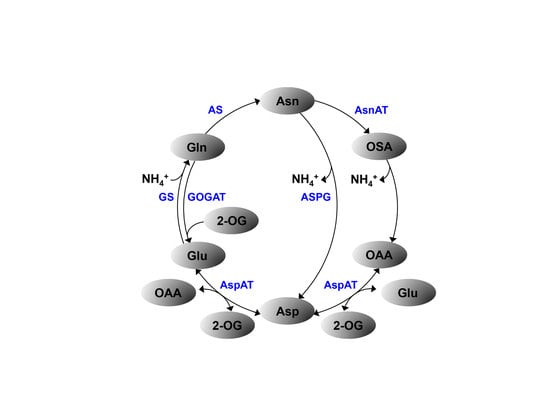
Impact of the Disruption of ASN3-Encoding Asparagine Synthetase on Arabidopsis Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Homozygous ASN3 T-DNA Insertion Lines
2.2. Plant Growth
2.3. Real-Time Quantitative RT-PCR Analysis
2.4. In Situ Hybridization
2.5. Western Blot Analysis
2.6. Chlorophylls and Ammonium Measurements
2.7. Quantitative Amino Acid PROFILING
2.8. 15N Labeling Analysis
3. Results
3.1. Characterization of asn3 Mutants
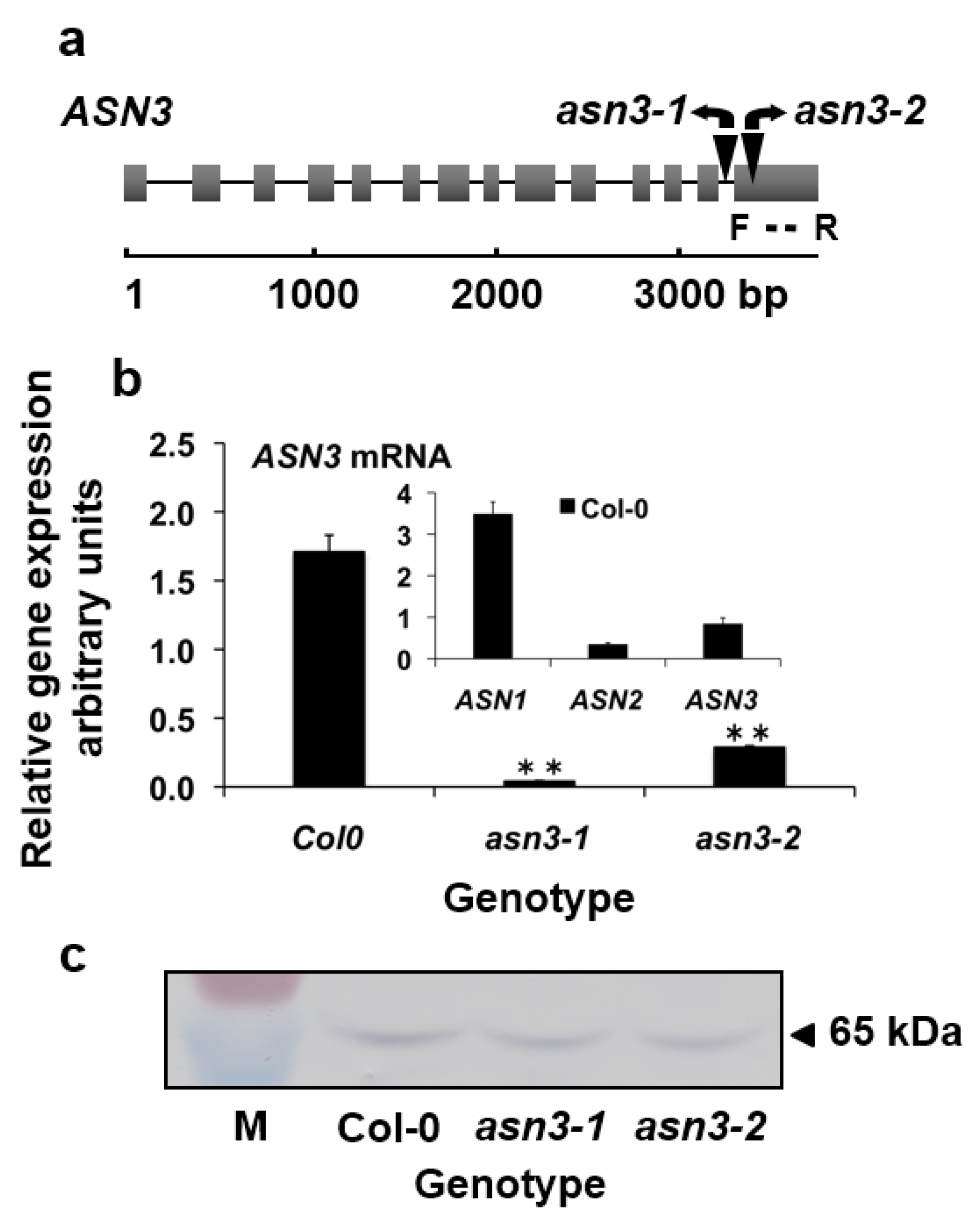
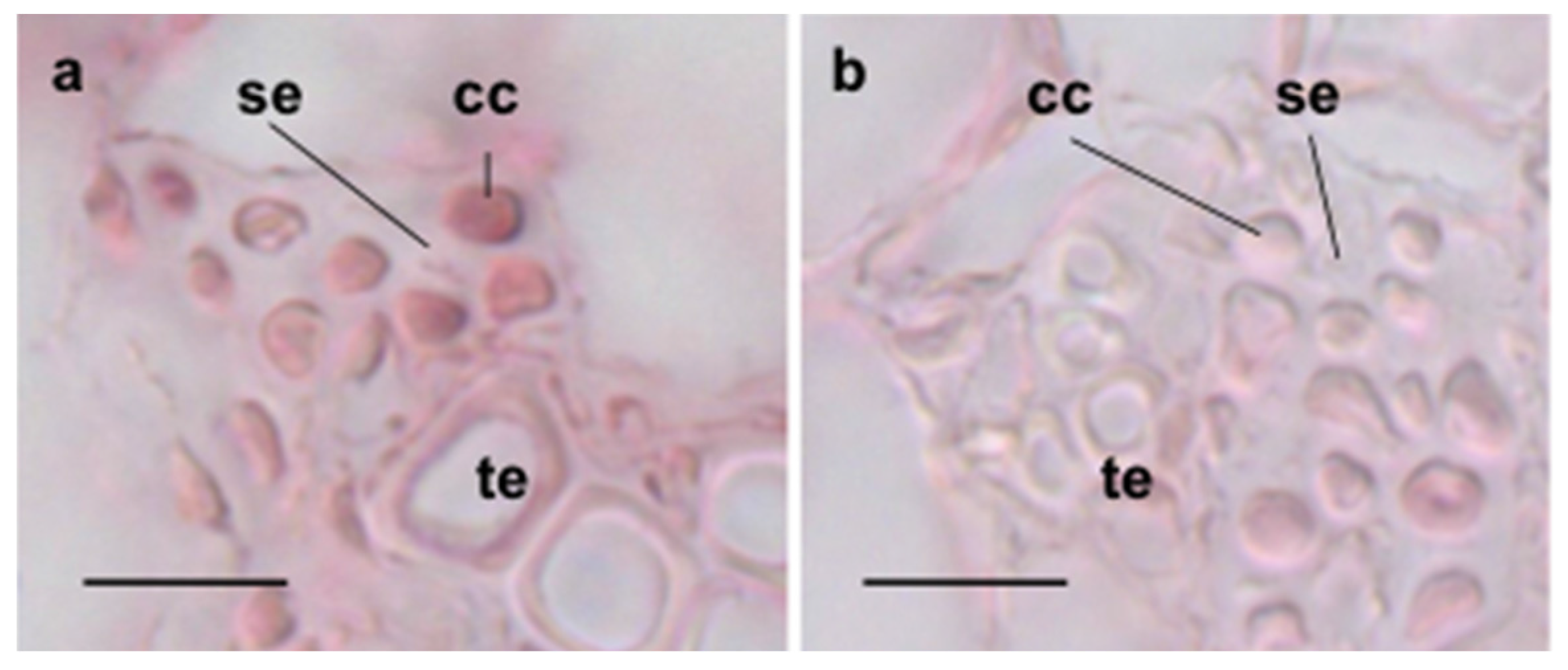
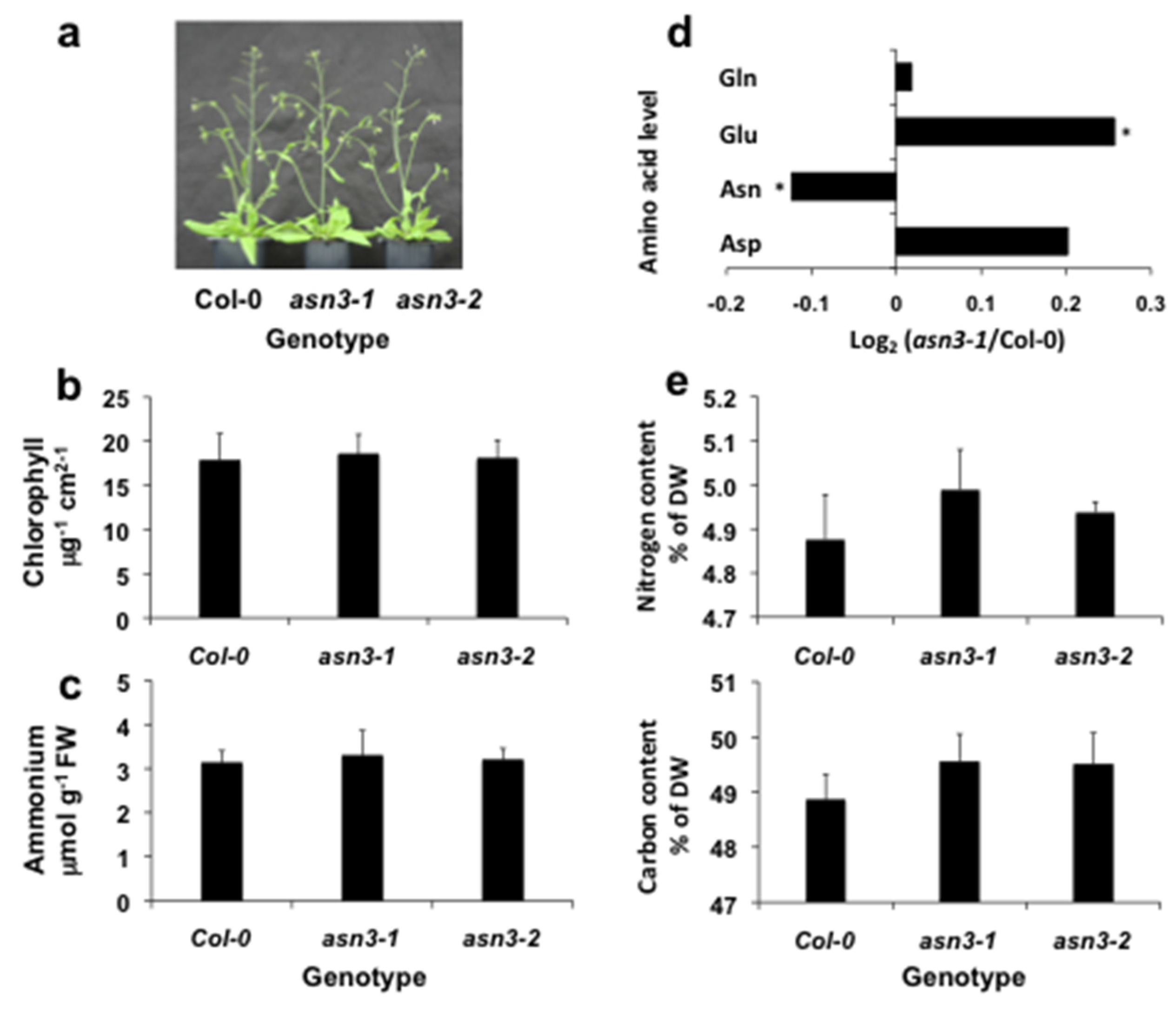
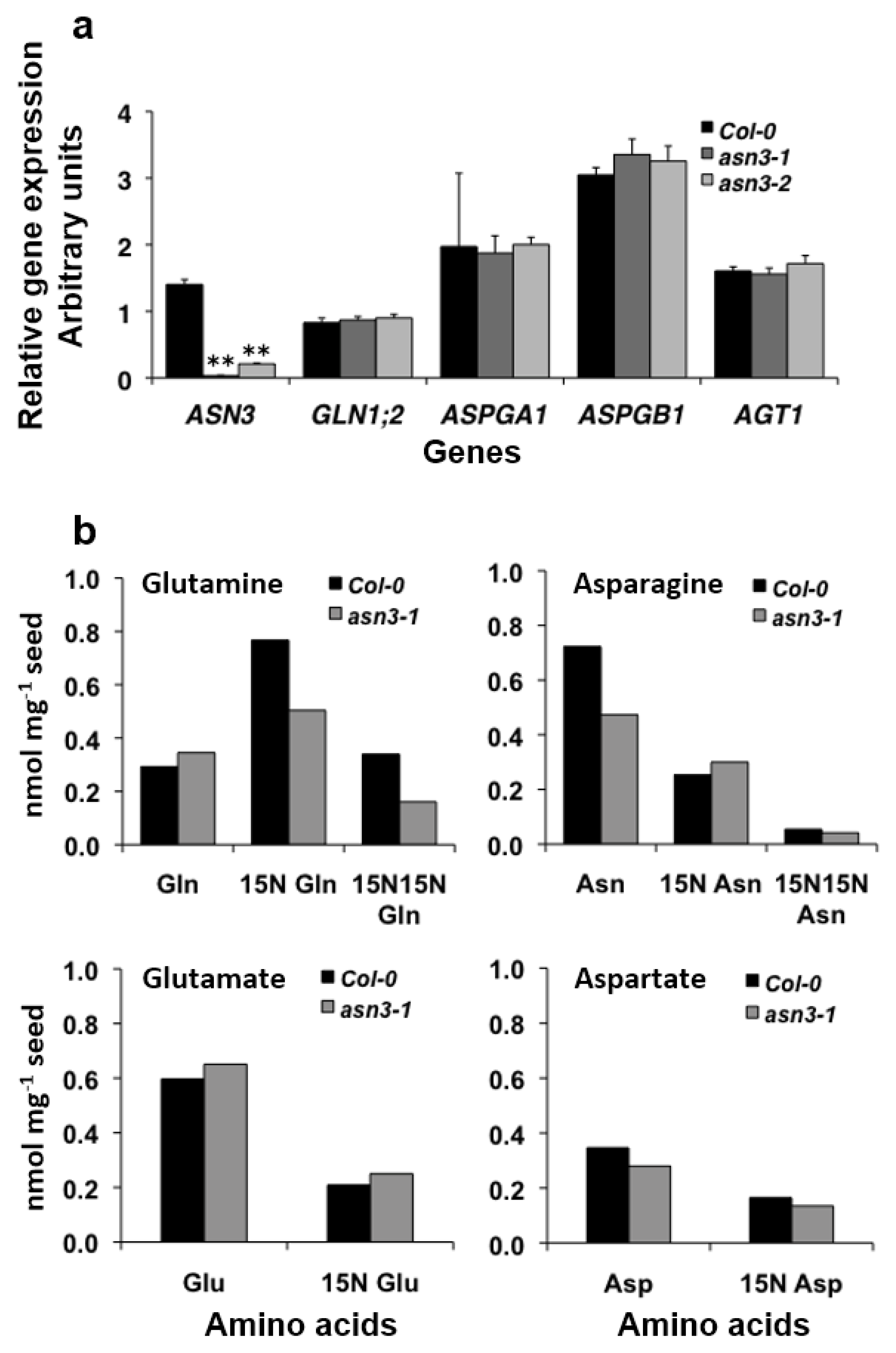
3.2. Cellular Expression of ASN3 mRNA and Phenotypic Analysis of asn3 Mutants
3.3. 15N Labeling of Amino Acids in Germinating asn3 Seeds

4. Discussion
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coruzzi, G.M. Primary N-assimilation into amino acids in Arabidopsis. In The Arabidopsis Book; Somerville, C., Meyerrowitz, E., Eds.; American Society of Plant Biologists: Rockville, MD, USA, 2003; pp. 1–17. [Google Scholar]
