Application of Two Bioenergy Byproducts with Contrasting Carbon Availability to a Prairie Soil: Three-Year Crop Response and Changes in Soil Biological and Chemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
| Property | Soil Depth (cm) † | ||
|---|---|---|---|
| 0–15 | 15–30 | 30–60 | |
| NO3−-N (mg·kg−1) | 3.9 ± 0.9 | 3.2 ± 0.8 | 3.1 ± 0.4 |
| NH4+-N (mg·kg−1) | 2.6 ± 0.1 | 2.6 ± 0.2 | 3.0 ± 0.1 |
| Avail. P (mg·kg−1) | 10.6 ± 1.4 | 8.4 ± 1.3 | 4.9 ± 0.8 |
| Avail. K (mg·kg−1) | 348 ± 36 | 279 ± 32 | 239 ± 22 |
| OC (%) | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.2 |
| pH | 7.9 ± 0.1 | 7.9 ± 0.0 | 8.0 ± 0.1 |
| EC (dS·m−1) | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| Precipitation | Mean Temperature | |||||||
|---|---|---|---|---|---|---|---|---|
| Month | 2009 | 2010 | 2011 | 30-Years Avg. | 2009 | 2010 | 2011 | 30-Years Avg. |
| Mm | °C | |||||||
| April | 10.2 | 34.1 | 4.5 | 22.9 | 2.6 | 6.2 | 3.2 | 4.6 |
| May | 19.6 | 124.4 | 31.2 | 51.8 | 9.3 | 9.0 | 10.3 | 11.6 |
| June | 47.7 | 75.3 | 96.1 | 67.9 | 14.9 | 16.4 | 15.0 | 16.2 |
| July | 80.9 | 55.7 | 52.5 | 63.6 | 16.3 | 17.8 | 18.8 | 18.5 |
| August | 48.5 | 43.6 | 14.7 | 44.1 | 15.8 | 16.8 | 18.1 | 17.9 |
| September | 14.6 | 55.4 | 5.2 | 31.9 | 16.6 | 10.9 | 14.4 | 11.7 |
2.2. Amendments Procurement, Preparation and Application
| Property | Value |
|---|---|
| C (%) | 71.4 |
| N (%) | 2.0 |
| P (%) | 2.5 |
| K (%) | 1.5 |
| S (%) | 0.1 |
| Na (%) | 0.8 |
| Ca (%) | 4.6 |
| Mg (%) | 0.2 |
| Cu (mg·kg−1) | 11.5 |
| Fe (%) | 0.4 |
| Mn (mg·kg−1) | 109 |
