1. Introduction
Soil is the foundation of the Earth’s ecosystems and is critical for sustaining agricultural production and ensuring food security. However, with the rapid development of industrialization and intensive agriculture, soil heavy metal (HM) pollution has become increasingly severe [
1]. Globally, approximately 14% to 17% of farmlands have toxic metal concentrations exceeding agricultural thresholds [
2]. HMs such as cadmium (Cd), lead (Pb), and mercury (Hg) are highly toxic, persistent, and non-degradable [
3], severely disrupting soil microbial community structure [
4] and physicochemical properties [
5], as well as reducing soil fertility. More critically, HMs easily transfer through the “soil-crop” system into the food chain—for example, rice’s high Cd enrichment makes it the primary dietary source of Cd for humans [
6], inducing various health risks including cardiovascular diseases [
7], neurotoxicity [
8], and cancer [
9]. Therefore, effectively controlling the bioavailability of HMs in farmland soils and blocking their migration to crops is a major and urgent issue in agricultural environmental science.
Selenium (Se) is an essential trace element for humans, with important biological functions such as antioxidant activity [
10], anti-cancer effects [
11], and cardiovascular protection [
12]. Globally, the Se content in soils ranges from 0.01 to 2.0 mg/kg [
13]. Soils with a Se content below 0.125 mg/kg are considered Se-deficient [
14].Widespread Se deficiency globally increases the risk of diseases like Keshan disease [
15] and type II diabetes [
16]. Numerous studies have shown that agronomic biofortification measures (e.g., foliar Se spraying, soil Se fertilization) not only enhance crop Se content but also significantly reduce crop uptake and accumulation of HMs [
17]. The realization of this “remediation–nutrition” dual effect highly depends on the rhizosphere—a dynamic microecological interface composed of plant roots and the closely associated surrounding soil [
18]. However, Se may also naturally coexist with HMs in soils and exert toxic effects. For instance, in onion rhizosphere soils, the coexistence of Se and Cd has been confirmed to induce significant cytogenotoxicity in root tip meristems [
19]. The complex physicochemical environment (pH, redox potential (Eh)) [
20] and microbial activity [
21] in the rhizosphere directly determine the interaction between Se and HMs. Thus, whether Se can effectively inhibit plant uptake of HMs largely depends on a series of chain reactions it triggers in the rhizosphere.
Currently, reviews on Se-mediated alleviation of crop HM stress have mostly focused on crop damage mechanisms and individual rhizosphere processes (e.g., chemical precipitation, microbial regulation), with a lack of systematic integrative analysis of the intricate processes in rhizosphere soils. Against this backdrop, the present review constructs a comprehensive multi-dimensional regulatory network for Se-mediated HM stress alleviation, which comprises three interconnected core components: source passivation (geochemical immobilization), rhizosphere interception (microbial and root barriers), and intracellular sequestration (gene regulation and chelation). To conduct this review, literature was retrieved from the Web of Science database using the keywords Se, HM, rhizosphere, microorganism, and bioavailability. Relevant experimental studies, reviews, and meta-analyses published from 2015 to 2025 were synthesized, and the retrieved literature was further screened based on the depth of mechanistic elucidation and relevance to the research theme. Focusing on the rhizosphere microdomain as the core research focus and integrating key factors including Se speciation, soil types, and HM species, this review finally proposes precise HM remediation strategies applicable for field-scale applications.
2. Geochemical Immobilization of HMs by Se
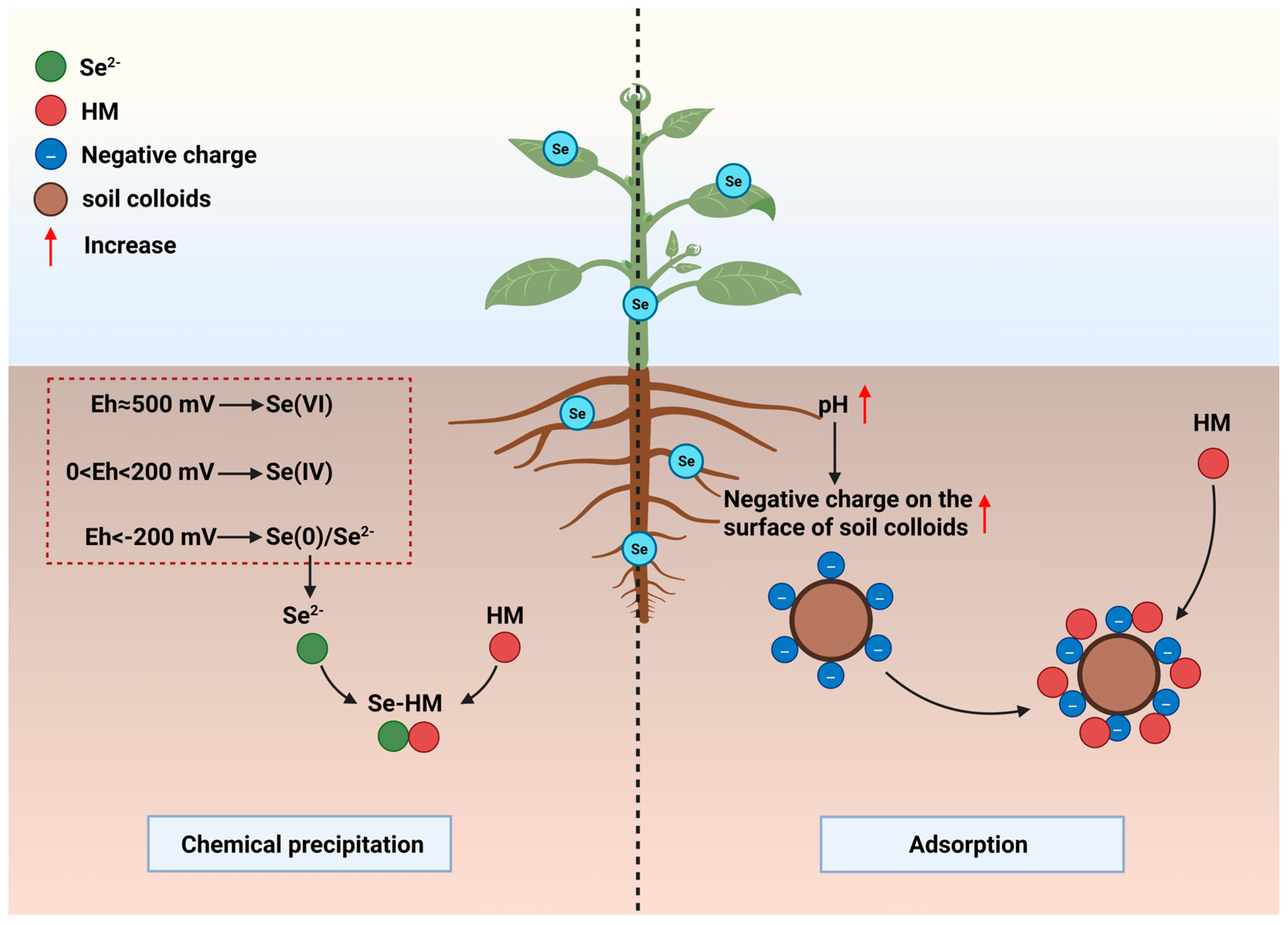
The rhizosphere is the first interaction interface between soil HMs and plant roots. Source interception before HMs come into contact with roots is the most direct and efficient defense strategy. Se can trigger geochemical reactions by regulating the rhizospheric physicochemical environment (Eh, pH), thereby achieving HM precipitation and adsorption. This process constitutes the “first line of defense” in the Se-mediated HM regulatory network, laying the foundation for subsequent microbial and root-related defense processes (
Figure 1).