- Lea, P.J.; Sodeck, L.; Parry, M.A.J.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Gaufichon, L.; Reisdorf-Cren, M.; Rothstein, S.J.; Chardon, F.; Suzuki, A. Biological functions of asparagine synthetase in plants. Plant Sci. 2010, 179, 141–153. [Google Scholar] [CrossRef]
- Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar]
- Herrera-Rodriguez, M.B.; Maldonado, J.M.; Perez-Vicente, R. Light and metabolic regulation of HAS1, HAS1.1 and HAS2, three asparagine synthetase genes in Helianthus annuus. Plant Physiol. Biochem. 2004, 42, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Bläsing, O.E.; Gibon, Y.; Günther, M.; Höhne, M.; Morcuende, R.; Osuna, D.; Thimm, O.; Björn, U.; Scheobe, W.-R.; Stitt, M. Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 2005, 17, 3257–3281. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.-M.; Wong, P.; Chan, H.-K.; Yam, K.-M.; Cheng, L.; Chow, C.-M.; Coruzzi, G.M. Overexpression of the ASN1 gene enhances nitrogen status in seeds of Arabidopsis. Plant Physiol. 2003, 132, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Gaufichon, L.; Masclaux-Daubresse, C.; Tcherkez, G.; Reisedorf-Cren, M.; Sakakibara, Y.; Hase, T.; Clément, G.; Avice, J.