| Zn (mg·kg−1) | 80 |
| Surface area (m2·g−1) | 13.4 |
2.3. Experimental Design
2.4. Plant and Soil Sample Collection and Analysis
2.5. Statistical Analysis
3. Results
3.1. Crop Yield and Nutrient Uptake
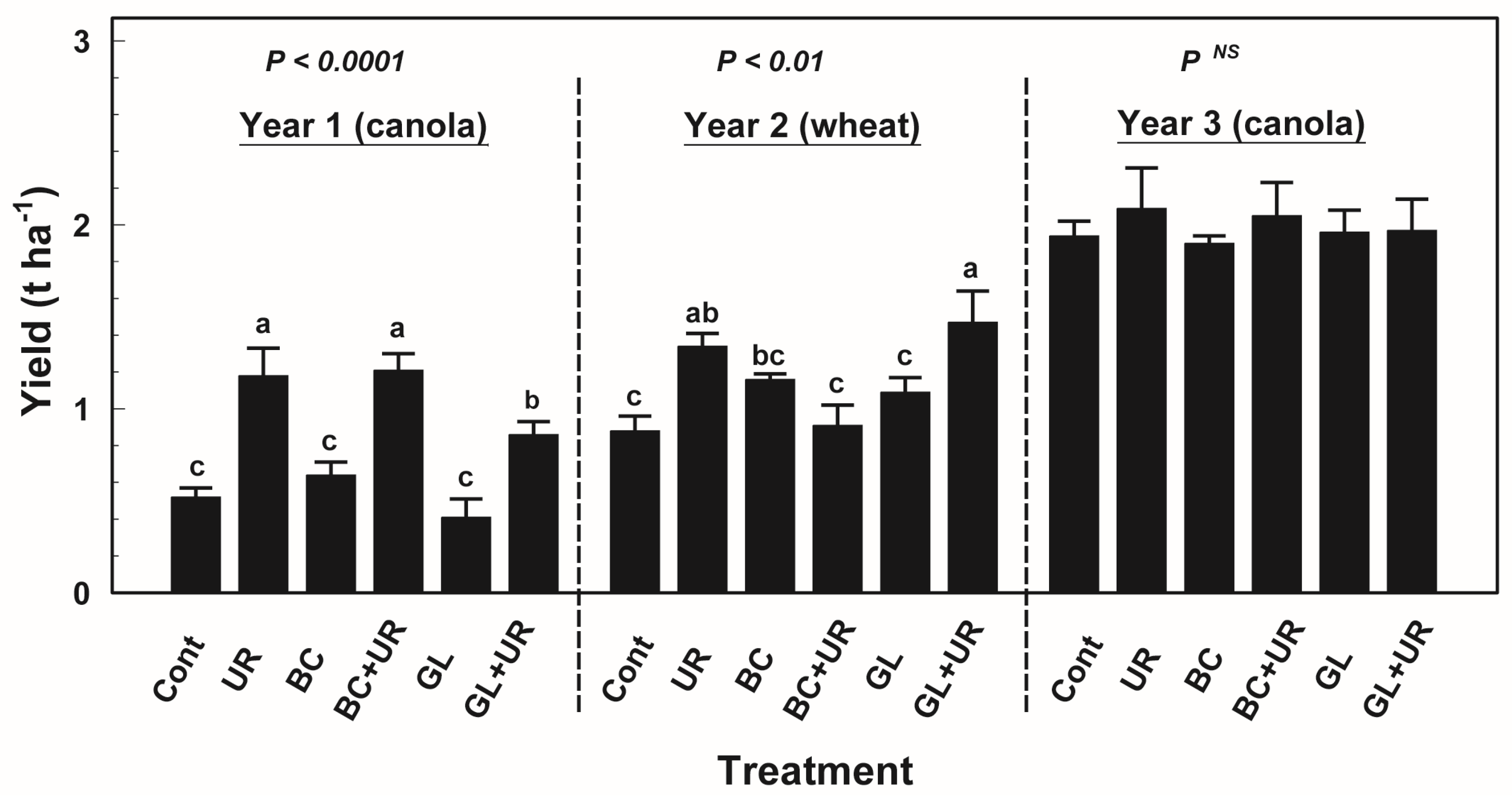
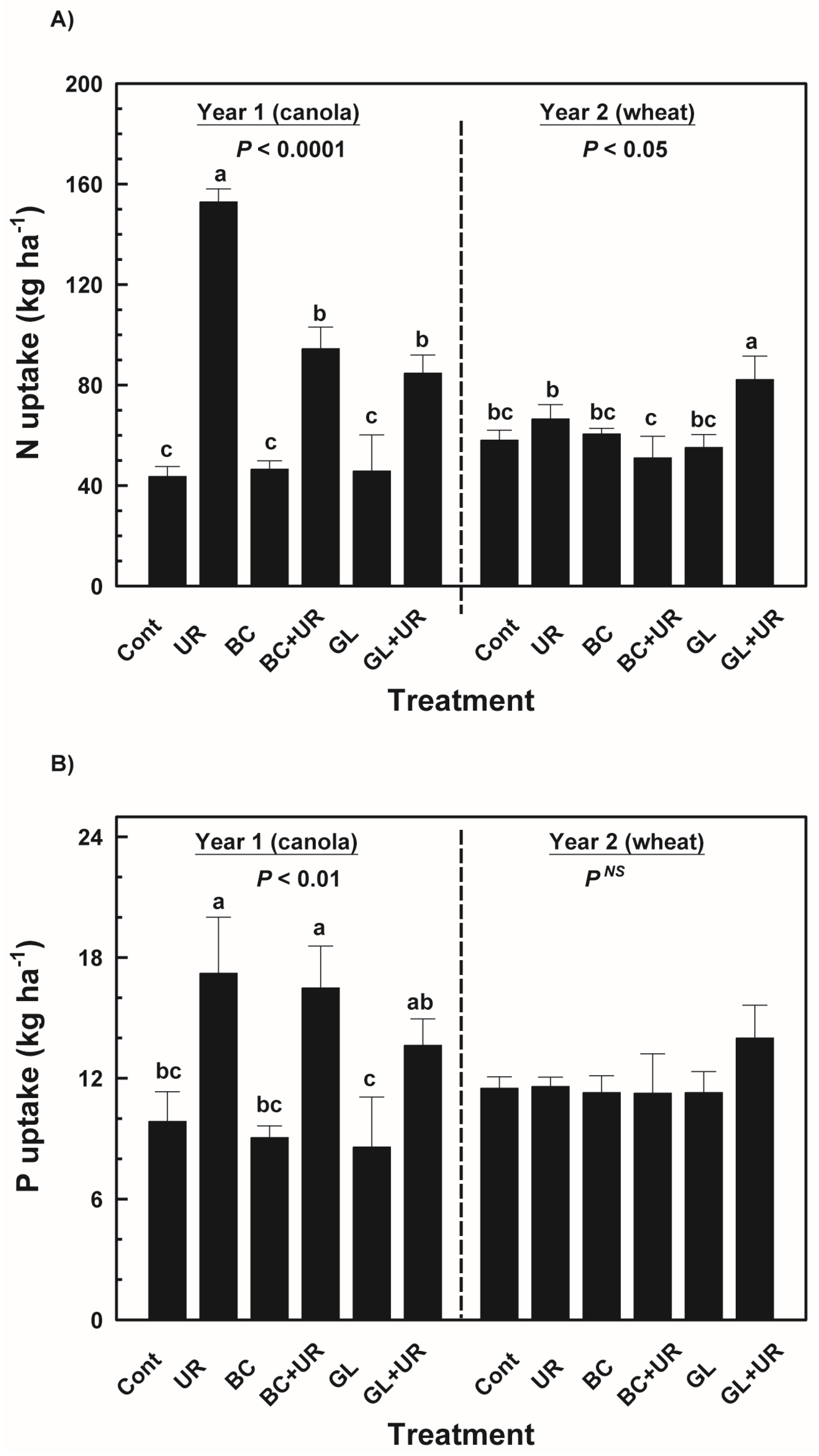
3.2. Dehydrogenase Activity and Microbial Biomass
| Treatment | Dehydrogenase Activity | Microbial Biomass C | Microbial Biomass N | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fall 2009 | Spring 2010 | Fall 2010 | Fall 2009 | Spring 2010 | Fall 2010 | Fall 2009 | Spring 2010 | Fall 2010 | |
| μg TPF·g −1 | μg·g −1 | ||||||||
| Control | 187 ± 8 b | 212 ± 10 | 299 ± 37 | 244 ± 34 a | 250 ± 63 | 205 ± 20 | 71 ± 13 a | 51 ± 19 | 30.5 ± 3.8 |
| Urea | 203 ± 19 b | 235 ± 21 | 289 ± 22 | 191 ± 21 a | 259 ± 54 | 211 ± 7 | 38 ± 6 bc | 57 ± 23 | 30.7 ± 0.9 |
| BC | 171 ± 15 b | 211 ± 10 | 279 ± 22 | 220 ± 57 a | 187 ± 79 | 207 ± 4 | 35 ± 9 bc | 53 ± 16 | 29.7 ± 1.0 |
| BC + UR | 199 ± 16 b | 199 ± 8 | 348 ± 52 | 109 ± 13 b | 220 ± 9 | 235 ± 33 | 24 ± 8 c | 40 ± 3 | 34.2 ± 5.4 |
| GL | 247 ± 20 a | 206 ± 9 | 324 ± 32 | 217 ± 14 a | 202 ± 30 | 223 ± 18 | 52 ± 7 ab | 41 ± 14 | 32.9 ± 3.2 |
| GL + UR | 252 ± 18 a | 238 ± 4 | 257 ± 13 | 191 ± 18 a | 287 ± 71 | 199 ± 8 | 48 ± 4 b | 69 ± 25 | 28.5 ± 2.6 |
| Treatment | 0.020 | NS | NS | 0.040 | NS | NS | 0.020 | NS | NS |
3.3. Selected Soil Chemical Properties
| 0–15 cm | ||||||
| Treatment | Fall 2009 | Spring 2010 | Fall 2010 | |||
| NH4+-N | NO3−-N | NH4+-N | NO3−-N | NH4+-N | NO3−-N | |
| μg·g−1 | ||||||