2.1. General Dynamics of Se in Soil
The chemical speciation transformation of Se in soil and its interactions with soil colloids and other ions directly determine its efficiency in immobilizing HM. Therefore, clarifying the overall kinetic characteristics of these processes can provide a critical theoretical basis for the geochemical immobilization mechanism.
Se in soil primarily exists in four chemical forms: selenate (Se(VI)), selenite (Se(IV)), elemental Se (Se(0)), and selenide (Se
2−). Their environmental behaviors differ significantly: among them, Se(VI) is the most soluble and mobile; Se(IV) is soluble but readily strongly adsorbed by soil components such as iron/aluminum oxides, thus its mobility is considerably reduced; Se(0) is chemically stable and water-insoluble, making it largely immobile in the environment; whereas the reduced form, Se
2−, is relatively reactive and tends to combine with HM ions to form insoluble coprecipitates, leading to its immobilization [
22].
Soil colloids (including clay minerals, metal oxides, and soil organic matter) are the core carriers regulating the Se adsorption–desorption processes. Clay minerals can adsorb Se oxyanions through electrostatic interactions [
23]; amorphous iron/aluminum oxides can form a strong affinity for Se oxyanions via their positive surface charges and high-density exchangeable surface ligand functional groups [
24]; soil organic matter binds Se through direct complexation and covalent bonding, and can also form stable soil organic matter–metal oxide–Se ternary complexes with HMs. These ternary complexes can create anaerobic microzones, leading to the reduction of Se(VI) and Se(IV) to insoluble Se(0) or Se
2− [
25,
26]. Notably, Se(VI) is predominantly adsorbed via weak electrostatic adsorption, whereas Se(IV) tends to undergo strong adsorption through ligand exchange [
27,
28]. Se desorption is influenced by pH: an increase in pH enhances the negative charge on colloid surfaces, resulting in electrostatic repulsion with Se oxyanions [
29].
Se oxyanions in soil often compete with other anions for adsorption sites, thereby affecting the bioavailability of Se. Phosphates, which are similar to Se oxyanions in chemical properties, exhibit the strongest competitiveness for ligand exchange sites and can significantly reduce Se adsorption [
30]. Sulfates mainly compete for electrostatic adsorption sites; in alkaline soils, they can attenuate Se adsorption and enhance its bioavailability [
31].
In summary, the dynamic interactions among Se speciation transformation, soil colloid chemistry, and competing anions determine the bioavailability of Se in soil solution and consequently, the efficiency and pathways of Se -mediated HM immobilization, which will be discussed in subsequent chapters.
2.2. Effect of Eh on Chemical Precipitation of Se and HMs
Chemical coprecipitation between Se and HMs is the most fundamental and long-lasting mechanism for Se-mediated HM immobilization, and this process is strictly constrained by the rhizospheric Eh. As a core factor regulating Se speciation transformation, Eh directly determines whether Se can be converted into forms that form precipitates with HMs. Meanwhile, it interacts with soil waterlogging conditions and microbial activities to jointly affect the stability of the precipitates.
Under high Eh conditions (≈500 mV), Se mainly exists as highly mobile Se(VI). As Eh decreases (0–200 mV), strongly adsorbed Se(IV) becomes dominant [
32]. In strongly reducing environments (Eh < −200 mV, e.g., waterlogged conditions), high-valence Se is reduced to insoluble Se(0) and ultimately to Se
2− [
33]. When Se exists in the Se
2− form in soil, it rapidly reacts with HM ions to form insoluble metal selenide particles, thereby reducing the bioavailability of HMs [
34]. For example, Mal J et al. found that in sludge systems with low Eh, Se(IV) was reduced to Se
2− and reacted with Pb(II) to form insoluble PbSe precipitates. However, decreasing Eh may also affect the chemical precipitation of anionic HMs such as arsenic (As) [
35]. Wan et al. found that selenite (0.5 mg/kg) decreased rice grain As by 27.5% under aerobic conditions, but increased it by 43.7% under flooded conditions [
36]. The anaerobic environment (low Eh) induced by flooding first promotes the reduction of As(V) to the more toxic As(III), and leads to the reduction of iron–manganese oxides, thereby releasing the adsorbed As and increasing its mobility [
37]. Meanwhile, under low Eh conditions, Se(IV) is reduced to insoluble Se(0) or Se
2− precipitates, which reduces Se bioavailability and thus significantly weakens the competitive effect of Se against As [
38]. Therefore, when using Se application to inhibit HM uptake by crops, especially in the case of combined HM contamination (e.g., Cd–As contamination), the soil flooding regime should be adjusted according to the specific HM to ensure the optimal remediation efficacy.
Eh not only directly regulates the chemical precipitation of HMs by Se but also exerts a distinct selection pressure on microbial communities via the rhizosphere redox microenvironment. Low Eh conditions selectively enrich functional microorganisms with both Se-reducing capacity and anaerobic metabolic characteristics, which can further promote the conversion of Se to Se
2− through their own metabolism to form precipitates with HMs [
35] In contrast, the oxidizing environment with high Eh is more conducive to the proliferation of Se-oxidizing bacteria, which convert Se(0) or organic Se into highly reactive Se(IV); this not only replenishes the rhizosphere available Se pool but also provides a material basis for subsequent microbe-mediated HM chelation [
39]. This achieves the synergistic coupling of geochemical processes and microbial functions, with the in-depth details discussed in subsequent sections.
