.; Wen, F.; Woo, H.H.; Zhu, Y. Function of root border cells in plant health: Pioneers in the rhizosphere. Annu. Rev. Phytopathol. 1998, 36, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Watson, B.S.; Bedair, M.F.; Urbanczyk-Wochniak, E.; Huhman, D.V.; Yang, D.S.; Allen, S.N.; Li, W.; Tang, Y.; Sumner, L.W. Integrated metabolomics and transcriptomics reveal enhanced specialized metabolism in Medicago truncatula root border cells. Plant Physiol. 2015, 167, 1699–1716. [Google Scholar] [CrossRef] [PubMed]
- Hawes, M.C.; Curlango-Rivera, G.; Wen, F.; White, G.J.; VanEtten, H.D.; Xiong, Z. Extracellular DNA: The tip of root defenses? Plant Sci. 2011, 180, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrach, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayanopoulos, V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Nasser, W.; Bersa, S.B.; Olsen, R.J.; Dean, M.A.; Rice, K.A.; Long, S.W.; Kristinsson, K.G.; Gottfredsson, M.; Vuopio, J.; Raisanen, K.; et al. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3615 genome sequences. Proc. Natl. Acad. Sci. USA 2014, 111, E1768–E1776. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, J.T.; Simpson, A.J.; Aziz, R.K.; Liu, G.Y.; Kristian, S.A.; Kotb, M.; Feramisco, J.; Nizet, V. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 2006, 16, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; VanEtten, H.D.; Tsaprailis, G.; Hawes, M.C. Extracellular proteins in Pisum sativum L. root tip and border cell exudates. Plant Physiol. 2007, 143, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; White, G.A.; Xiong, Z.; VanEtten, H.D.; Hawes, M.C. Extracellular DNA is required for root tip resistance to fungal infection. Plant Physiol. 2009, 151, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Tuan, T.; Hawes, M.C.; Allen, C. Extracellular DNases contribute to virulence of Ralstonia solanacearum. Phytopathology 2013, 103, 147–148. [Google Scholar]
- Liz, R.; Simard, J.; Leonardi, L.; Girard, D. Silver nanoparticles rapidly induce atypical human neutrophil cell death by a process involving inflammatory caspases and reactive oxygen species and induce neutrophil extracellular traps release upon cell adhesion. Int. Immunopharmacol. 2015, 28, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Mushtakova, V.M.; Fomina, V.A.; Rogovin, V.V. Toxic effect of heavy metals on human blood neutrophils. Biol. Bulletin 2005, 32, 276–278. [Google Scholar] [CrossRef]
- Niermiec, J.J.; de Samber, B.; Garrevoet, J.; Vergucht, E.; Vekemans, B.; de Rycke, R.; Björn, E.; Sandblad, L.; Wellenreuther, G.; Falkenberg, G.; et al. Trace element landscape of resting and activated human neutrophils on the sub-micrometer level. Metallomics 2015, 7, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.; Delhaize, E. Adaptations to aluminium toxicity. In Plant Stress Physiology; CABI: Wallingford, UK, 2012; pp. 171–193. [Google Scholar]
- Klug, B.; Kirchner, T.W.; Horst, W.J. Differences in aluminum accumulation and resistance between genotypes of the genus Fagopyrum. Agronomy 2015, 5, 418–434. [Google Scholar] [CrossRef]
- Richard, C.; Munyinda, K.; Kinkese, T.; Osiru, D.S. Genotypic variation in seedling tolerance to aluminum toxicity in historical maize inbred lines of Zambia. Agronomy 2015, 5, 200–219. [Google Scholar] [CrossRef]
- Ryan, P.R.; Ditomaso, J.M.; Kochian, L.V. Aluminum toxicity in roots: An investigation of spatial sensitivity and the role of the root cap. J. Exp. Bot. 1993, 44, 437–446. [Google Scholar] [CrossRef]
- Miyasaka, S.; Hawes, M.C. Possible role of root border cells in detection and avoidance of aluminum toxicity. Plant Physiol. 2013, 125, 1978–1987. [Google Scholar] [CrossRef]
- Brigham, L.A.; Miyasaka, S.; Hawes, M.C. Avoidance of aluminum toxicity: Role of root border cells. Plant Nut. Dev. 2001, 92, 452–453. [Google Scholar]