| Control | 6.4 ± 1.5 | 3.4 ± 0.6 | 3.0 ± 0.3 | 5.0 ± 0.8 | 6.1 ± 1.1 | 4.8 ± 0.5 |
| Urea | 6.3 ± 0.8 | 2.9 ± 0.8 | 3.6 ± 0.6 | 5.9 ± 0.5 | 7.3 ± 1.5 | 3.7 ± 0.3 |
| BC | 7.6 ± 1.4 | 2.9 ± 0.7 | 3.3 ± 0.3 | 4.0 ± 1.2 | 6.0 ± 0.9 | 4.1 ± 0.2 |
| BC + UR | 6.6 ± 1.2 | 2.0 ± 0.3 | 3.3 ± 0.4 | 5.0 ± 1.2 | 5.5 ± 1.1 | 4.2 ± 0.9 |
| GL | 6.3 ± 1.2 | 3.3 ± 0.7 | 3.3 ± 0.4 | 5.9 ± 0.6 | 6.0 ± 1.1 | 3.9 ± 0.2 |
| GL + UR | 6.4 ± 1.0 | 3.3 ± 0.9 | 3.3 ± 0.1 | 7.3 ± 0.5 | 6.3 ± 1.5 | 3.6 ± 0.5 |
| ANOVA | ||||||
| Treatment | NS | NS | NS | NS | NS | NS |
| 15–30 cm | ||||||
| Control | 7.0 ± 1.1 | 2.6 ± 0.6 | 3.3 ± 0.4 | 4.4 ± 1.1 | 7.1 ± 1.2 | 2.2 ± 0.2 |
| Urea | 7.2 ± 1.2 | 1.7 ± 0.3 | 3.4 ± 0.6 | 4.4 ± 1.5 | 8.4 ± 1.6 | 2.0 ± 0.2 |
| BC | 9.7 ± 1.1 | 2.5 ± 0.8 | 3.4 ± 0.3 | 2.5 ± 0.7 | 6.2 ± 0.8 | 2.4 ± 0.3 |
| BC + UR | 5.3 ± 1.5 | 2.3 ± 0.5 | 3.9 ± 0.4 | 4.4 ± 1.5 | 6.0 ± 0.9 | 3.0 ± 0.8 |
| GL | 8.4 ± 0.2 | 2.9 ± 0.6 | 6.5 ± 3.6 | 5.4 ± 0.2 | 7.1 ± 1.1 | 2.7 ± 0.4 |
| GL + UR | 7.8 ± 1.0 | 2.1 ± 0.7 | 3.3 ± 0.3 | 4.4 ± 0.7 | 6.6 ± 1.4 | 2.1 ± 0.1 |
| ANOVA | ||||||
| Treatment | NS | NS | NS | NS | NS | NS |
| Treatment | Fall 2009 | Fall 2010 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Extractable P | Extractable K | Organic C | pH | EC | Total N | Total P | Extractable P | Extractable K | Organic C | |
| μg·g−1 | % | dS·m−1 | μg·g−1 | % | ||||||
| Control | 7.2 ± 2.7 | 291 ± 24 | 1.05 ± 0.10 | 7.6 ± 0.2 | 0.29 ± 0.13 a | 1070 ± 19 | 441 ± 8 | 13.7 ± 1.7 | 323 ± 26 | 1.16 ± 0.05 |