In addition to soil Eh, the bioavailable molar ratio of Se to the HM in the soil is another critical factor affecting the formation of insoluble metal selenides. Studies have found that when the bioavailable molar ratio of Se to Cd exceeds 0.7, bioavailable Se can be reduced to Se
2−, followed by the formation of insoluble CdSe, which significantly reduces the Cd content in crops [
40]. Conversely, Cd may be more efficiently absorbed by roots in the form of CdSeO
3 and CdSeO
4 [
40]. Wang et al. also observed that an effective molar ratio of 1 between Se and Hg in soil leads to the formation of insoluble HgSe [
41].
It is noteworthy that the insoluble metal selenides formed in suitable reductive environments can remain stable over long term. However, when Eh rises to oxidizing conditions (a typical example being the drainage and sunning of paddy soils), these metal selenides are gradually oxidized and eventually generate soluble Se(IV) and Se(VI), which causes the dissolution of selenide precipitates [
42]. Therefore, maintaining a reductive environment with low Eh is the key to ensuring the long-term stability of metal selenide precipitates.
2.3. Effect of Rhizosphere pH on HM Adsorption by Soil Colloids
In addition to the chemical precipitation of Se and HM directly regulated by Eh, pH can also regulate the adsorption of Se and HMs by soil colloids. Soil pH changes directly affect the solubility of HMs in soil. Se application can effectively regulate rhizosphere soil pH, and variations in soil pH directly influence the surface charge properties of soil colloids (e.g., clay minerals, organic matter), thereby altering the adsorption and desorption behaviors of HM ions. Studies have shown that an increase in pH generates hydroxide ions (OH
−), which enhance the negative charge content on the surface of soil colloids—this reduces the bioavailability of HM cations such as Cd, Pb, Mn, and Hg, but increases the mobility of oxyanions like As, Sb, and Se [
37].
Thus, increasing soil pH can enhance the effect of Se on inhibiting cationic HM uptake. For example, Wan et al. found that elevated pH reduced the adsorption of Se(IV) by soil minerals by increasing the negative charge on their surfaces, thereby raising the effective concentration of Se in soil solution and ultimately enhancing the immobilization efficiency of Cd by Se [
43]. Research on rice also clearly demonstrated that Se(IV) application significantly increased rhizosphere soil pH; this pH elevation directly reduced the mobility of Cd in soil and ultimately decreased Cd accumulation in plants [
20]. This pH-regulating effect has important practical implications—for instance, combining Se with traditional soil amendments that increase pH (e.g., lime) can further immobilize Cd in soil, thereby strengthening the inhibition of Cd uptake by rice [
44]. But the increased mobility of As and Sb induced by elevated pH cannot be ignored.
It should be pointed out that the formation of metal selenides and the adsorption of HMs by soil colloids have been primarily verified under laboratory-controlled conditions or in specific soil types. Their long-term stability and potential risks in diverse, dynamically changing field environments (especially those with fluctuating Eh and pH values) still require systematic verification at the field scale.
3. Rhizosphere Microbe-Mediated Immobilization of HM by Se
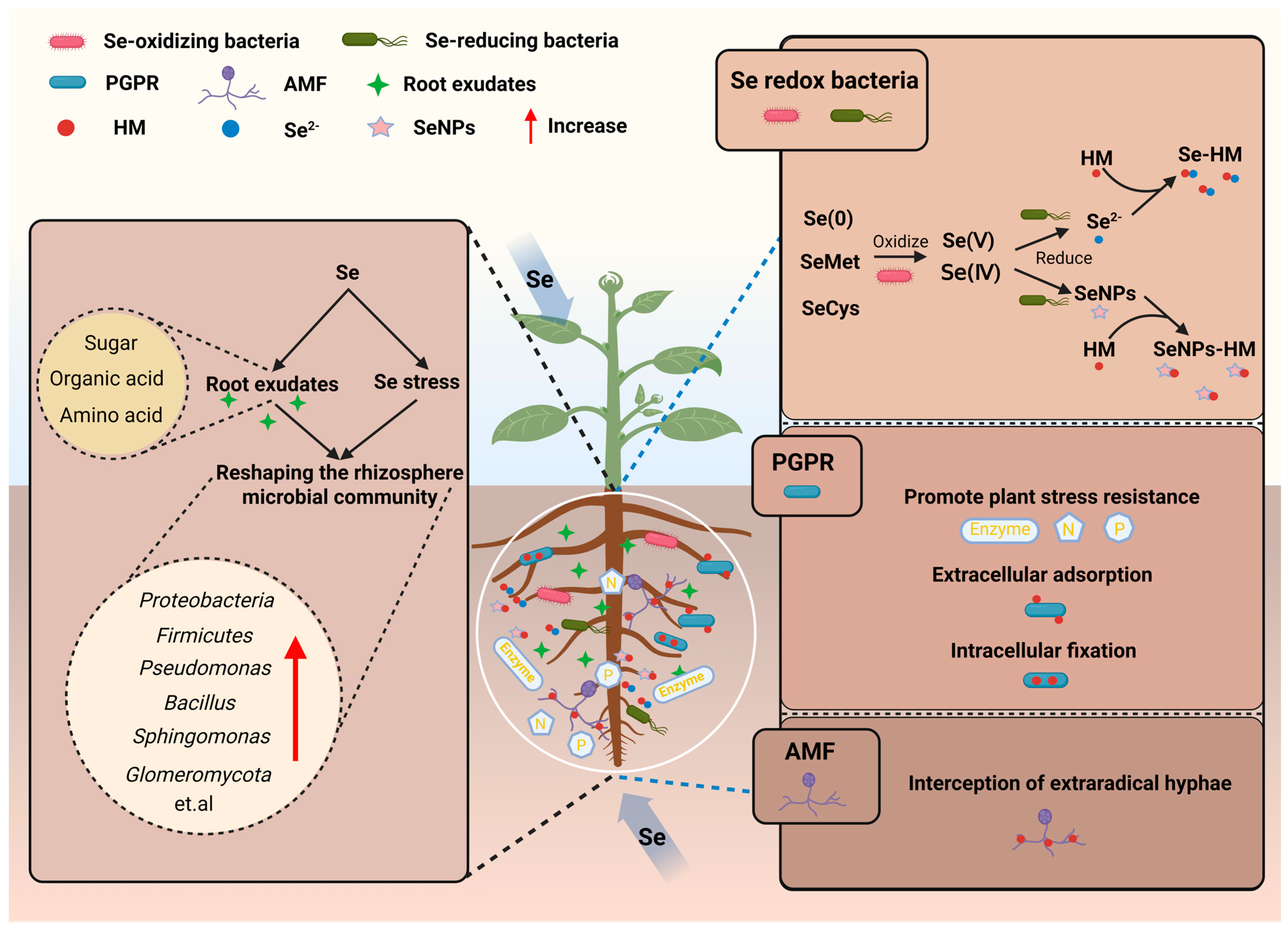
As the core hub connecting soil and plants, the rhizospheric microbiome, with its functional diversity, can effectively compensate for the limitations of geochemical processes. Se reshapes the rhizospheric microbial community through dual selection pressures (its own toxicity and root exudates), enriching functional microorganisms involved in Se speciation transformation and HM immobilization. This constitutes the “second line of defense” in the Se-mediated HM regulatory network, synergistically enhancing the stability and efficiency of HM immobilization together with geochemical processes (
Figure 2).