- Dyrssen, D.; Haraldsson, C.; Nyberg, E.; Wedborg, M. Complexation of aluminum with DNA. J. Inorg. Biochem. 1987, 29, 67–75. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Ahn, S.; Matsumoto, H. Inhibition of growth and development of root border cells in wheat by Al. Physiol. Plant. 2003, 117, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Llugany, M.; Lombini, A.; Poschenrieder, C.; Dinelli, E.; Barcelo, J. Different mechanisms account for enhanced copper resistance in Silene armeria ecotypes from mine spoil and serpentine sites. Plant Soil 2003, 251, 55–63. [Google Scholar] [CrossRef]
- Zelko, I.; Lux, A. Effect of cadmium on Karwinskia humboldtiana roots. Biologia 2003, 59, 205–209. [Google Scholar]
- Pan, J.; Ye, D.; Wang, L.; Hua, J.; Zhao, G.; Pan, W.; Han, N.; Zhu, M. Root border cell development is a temperature-insensitive and Al-sensitive process in barley. Plant Cell Physiol. 2004, 45, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Tamas, L.; Budikova, S.; Huttova, J.; Mistrik, I.; Simonovicova, M. Aluminum-induced cell death of barley-root border cells is correlated with peroxidase- and oxalate oxidase-mediated hydrogen peroxide production. Plant Cell Rep. 2005, 24, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Feng, Y.M.; Goldbach, H.E. Mist culture for mass harvesting of root border cells: Aluminum effects. J. Plant Nutr. Soil Sci. 2006, 169, 670–674. [Google Scholar] [CrossRef]
- Yu, M.; Goldbach, H.E. Influence of boron on Al absorption and Ca release of root border cells of pea (Pisum sativum). In Advances in Plant and Animal Boron Nutrition; Springer: Dordrecht, The Netherlands, 2007; pp. 63–68. [Google Scholar]
- Chen, W.; Liu, P.; Xu, G.; Cai, M.; Yu, H.; Chen, M. Effects of Al3+ on the biological characteristics of cowpea root border cells. Acta Physiol. Plant. 2008, 30, 303–308. [Google Scholar] [CrossRef]
- Xing, C.; Zhu, M.; Cai, M.; Liu, P.; Xu, G.; Wu, S. Developmental characteristics and response to iron toxicity of root border cells in rice seedlings. J. Zhejiang Univ. Sci. B. 2008, 9, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhu, L.; Liu, X.Y.; Zhang, Y.; Zhao, N. Individual and joint effects of lead and mercury on the viability of root border cells in mung bean (Vigna radiata). In Proceedings of the International Symposium on Environmental Science and Technology, Shanghai, China, 2–5 June 2009; pp. 254–258.
- Yu, M.; Shen, R.; Xiao, H.; Xu, J.; Wang, H.; Wang, H.; Zeng, Q.; Bien, J. Boron alleviates aluminum toxicity in pea (Pisum sativum). Plant Soil 2009, 314, 87–98. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, S.; Xing, C.; Wang, F.; Wang, N.; Zhu, L. Developmental characteristics and aluminum resistance of root border cells in rice seedlings. Plant Sci. 2008, 180, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Wang, F.; Li, R.; Zhang, S.; Wang, W.; Xu, G. Response and tolerance of root border cells to aluminum toxicity in soybean seedlings. J. Inorg. Biochem. 2011, 105, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; Menzies, N.W.; de Jonge, M.D.; McKenna, B.D.; McKenna, B.A.; Donner, E.; Webb, R.I.; Paterson, D.J.; Howard, D.L.; Ryan, C.G.; et al. In situ distribution and speciation of toxic Cu, Ni and Zn in hydrated roots of cowpea. Plant Physiol. 2011, 156, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, G.H.; Liu, P.; Song, J.M.; Xu, G.D.; Cai, M.Z. Morphological and physiological responses of root tip cells to Fe2+ toxicity in rice. Acta Physiol. Plant. 2011, 33, 683–689. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.P.; Liu, P.; Song, J.M.; Xu, G.D.; Zheng, G.H. Effect of toxic Fe2+ levels on the biological characteristics of rice root border cells. Russ. J. Plant Physiol. 2012, 59, 766–771. [Google Scholar] [CrossRef]