-C.; Grandjean, O.; Marmagne, A.; et al. Arabidopsis thaliana ASN2 encoding asparagine synthetase is involved in the control of nitrogen assimilation and export during vegetative growth. Plant Cell Environ. 2013, 36, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.-K.; Chan, H.-K.; Coruzzi, G.M.; Lam, H.-M. Correlation of ASN2 gene expression with ammonium metabolisms in Arabidopsis. Plant Physiol. 2004, 134, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Coïc, Y.; Lessaint, C. Comment assurer une bonne nutrition en eau et en ions minéreaux en holticulture? Holticulture Fr. 1971, 8, 11–14. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris L. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolic profiling in Arabidopsis. Method Mol. Biol. 2006, 323, 439–447. [Google Scholar]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system from microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [PubMed]
- Boyes, D.C.; Zayed, A.M.; Ascenzi, R.; McCaskill, A.J.; Hoffman, N.E.; Davis, K.R. Growth stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell 2001, 13, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Hayashi, M.; Goto, F.; Sato, S.; Soga, T.; Nishioka, T.; Tomita, M.; Kawai-Yamada, M.; Uchimiya, H. Evaluation of metabolic alteration in transgenic rice overexpressing dihydroflavonol-4-reductase. Ann. Bot. 2006, 98, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, K.; Job, C.; Groot, S.P.C.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomics of Arabidopsis seed germination. A comparative study of wild-type and gibberellin-deficient seeds. Plant Physiol. 2007, 129, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Ireland, R.J.; Lea, P.J. The enzymes of glutamine, glutamate, asparagine and aspartate metabolism. In Plant Amino Acids; Singh, B.K., Ed.; Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland; Hong Kong, China, 1999; pp. 49–109. [Google Scholar]
- Schmid, M.; Davidson, T.S.; Hnz, S.; Pap, U.J.; Demar, M.; Vingron, M.; Schölkopf, B.; Weigel, D.; Lohmann, J.U. A gene expression map of Arabidopsis thaliana development. Nat. Genet. 2005, 37, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Marsolais, F. Identification and characterization of omega-amidase as an enzyme metabolically linked to asparagine transamination in Arabidopsis. Phytochemisty 2014, 99, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.; Kameka, A.; Pajak, A.; Bruneau, L.; Beyaert, R.; Hernández-Sebastià, C.; Marsolais, F. Arabidopsis mutants lacking asparaginases develop normally but exhibit enhanced root inhibition by exogenous asparagine. Amino Acids 2012, 42, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Joy, K.W.; Prabha, C. The role of transamination in the synthesis of homoserine in peas. Plant Physiol. 1986, 82, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Liepman, A.H.; Olsen, L.J. Peroxisomal alanine:glyoxylate aminotransferase (AGT1) is a photorespiratory enzyme with multiple substrates in Arabidopsis thaliana. Plant J. 2001, 25, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lee, J.; Pandurangan, S.; Clark, M.; Pajak, A.; Marsolas, F. Characterization of Arabidopsis serine: Glyoxylate aminotransferase, AGT1, as an asparagine aminotransferase. Phytochemistry 2013, 85, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.; Telmer, P.G.; Marsolais, F. Role of asparaginase variable loop at the carboxyl terminal of the alpha subunit in the determination of substrate preference in plants. Planta 2012, 235, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaufichon, L.; Marmagne, A.; Yoneyama, T.; Hase, T.; Clément, G.; Trassaert, M.; Xu, X.; Shakibaei, M.; Najihi, A.; Suzuki, A. Impact of the Disruption of ASN3-Encoding Asparagine Synthetase on Arabidopsis Development. Agronomy 2016, 6, 12. https://doi.org/10.3390/agronomy6010012
Gaufichon L, Marmagne A, Yoneyama T, Hase T, Clément G, Trassaert M, Xu X, Shakibaei M, Najihi A, Suzuki A. Impact of the Disruption of ASN3-Encoding Asparagine Synthetase on Arabidopsis Development. Agronomy. 2016; 6(1):12. https://doi.org/10.3390/agronomy6010012
Chicago/Turabian StyleGaufichon, Laure, Anne Marmagne, Tadakatsu Yoneyama, Toshiharu Hase, Gilles Clément, Marion Trassaert, Xiaole Xu, Maryam Shakibaei, Amina Najihi, and Akira Suzuki. 2016. "Impact of the Disruption of ASN3-Encoding Asparagine Synthetase on Arabidopsis Development" Agronomy 6, no. 1: 12. https://doi.org/10.3390/agronomy6010012
APA StyleGaufichon, L., Marmagne, A., Yoneyama, T., Hase, T., Clément, G., Trassaert, M., Xu, X., Shakibaei, M., Najihi, A., & Suzuki, A. (2016). Impact of the Disruption of ASN3-Encoding Asparagine Synthetase on Arabidopsis Development. Agronomy, 6(1), 12. https://doi.org/10.3390/agronomy6010012