3.1. Se-Driven Construction of Rhizosphere Microbial Communities
The rhizospheric microbial community is highly sensitive to environmental changes, and the specific selection pressure induced by Se application directionally reshape its community composition. In the context of HM pollution, Se application can construct a functional microbial community capable of efficiently coping with HM stress. Microbes with both Se metabolism and HM detoxification capabilities—such as
Bacillus [
45],
Pseudomonas [
46] and
Sphingomonas [
47]—proliferate extensively and become dominant populations [
48]. To reshape the composition and function of the rhizosphere microbial community, Se primarily acts through a dual pathway mediated by self-induced selection pressure and root exudates.
On the one hand, Se at a certain concentration is toxic to many microbes, creating a strong environmental selection pressure. Only microbes with Se resistance or efficient detoxification mechanisms (e.g., reduction, methylation) can survive and become dominant. For example, in high-Se soils, the relative abundance of Proteobacteria and Firmicutes increases, with
Pseudomonas and
Bacillus becoming dominant genera [
49].
On the other hand, Se can alter the composition of root exudates by influencing plant physiological status. Root exudates (e.g., sugars, organic acids, amino acids) are not only the main nutrient source for rhizosphere microbes but also signal molecules mediating microbe-plant interactions; their changes directly drive shifts in microbial communities [
50]. Metabolomics studies confirm that Se treatment increases the concentration of organic acids (citric acid, malic acid) and decreases sugars (glucose, fructose) in
Arabidopsis thaliana root exudates, leading to a significant increase in the abundance of carbon source-preferring microbes (e.g.,
Sphingomonas) [
51]. Li et al. found that under Cd-contaminated conditions, nano-Se treatment significantly increased the release of sugars (e.g., glucose, fructose, sucrose) and phenolic acids (e.g., vanillic acid, p-hydroxybenzoic acid, and syringic acid) from pepper roots. These exudates effectively recruited and enriched beneficial microbes such as Gammaproteobacteria, Alphaproteobacteria, and Bacteroidota in the rhizosphere, thereby reducing the bioavailability of Cd and its accumulation in pepper plants [
52].
3.2. Rhizosphere Microbe-Mediated Immobilization of HM
The rhizospheric microbial community reshaped by Se exerts HM immobilization effects mainly through two pathways: first, indirectly immobilizing HM by regulating Se speciation; second, directly immobilizing HM through the microbes’ own adsorption, chelation, or physical interception. These two pathways cooperate with each other to further enhance the stability of HM immobilization.
3.2.1. HM Immobilization via Microbial Regulation of Se Speciation
Rhizosphere microbes can directly alter the chemical speciation of Se—the most direct manifestation of their regulatory role. Through a series of redox reactions, microbes convert highly toxic, soluble Se(IV)/Se(VI) into low-toxicity, insoluble Se(0), Se nanoparticles (SeNPs), and Se
2−, thereby indirectly achieving HM immobilization [
53] (
Table 1).
Table 1.
Immobilization of HM by rhizosphere microbe-mediated Se.
Table 1.
Immobilization of HM by rhizosphere microbe-mediated Se.
| Microbial Species | Microbial Name | Core Function(s) | Reference |
|---|
| Se-reducing Bacteria | Pseudomonas spp. | Reduces Se (VI)/Se (IV) to Se (0) or Se2− | [54] |
| Rhizobium sp. | Reduces Se (IV) to SeNPs | [55] |
| Burkholderia fungorum | Reduces Se (IV) to SeNPs or Se2− | [56] |
| Paenirhodobacter enshiensis | Reduces Se (IV) to SeNPs | [57] |
| Comamonas testosteroni S44 | Reduces Se (IV) to Se (0) or Se2− | [58] |
| Bacillus megaterium | Reduces Se (IV)/Se (0) to Se2− | [59] |
| Streptomyces sp. ES2-5 | Reduces Se (IV) to Se (0) nanoparticles | [60] |
| Thiobacillus ferrooxidans | Reduces Se (0) to Se2− | [61] |
| Bacillus selenitireducens | Reduces Se (IV) to SeNPs |
| Se-oxidizing Bacteria | LX-1 | Oxidizes Se (0), SeMet and SeCys to Se (IV) | [62] |
| LX-100 | Oxidizes Se (0), SeMet and SeCys to Se (IV) |
| T3F4 | Oxidizes Se (0), SeMet and SeCys to Se (IV) |
| PGPR (Plant Growth-Promoting Rhizobacteria) | Bacillus proteolyticus SES | Enhances HM immobilization by secreting metabolites | [45] |
| Bacillus cereus RC-1 | Adsorbs Cd through the cell wall and chelates Cd via intracellular MTs | [63] |
| AMF (Arbuscular Mycorrhizal Fungi) | Rhizophagus intraradices | Physically intercepts and adsorbs HMs through hyphae | [64] |
Microbial Se reduction is one of the most important mechanisms for HM immobilization in the rhizosphere. Se-reducing bacteria are widely distributed: most known Se(IV)-reducing bacteria belong to the phyla Proteobacteria, Firmicutes, and Actinobacteria (
Table 1). These microbial groups are rich in thiol-containing compounds and can efficiently reduce mobile, high-valence Se to low-valence Se(0) [
65]. For instance, studies have shown that Brevundimonas diminuta achieves a Se(IV) reduction rate of up to 99.08%, accompanied by the biosynthesis of SeNPs [
66]. In the presence of HMs, Se(0) can be further reduced to Se
2− by microorganisms to form insoluble metal selenides, as demonstrated by species such as Acidithiobacillus ferrooxidans and Bacillus selenitireducens [
61]. This reductive mechanism enables Se fertilizers to act as efficient precipitants, forming highly insoluble metal selenides with HMs in soil solution, thereby reducing the bioavailability of rhizospheric HMs. It should be noted, however, that the tendency of microorganisms to produce metal selenides via reduction is influenced by the aforementioned environmental factor—Eh [
35].