- Kopittke, P.M.; de Jonge, M.D.; Menzies, N.W.; Wang, P.; Donner, E.; McKenna, B.A.; Paterson, D.; Howard, D.L.; Lombi, E. Examination of the distribution of arsenic in hydrated and fresh cowpea roots using two- and three-dimensional techniques. Plant Physiol. 2012, 159, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zhang, S.; Xing, C.; Wang, F.; Zhu, L.; Wang, N.; Liu, L. Interaction between iron plaque and root border cells ameliorates aluminum toxicity of Oryza sativa differing in aluminum tolerance. Plant Soil. 2012, 353, 155–167. [Google Scholar] [CrossRef]
- Radmer, L.; Tesfaye, M.; Somers, D.A.; Temple, S.J.; Vance, C.P.; Samac, D.A. Aluminum resistance mechanisms in oat (Avena sativa L.). Plant Soil 2012, 351, 121–134. [Google Scholar] [CrossRef]
- Forino, L.M.C.; Castiglione, M.R.; Bartoli, G.; Balestri, M.; Andreuci, A.; Tagliasacchi, A.M. Arsenic-induced morphogenic response in roots of arsenic hyperaccumulator fern Pteris vittata. J. Hazard. Mater. 2012, 235, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, M.; Wang, C. Influence of boron and aluminum on production and viability of root border cells of pea (Pisum sativum). Adv. Plant Anim. Boron Nut. 2013, 30, 69–74. [Google Scholar]
- Cai, M.; Wang, N.; Xing, C.; Wang, F.; Wu, K.; Du, X. Immobilization of aluminum with mucilage secreted by root cap and root border cells is related to aluminum resistance in Glycine max L. Environ. Sci. Pollut. Res. 2013, 20, 8924–8933. [Google Scholar] [CrossRef] [PubMed]
- Curlango-Rivera, G.; Huskey, D.A.; Mostafa, A.; Kessler, J.O.; Xiong, Z.; Hawes, M.C. Intraspecies variation in cotton border cell production: Rhizosphere microbiome implications. Am. J. Bot. 2013, 100, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Balestri, M.; Ceccarini, A.; Forino, L.M.C.; Zelko, I.; Martinka, M.; Lux, A.; Ruffini Castiglione, M. Cadmium uptake, localization and stress-induced morphogenic response in the fern Pteris vittata. Planta 2014, 239, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Curlango-Rivera, G.; Duclos, D.V.; Ebolo, J.J.; Hawes, M.C. Transient exposure of root tips to primary and secondary metabolites: Impact on root growth and production of border cells. Plant Soil. 2010, 306, 206–216. [Google Scholar] [CrossRef]
- Hawes, M.C.; Curlango-Rivera, G.; Xiong, Z.; Kessler, J.O. Roles of root border cells in plant defense and regulation of rhizosphere microbial populations by extracellular DNA “trapping”. Plant Soil 2012, 355, 1–16. [Google Scholar] [CrossRef]
- Odell, R.E.; Dumlao, M.R.; Samar, D.; Silk, W.K. Stage-dependent border cell and carbon flow from roots to rhizosphere. Am. J. Bot. 2008, 95, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Shen, R.; Liu, J.; Chen, R.; Xu, M.; Yang, Y.; Xiao, H.; Wang, H.; Wang, H.; Wang, C. The role of root border cells in aluminum resistance of pea (Pisum sativum) grown in mist culture. J. Plant Nutr. Soil Sci. 2009, 172, 528–534. [Google Scholar] [CrossRef]
- Ramirez-Andreotta, M.D.; Brusseau, M.L.; Artiola, J.F.; Maier, R.M. A greenhouse and field-based study to determine the accumulation of arsenic in common homegrown vegetables grown in mining-affected soils. Sci. Total Environ. 2013, 443, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Driouich, A.; Follet-Gueye, M.; Vicre-Gibouin, M.; Hawes, M.C. Root border cells and secretions as critical elements in plant host defense. Curr. Opin. Plant. Biol. 2013, 16, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hawes, M.C.; Pueppke, S.G. Sloughed peripheral root cap cells: Yield from different species and callus formation from single cells. Am. J. Bot. 1986, 73, 1466–1473. [Google Scholar] [CrossRef]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. Res. 2009, 16, 765–794. [Google Scholar] [CrossRef] [PubMed]
- Interstate Technology & Regulatory Council (ITRC). Phytotechnology Technical and Regulatory Guidance and Decision Trees; PHYTO-3. Interstate Technology & Regulatory Council, Phytotechnologies Team: Washington, DC, USA, 2009. Available online: http://www.itrcweb.org (accessed on 30 October 2015).