| Urea | 7.5 ± 1.5 | 306 ± 10 | 1.02 ± 0.03 | 7.3 ± 0.2 | 0.15 ± 0.02 b | 1066 ± 38 | 440 ± 98 | 14.4 ± 2.5 | 329 ± 19 | 1.23 ± 0.03 |
| GL | 5.7 ± 1.0 | 343 ± 24 | 1.06 ± 0.06 | 7.5 ± 0.1 | 0.13 ± 0.01 b | 1087 ± 27 | 448 ± 8 | 12.1 ± 1.5 | 364 ± 28 | 1.19 ± 0.04 |
| GL + UR | 6.5 ± 1.5 | 330 ± 20 | 1.06 ± 0.09 | 7.3 ± 0.3 | 0.17 ± 0.03 b | 996 ± 10 | 418 ± 7 | 12.7 ± 1.5 | 336 ± 20 | 1.15 ± 0.05 |
| BC | 9.0 ± 2.0 | 325 ± 34 | 1.09 ± 0.07 | 7.4 ± 0.1 | 0.13 ± 0.01 b | 1092 ± 26 | 443 ± 6 | 14.5 ± 1.7 | 354 ± 27 | 1.25 ± 0.04 |
| BC + UR | 5.1 ± 0.8 | 357 ± 11 | 1.08 ± 0.01 | 7.5 ± 0.2 | 0.14 ± 0.02 b | 1105 ± 52 | 450 ± 13 | 11.6 ± 0.7 | 399 ± 23 | 1.27 ± 0.08 |
| ANOVA | ||||||||||
| Treatment | NS | NS | NS | NS | 0.10 | NS | NS | NS | NS | NS |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cayuela, M.L.; Oenema, O.; Kuikma, P.J.; Bakker, R.R.; van Groenigen, J.W. Bioenergy by-products as soil amendments? Implications for carbon sequestration and greenhouse gas emissions. GCB Bioenergy 2010, 2, 201–213. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2015; pp. 1–14. [Google Scholar]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Redmile-Gordon, M.A.; Armenise, E.; Hirsch, P.R.; Brookes, P.C. Biodiesel co-product (BCP) decreases soil nitrogen (N) losses to groundwater. Water Air Soil Pollut. 2014, 225, 1831. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, S.; Verheijen, F.; van der velde, M.; Bastos, A. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Bruun, E.W.; Arthur, E.; de Jonge, L.W.; Moldrup, P.; Hauggaard-Nielsen, H.; Elsgaard, L. Effect of biochar on aerobic processes, enzyme activity, and crop yields in two sandy loam soils. Biol. Fertil. Soils 2014, 50, 1087–1097. [Google Scholar] [CrossRef]