In addition, microbially synthesized SeNPs exhibit unique advantages in HM immobilization. Typically spherical in shape, these SeNPs possess a high specific surface area and negative surface charge. These physicochemical properties enable them to adsorb HM ions effectively and form stable SeNPs–HM complexes, significantly decreasing HM bioavailability [
67]. Owing to their nanoscale characteristics, SeNPs can also be readily taken up by plants, thereby regulating the expression of metal transporter protein genes and other related genes. For example, in wheat, SeNPs can significantly reduce Cd accumulation in roots and shoots, primarily by modulating the expression of genes associated with Cd uptake and translocation [
68]. The specific molecular regulatory mechanisms are discussed later in this paper. Furthermore, SeNPs feature sustained-release properties, high stability, strong biological activity, and environmental friendliness, which endow them with broad application prospects in the field of environmental remediation [
69].
While Se oxidation proceeds much slower than efficient Se reduction, it remains an important pathway to increase the concentration of bioavailable Se in soil [
70]. Se(0) and organic Se (e.g., selenomethionine (SeMet) and selenocysteine (SeCys)) are naturally occurring Se forms in soil but have extremely low bioavailability: Se(0) cannot be directly absorbed by plants; SeMet and SeCys are mostly bound to soil organic matter, making them difficult to release into soil solution for interaction with HMs [
71]. Certain Se-oxidizing bacteria can oxidize low-valence, recalcitrant Se in soil to more mobile high-valence Se, thereby enhancing soil Se bioavailability [
72]. High-valence Se is not only easily absorbed by plants but also readily reduced by Se-reducing bacteria to Se
2− or SeNPs for HM binding and precipitation. Studies have shown that three Se-oxidizing bacteria (LX-1, LX-100, and T3F4) can oxidize soil SeMet, SeCys, and Se(0) to Se(IV), increasing the available Se content for rapeseed uptake while inhibiting Cd absorption and increasing Se content in aboveground plant parts [
62]. In pak choi (
Brassica rapa L.), application of the Se-oxidizing bacterium T3F4 was also shown to improve soil Se mobility, thereby promoting Se uptake and reducing As accumulation [
73].
3.2.2. Other Microbe-Mediated HM Immobilization Mechanisms
Rhizosphere microbial communities co-selected by Se and HMs often include efficient plant growth-promoting rhizobacteria (PGPR). These microbes enhance plant stress resistance through multiple pathways—e.g., secreting ACC deaminase to reduce ethylene stress [
74], and strengthening nutrient cycling (e.g., N, P)—thereby mitigating HM toxicity [
75]. Additionally, some tolerant strains (e.g.,
Bacillus cereus RC-1) can immobilize HM via two mechanisms: adsorption by hydroxyl-rich cell walls, and intracellular binding to proteins such as metallothioneins (MTs) after HM ions are transported into cells [
63].
In addition to abundant PGPR, rhizosphere soil also contains mycorrhizal fungi [
76]. Among them, arbuscular mycorrhizal fungi (AMF) are closely associated with plant tolerance to HM stress: their extraradical hyphae not only form a network to block HMs but also adsorb and immobilize HMs via cell wall components (e.g., polysaccharides, chitin), reducing HM migration to plant roots [
77].
Based on the above-described interactions between Se and rhizosphere microbes, combining exogenous Se application with inoculation of specific microbial agents (e.g., PGPR, AMF) that have efficient HM resistance and transformation capabilities often produces a synergistic effect superior to single treatments. This strategy integrates the chemical and biological regulatory effects of Se with microbial bioaugmentation, making it the most promising application approach to date. Liu et al. found in a pot experiment that the combined application of
Rhizophagus intraradices and selenite successfully reduced soil Cd bioavailability and Cd accumulation in wheat under Cd-stressed conditions, with the reduction efficacy being 47.3% higher than that of the control [
64].
4. Regulation of Plant Root Structure and Function by Se
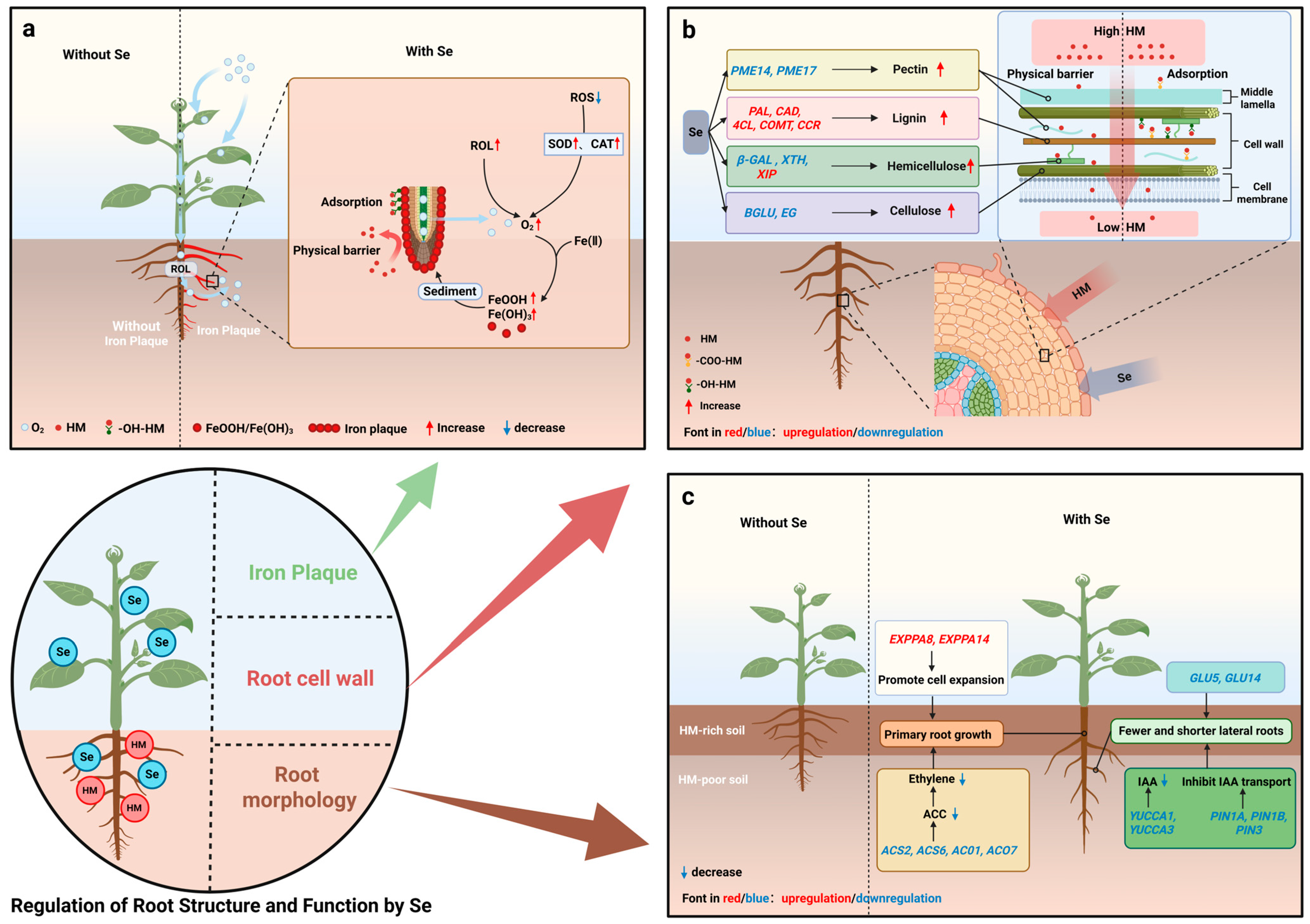
Even with the dual barriers of geochemical immobilization and microbial interception, some HM ions may still reach the root surface or even penetrate the rhizosphere. At this point, the plant’s own root system serves as the “third line of defense” in the Se-mediated regulatory network. Se can actively regulate root structure and function to establish physical and chemical dual barriers, blocking HM entry. By inducing iron plaque formation on the root surface, reshaping root morphology, and enhancing cell wall components, Se significantly improves the root system’s resistance to HM invasion, compensating for the deficiencies of upstream defense processes.