- Dushenkov, V.; Nanda, K.; Motto, H.; Raskin, I. Rhizofiltration: The use of plants to remove heavy metals from aqueous streams. Environ. Sci. Technol. 1995, 30, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Anawar, H.M.; Garcia-Sanchez, A.; Tari Kul Alam, M.; Rahman, M.M. Phytofiltration of water polluted with arsenic and heavy metals. Int. J. Environ. Pollut. 2008, 33, 292–312. [Google Scholar] [CrossRef]
- Arthur, E.L.; Rice, P.J.; Rice, P.J.; Anderson, T.A.; Baladie, S.M.; Henderson, K.D.; Coats, J.R. Phytoremediation—An overview. Crit. Rev. Plant. Sci. 2005, 24, 109–122. [Google Scholar] [CrossRef]
- Cheng, S.P. Heavy metals in plants and phytoremediation. Environ. Sci. Pollut. Res. 2003, 10, 335–340. [Google Scholar] [CrossRef]
- Cooney, C.M. Sunflowers remove radionuclides from water in ongoing phytoremediation field tests. Environ. Sci. Technol. 1996, 30, 194. [Google Scholar] [CrossRef]
- Meagher, R.B.; Heaton, A.C.P. Strategies for the engineered phytoremediation of toxic element pollution: Mercury and arsenic. J. Ind. Microbiol. Biotech. 2004, 32, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Page, V.; Feller, U. Heavy metals in crop plants: Transport and redistribution processes on the whole plant level. Agronomy 2015, 5, 447–463. [Google Scholar] [CrossRef]
- Raskin, I. Phytoremediation of metals: Using plants to remove pollutants from the environment. Curr. Opin. Biotechnol. 1997, 8, 221–226. [Google Scholar] [CrossRef]
- Shah, K.; Nongkynrih, J.M. Metal hyperaccumulation and bioremediation. Biol. Plant. 2007, 51, 618–634. [Google Scholar] [CrossRef]
- Cooper, P.C.; Palmer, L.J.; Chapple, I.L.C. Neutrophil extracellular traps as a new paradigm in innate immunity: Friend or foe? Periodontol. 2000 2013, 63, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Curlango-Rivera, G.; Flores-Lara, Y.; Cho, I.; Huskey, D.A.; Xiong, Z.; Hawes, M.C. Signals controlling extracellular trap formation in plant and animal immune responses. Clin. Microbiol. 2014, 3, 5–7. [Google Scholar]
- Haichar, F.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Biol. Biochem. 2015, 77, 69–80. [Google Scholar] [CrossRef]
- Hawes, M.C.; Brigham, L.A. Impact of root border cells on microbial populations in the rhizosphere. Adv. Plant Pathol. 1992, 8, 118–148. [Google Scholar]
- Durand, C.; Vicre-Gibouin, M.; Follet-Gueye, M.L.; Duponchel, L.; Moreau, M.; Lerouge, P.; Driouich, A. The organization pattern of root border-like cells of Arabidopsis is dependent on cell wall homogalacturonan. Plant Physiol. 2009, 150, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Driouich, A.; Durnad, C.; Cannesan, M.A.; Percoco, G.; Vicré-Gibouin, M. Border cells versus border-like cells: Are they alike? J. Exp. Bot. 2010, 61, 3827–3831. [Google Scholar] [CrossRef] [PubMed]
- Endo, I.; Tange, T.; Osawa, H. A cell-type-specific defect in border cell formation in the Acacia mangium root cap developing an extraordinary sheath of sloughed-off cells. Ann. Bot. 2011, 108, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Nguema-Ona, E.; Vicre-Gibouin, M.; Cannesan, M.; Driouich, A. Arabinogalactan proteins in root-microbe interactions. Trends Plant Sci. 2013, 18, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Cannesan, M.; Durand, C.; Burel, C.; Gangneux, C.; Lerouge, P.; Ishii, T.; Laval, K.; Follet-Gueye, M.L.; Driouich, A.; Vicré-Gibouin, M. Effect of arabinogalactan proteins from the root caps of pea and Brassica napus on Aphanomyces euteiches zoospore chemotaxis and germination. Plant Physiol. 2012, 159, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Cannesan, M.A.; Gangneux, C.; Lanoue, A.; Giron, D.; Laval, K.; Hawes, M.; Driouich, A.; Vicré-Gibouin, M. Association between border cell responses and localized root infection by pathogenic Aphanomyces euteiches. Ann. Bot. 2011, 108, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.F.; Burken, J.G.; Maier, R.M.; Newman, L.A.; Rock, S.; Schnoor, J.L.; Suk, W.A. Phytotechnologies—Preventing Exposures, Improving Public Health. Int. J. Phytoremediation 2013, 15, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Colzi, I.; Pignattelli, S.; Glorni, E.; Papini, A.; Connelli, C. Linking root traits to copper exclusion mechanisms in Silene paradoxa L. (Caryophyllaceae). Plant Soil 2015, 390, 1–15. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).