- Ameloot, N; Sleutel, S.; Case, S.D.C.; Alberti, G.; McNamara, N.P.; Zavalloni, C.; Vervisch, B.; Vedove, G.; Neve, S.D. C mineralization and microbial activity in four biochar field experiments several years after incorporation. Soil Biol. Biochem. 2014, 78, 195–203. [Google Scholar]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Burton, R.; Zhou, Y. Glycerol (byproduct of biodiesel production) as a source for fuels and chemicals—Mini review. Open Fuels Energy Sci. J. 2010, 3, 17–22. [Google Scholar] [CrossRef]
- Groesbeck, C.N.; McKinney, L.J.; DeRouchey, J.M.; Tokach, M.D.; Goodband, R.D.; Dritz, S.S.; Nelssen, J.L.; Duttlinger, A.W.; Fahrenholz, A.C.; Behnke, K.C. Effect of crude glycerol on pellet mill production and nursery pig growth performance. J. Anim. Sci. 2008, 86, 2228–2236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lammers, P.J.; Kerr, B.J.; Weber, T.E.; Dozier, W.A.; Kidd, M.T.; Bregendahl, K.; Honeyman, M.S. Digestible and metabolizable energy of crude glycerol for growing pigs. J. Anim. Sci. 2007, 86, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Kolesárová, N.; Hutňan, M.; Špalková, V.; Lazor, M. Biodiesel by-products as potential substrates for biogas production. In Proceedings of the 37th International Conference of Slovak Society of Chemical Engineering, Tatranské Matliare, Slovakia, 24–28 May 2010; Markoš, J., Ed.; pp. 1126–1139.