4.1. Se-Induced Formation of Root Iron Plaque
Iron plaque (IP) is a unique HM defense structure formed by wetland plants (e.g., rice) in a waterlogged environment, existing as a reddish-brown gelatinous film attached to the root surface. Specifically, plants transport oxygen from aboveground tissues to roots via unique aerenchyma, then release it into the rhizosphere microenvironment through radial oxygen loss (ROL), creating an oxidizing environment that promotes the oxidation of soluble Fe(II) to Fe(III) and its deposition as amorphous iron oxide (FeOOH) and iron hydroxide (Fe(OH)
3) on root surfaces [
78]. Iron plaque significantly restricts HM migration from soil to roots through two mechanisms: tightly covered iron plaques directly block HM from entering root cells [
79]; abundant hydroxyl groups (-OH) on the plaque surface immobilize HM ions via electrostatic interactions [
80] (
Figure 3a).
In the presence of Fe
2+, Se can influence root iron plaque formation by regulating root ROL levels, thereby blocking HM migration to roots [
81]. First, Se alleviates physiological damage to rice root aerenchyma caused by HMs, increasing root porosity and providing structural support for downward oxygen transport, which directly enhances ROL intensity at root tips [
82]. Second, Se activates the activity of antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT), accelerating the degradation of reactive oxygen species (ROS, e.g., O
2−, H
2O
2) induced by HM stress. This process not only mitigates root damage but also produces O
2, indirectly increasing internal root oxygen content and providing an oxygen source for ROL [
83].
The form of Se, application timing, and chemical form and content of HM all affect the effect of Se on root iron plaque formation and HM immobilization.
Effects of Se forms on regulating iron plaque function differ significantly. Se(IV) promotes As transport from iron plaque to rice roots, while Se(VI) significantly inhibits this process, though the underlying mechanism remains unclear [
84].
Timing of Se application is crucial for maximizing HM immobilization mediated by iron plaque. For instance, applying Se during the rice tillering stage (the peak period of rhizosphere oxygen release via radial oxygen loss, ROL) significantly induces extensive iron plaque formation on root surfaces at the heading stage, thereby achieving the maximum inhibition of root Cd uptake [
82].
The chemical form and content of HMs further modulate the efficacy of Se-mediated iron plaque formation. Se can promote iron plaque formation on rice roots only under Sb(III) stress, with no significant effect on Sb(V)-exposed groups [
85]. A study on rice found that Se treatment alone could not break through the Cd adsorption threshold of iron plaque (53 mg/kg), but the combined application of Se and lime could restore iron plaque function to cope with high Cd pollution by reducing soil available Cd concentration [
44].
4.2. Se Reshapes Root Morphology to Avoid HM Pollution
Root morphology plays a crucial role in plant resistance to HM toxicity and stress. A larger root surface area may lead to higher HM accumulation; thus, reducing lateral root number and increasing primary root dominance helps plants decrease HM uptake [
86].
Numerous studies have shown that Se can induce changes in plant root morphology, thereby reducing HM absorption and accumulation. Huang et al. reported that pepper cultivars with larger root surface area, longer root length, and more root tips showed higher Cd accumulation, suggesting that reducing these root traits can decrease HM uptake [
87]. The core mechanism of Se-regulated root morphology may involve the regulation of auxin (IAA) and ethylene biosynthesis, but there are significant differences in the regulatory mechanisms for primary and lateral roots. Se downregulates ethylene synthesis genes (
ACS2/6,
ACO1/7) to reduce the concentration of the ethylene precursor ACC, ultimately decreasing root ethylene levels; it also upregulates cell expansion genes (
EXPA8/14,
EXPB2/3) to promote primary root cell elongation, resulting in increased primary root length. For lateral roots: Se downregulates auxin synthesis genes (
YUCCA1/3) to reduce IAA accumulation, while also downregulating auxin transport genes (
PIN1A/B,
PIN3) and key lateral root formation genes (
GLU5/14), leading to reduced lateral root number and length [
88] (
Table 2).
Thus, Se treatment can both adjust the ratio of primary to lateral roots (reducing root surface area and thus HM uptake sites) and increase primary root length (guiding roots to migrate from the surface to deeper soil layers, actively avoiding HM-enriched topsoil and reducing exposure intensity) (
Figure 3b). However, some studies have reported contrasting results. Zhao et al. found that 0.1 mg/L of selenite increased the root surface area, root volume, and root tip number of
Brassica campestris L. seedlings under Cr stress by 109%, 150%, and 84%, respectively; at the harvest stage, the root tip number was increased by 120%, which expanded the contact area with Cr [
89]. This might be related to the Se concentration. A study indicated that 0.2 mg/L of selenite (a low concentration) increased the root surface area by 85.8%, whereas 0.8 mg/L of selenite (a high concentration) decreased the root surface area by 16.1% [
90].
Notably, most existing studies on root morphological changes have been limited to hydroponic experiments conducted at a single growth stage. Technical barriers such as easy root damage and difficult observation under soil conditions have restricted the relevant research. In addition, the persistence of experiment-induced root morphological changes and the aforementioned iron plaque formation throughout the entire crop growth cycle, as well as their agronomic application value, remain to be further investigated.
Table 2.
Influence of Se on the expression of genes related to root structure and function in crops.
Table 2.
Influence of Se on the expression of genes related to root structure and function in crops.
| Crop Species | Gene | Gene Function(s) | Gene Expression After Se Application | Reference |
|---|
| Oryza sativa L. | EXPA8/14 | Promotes elongation of primary root cells | Upregulation | [88] |
| EXPB2/3 | Promotes elongation of primary root cells |
| ACS2/6 | Synthesizes ethylene and promotes lateral root development | Downregulation |
| ACO1/7 | Synthesizes ethylene and promotes lateral root development |
| YUCCA1/3 | Synthesizes auxin and promotes lateral root development |
| PIN1A/B | Transports auxin and promotes lateral root formation |
| PIN3 | Transports auxin and promotes lateral root formation |
| GLU5/14 | Promotes the formation and development of lateral root primordia, increasing the number and length of lateral roots |
| XIP | Inhibits xylanase from cleaving xylan chains in hemicellulose | Upregulation | [91] |
| PME14/17 | Catalyzes pectin demethylation to expose carboxyl groups | Downregulation |
| Capsicum annuum L. | PAL | Catalyzes the conversion of phenylalanine to cinnamic acid, providing precursor substances for lignin synthesis | Upregulation | [92] |
| CAD | Involved in lignin monomer synthesis |
| 4CL | Catalyzes the conversion of coumaric acid to coumaryl-CoA, providing precursors for lignin synthesis |
| COMT | Catalyzes methylation reactions in lignin synthesis |
| Triticum aestivum L. | CCR | Catalyzes lignin monomer synthesis | Upregulation | [68] |
| β-GAL | Hydrolyzes galactose residues in hemicellulose and participates in cell wall polysaccharide remodeling | Downregulation |
| XTH | Hydrolyzes xyloglucan in hemicellulose |
| BGLU | Hydrolyzes cellobiose and decomposes cellulose |
| EG | Randomly cleaves cellulose polymer chains and degrades cellulose |
4.3. Se Enhances the HM Barrier Capacity of Root Cell Walls
The root cell wall is the last physical barrier for HM before they enter plant cells. Even if HMs pass through the iron plaque and come into contact with the root surface, the cell wall can still achieve interception through adsorption and physical blocking. The cell wall is mainly composed of lignin, cellulose, hemicellulose, and pectin; the middle lamella adjacent to the cell wall is also pectin-based [
93]. Se can intercept HMs from entering root cells through regulating genes related to root cell wall components and leveraging the cell wall’s physical barrier and adsorption functions [
68] (
Figure 3c,
Table 2).
4.3.1. Se Promotes Lignin Synthesis in Root Cell Walls
Lignin, synthesized via the phenylpropanoid pathway, is a hydrophobic polymer critical for plant resistance to HM stress [
94]. HM stress induces phenolic compound accumulation, enhancing lignin deposition to form a physical barrier; additionally, carboxyl and phenolic hydroxyl groups in lignin can adsorb HMs [
95]. Studies show that Se treatment significantly upregulates the expression of key lignin synthesis genes (e.g.,
PAL,
CAD,
4CL,
COMT), activating the phenylpropanoid metabolic pathway, increasing lignin content in root cell walls, maintaining cell wall integrity, and hindering further infiltration of HM ions into the cytoplasm [
92]. In wheat, key lignin synthesis genes
CAD and
CCR are also upregulated by SeNPs, thereby enhancing the Cd barrier capacity [
68].
4.3.2. Se Increases Cell Wall Polysaccharide Content
Se can significantly increase the content of cell wall polysaccharides (pectin, hemicellulose, cellulose).
Pectin is mainly composed of galacturonic acid with abundant carboxyl groups. Pectin methylesterase (PME) catalyzes the hydrolysis of methyl ester bonds in pectin’s galacturonic acid residues (demethylation), exposing free carboxyl groups (-COOH) for binding to HM [
96]. Se treatment promotes pectin demethylation by PME through downregulating the synthesis genes
PME14 and
PME17 of PMEI (PME inhibitor), thereby enhancing the HM binding capacity of pectin in the cell wall [
91].
Hemicellulose is a heteropolysaccharide composed of xylan, mannan, and other polymers, whose hydroxyl groups can adsorb a certain amount of HM ions. Studies show that Se regulates genes related to hemicellulose biosynthesis: it downregulates β-GAL (encoding β-galactosidase) to reduce hydrolysis of galactose residues, ensuring binding stability between hemicellulose and other components; it downregulates XTH (encoding xyloglucan hydrolase) to inhibit xyloglucan hydrolysis, avoiding structural damage [
68]; it upregulates XIP (encoding xylanase inhibitor protein) to directly inhibit xylanase cleavage of the xylan backbone, preventing xylan degradation and maintaining hemicellulose stability [
91].
Cellulose is the framework component of the cell wall, forming a fibrous network of β-glucan chains via hydrogen bonds. It binds HMs through its own hydroxyl groups, and enhances the physical barrier function of the cell wall against HMs through tight cross-linking with hemicellulose [
97]. Research indicates that nano-Se inhibits cellulose degradation by downregulating the β-glucosidase-encoding gene
BGLU and endoglucanase-encoding gene
EG, thereby improving Cd immobilization capacity [
68].