- Fountoulakis, M.S.; Manios, T. Enhanced methane and hydrogen production from municipal solid waste and agro-industrial by-products co-digested with crude glycerol. Bioresour. Technol. 2009, 100, 3043–3047. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; van Wambeke, M.; Carballa, M.; Verstraete, W. Improvement of the anaerobic treatment of potato processing wastewater in a UASB reactor by co-digestion with glycerol. Biotechnol. Lett. 2008, 30, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.; Schoenau, J.; Urton, R. Effect of soil amendment with thin stillage and glycerol on plant growth and soil properties. J. Plant Nutr. 2011, 34, 2206–2221. [Google Scholar] [CrossRef]
- Alotaibi, K.D.; Schoenau, J.J. Enzymatic activity and microbial biomass in soil amended with biofuel production byproducts. Appl. Soil Ecol. 2011, 48, 227–235. [Google Scholar] [CrossRef]
- Alotaibi, K.D.; Schoenau, J.J. Greenhouse gas emissions and nutrient supply rates in soil amended with biouel production by-products. Biol. Fertil. Soils 2013, 49, 129–141. [Google Scholar] [CrossRef]
- Qian, P.; Schoenau, J.J.; King, T.; Fatteicher, C. Effect of soil amendment with alfalfa powders and distillers grains on nutrition and growth of canoal. J. Plant Nutr. 2011, 34, 1403–1417. [Google Scholar] [CrossRef]
- Thomas, R.L.; Sheard, R.W.; Moyer, J.R. Comparision of conventional and automated procedures for nitrogen, phosphorus and potassium analysis of plant material using a single digestion. Agron. J. 1967, 59, 240–243. [Google Scholar] [CrossRef]
- Technicon Industrial Systems. Ammonium in Water and Seawater; Industrial Method 154–71 W/B Technicon Industrial Systems: Tarrytown, NY, USA, 1978. [Google Scholar]
- Keeney, D.R.; Nelson, D. Nitrogen-Inorganic Forms, in Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Qian, P.; Schoenau, J.J.; Karamanos, R.E. Simultaneous extraction of available phosphorus and potassium with a new test: A modification of Kelowna extraction. Commun. Soil Sci. Plant Anal. 1994, 25, 627–636. [Google Scholar] [CrossRef]
- Wang, D.; Anderson, D.W. Direct measurement of organic carbon content in soils by the Leco CR-12 carbon analyzer. Commun. Soil Sci. Plant Anal. 1998, 29, 15–21. [Google Scholar] [CrossRef]
- Casida, L.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Serra-Wittling, C.; Houot, S.; Barriuso, E. Soil enzymatic response to addition of municipal solid-waste compost. Biol. Fertil. Soils 1995, 20, 226–236. [Google Scholar] [CrossRef]
- Voroney, R.; Brookes, P.; Beyaert, R. Soil microbial biomass C, N, P, and S. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 637–651. [Google Scholar]
- Wu, J.; Joergensen, R.; Pommerening, B.; Chaussod, R.; Brookes, P. Measurement of soil microbial biomass C by fumigation-extraction-an automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Joergensen, R.G. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the KEC value. Soil Biol. Biochem. 1996, 28, 25–31. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Mueller, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the KEN value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- Stumborg, C.; Schoenau, J.J. Evaluating phosphorus loading from repeated manure applications to two Saskatchwan soils. Can. J. Soil Sci. 2008, 88, 377–387. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil. 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Speir, R.A.; Harris, K.; Das, K.; Lee, R.D.; Morris, L.A.; Fisher, D.S. Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron. J. 2010, 102, 623–633. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. Biochar adsorbed ammonia is bioavailable. Plant Soil. 2012, 350, 57–69. [Google Scholar] [CrossRef]
- Tabatabai, M. Soil enzymes. Part 2, microbiological and biochemical properties. In Methods of Soil Analysis; Weaver, R.W., Angle, J.S., Bottomley, P.S., Eds.; SSSA Inc.: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Moeskops, B.; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; de Neve, S. Soil microbial communities and activities under intensive organic and conventional vegetable farming in West Java, Indonesia. Appl. Soil Ecol. 2010, 45, 112–120. [Google Scholar] [CrossRef]
- Ameloot, N.; de Neve, S.; Jegajeevagan, K.; Yildiz, G.; Buchan, D.; Funkuin, Y.N.; Prins, W.; Bouckaert, L.; Sleutel, S. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. Biochem. 2012, 57, 401–410. [Google Scholar] [CrossRef]
- Smith, J.L.; Collins, H.P.; Bailey, V.L. The effect of young biochar on soil respiration. Soil Biol. Biochem. 2010, 42, 2345–2347. [Google Scholar] [CrossRef]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Wu, F.; Jia, Z.; Wang, S.; Chang, S.X.; Startsev, A. Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil. Biol. Fertil. Soils 2013, 49, 555–565. [Google Scholar] [CrossRef]
- Moore, J.; Klose, S.; Tabatabai, M. Soil microbial biomass carbon and nitrogen as affected by cropping systems. Biol. Fertil. Soils 2000, 31, 200–210. [Google Scholar] [CrossRef]
- Powlson, D.; Prookes, P.; Christensen, B.T. Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol. Biochem. 1987, 19, 159–164. [Google Scholar] [CrossRef]
- Kolb, S.E.; Fermanich, K.J.; Dornbush, M.E. Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci. Soc. Am. J. 2009, 73, 1173–1181. [Google Scholar] [CrossRef]
- Dempster, D.; Gleeson, D.; Solaiman, Z.; Jones, D.; Murphy, D. Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 2012, 354, 311–324. [Google Scholar] [CrossRef]
- Brookes, P.C.; Cayuela, M.L.; Contin, M.; de Nobili, M.; Kemmitt, S.J.; Mondini, C. The mineralization of fresh and humified soil organic matter by the soil microbial biomass. Waste Manag. 2008, 28, 716–722. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, K.D.; Schoenau, J.J. Application of Two Bioenergy Byproducts with Contrasting Carbon Availability to a Prairie Soil: Three-Year Crop Response and Changes in Soil Biological and Chemical Properties. Agronomy 2016, 6, 13. https://doi.org/10.3390/agronomy6010013
Alotaibi KD, Schoenau JJ. Application of Two Bioenergy Byproducts with Contrasting Carbon Availability to a Prairie Soil: Three-Year Crop Response and Changes in Soil Biological and Chemical Properties. Agronomy. 2016; 6(1):13. https://doi.org/10.3390/agronomy6010013
Chicago/Turabian StyleAlotaibi, Khaled D., and Jeff J. Schoenau. 2016. "Application of Two Bioenergy Byproducts with Contrasting Carbon Availability to a Prairie Soil: Three-Year Crop Response and Changes in Soil Biological and Chemical Properties" Agronomy 6, no. 1: 13. https://doi.org/10.3390/agronomy6010013
APA StyleAlotaibi, K. D., & Schoenau, J. J. (2016). Application of Two Bioenergy Byproducts with Contrasting Carbon Availability to a Prairie Soil: Three-Year Crop Response and Changes in Soil Biological and Chemical Properties. Agronomy, 6(1), 13. https://doi.org/10.3390/agronomy6010013