5. Se Regulates HM Transport in Root Cells
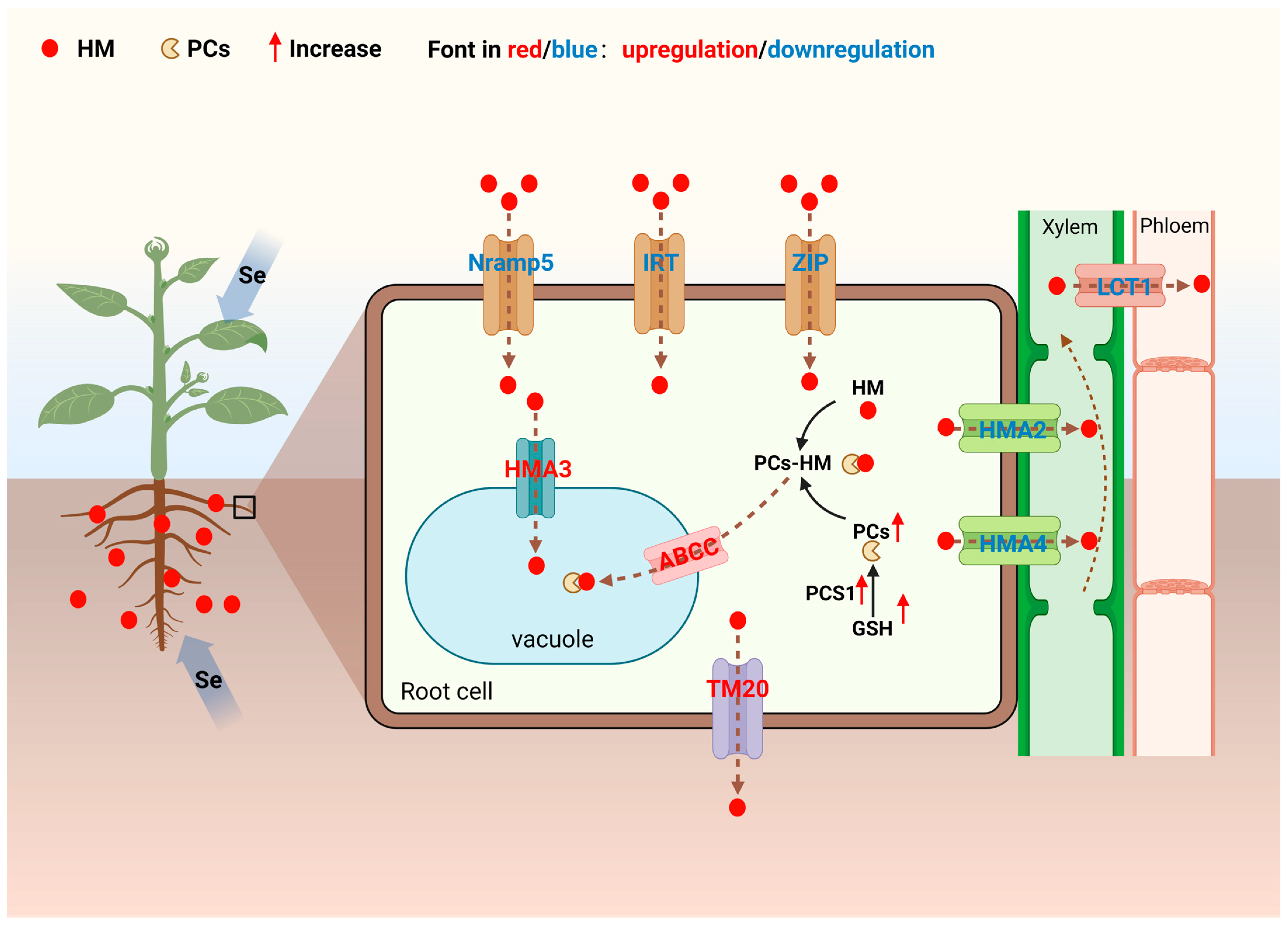
Although geochemical immobilization, microbial interception, and root structure regulation constitute three tight defense lines, a small number of HM ions may still break through these defenses and enter root cells. At this point, Se activates the “fourth line of defense” in the regulatory network—intracellular transport regulation. By precisely regulating the expression of genes related to HM uptake, efflux, transport, and sequestration, Se not only reduces HM entry into cells but also promotes the efflux of intracellular HM or their vacuolar sequestration, blocking the translocation of HMs to aboveground edible parts and filling the final gap in the Se-mediated HM regulatory network (
Table 3,
Figure 4).
Table 3.
Influence of Se on the expression of genes related to HM uptake, transport, and immobilization in crop roots.
Table 3.
Influence of Se on the expression of genes related to HM uptake, transport, and immobilization in crop roots.
| Crop Species | Gene Name | Gene Function(s) | Gene Expression After Se Application | Reference |
|---|
| Oryza sativa L. | OsNramp5 | Mediates Cd uptake by root cells | Downregulation | [98] |
| OsIRT1 | Mediates Fe and Cd uptake by root cells |
| OsIRT2 | Mediates Fe and Cd uptake by root cells |
| OsZIP1 | Mediates Fe and Cd uptake by root cells | Downregulation | [99] |
| OsPCS1 | Promotes the synthesis of PCs | Upregulation |
| OsHMA2 | Transports root Cd into the xylem | Downregulation | [100] |
| OsHMA4 | Transports root Cd into the xylem |
| OsLCT1 | Transports Cd to leaves and grains | Downregulation | [101] |
| OsHMA3 | Transports Cd to vacuoles | Upregulation | [102] |
| Triticum aestivum L. | TaTM20 | Mediates Cd efflux from root cells | Upregulation | [103] |
| Brassica juncea L. | ABCC | Transports PCs-Cd complexes to vacuoles | Upregulation | [104] |
5.1. Se Inhibits HM Uptake and Transport in Root Cells
HM ions often enter root cells by competing for the transport channels of essential mineral elements such as Fe2+ and Zn2+, due to their similar physicochemical properties to these elements. Se can block the intracellular migration of HMs through two mechanisms: first, downregulating the gene expression of these shared transporters to reduce HM uptake; second, upregulating the genes encoding HM efflux proteins to promote the excretion of HMs that have already entered the cells. Additionally, Se can inhibit the long-distance translocation of HMs from roots to aboveground parts, thereby reducing HM accumulation in edible parts.
First, Se can inhibit HM uptake by plant root cells. The manganese transporter Nramp5, a member of the natural resistance-associated macrophage protein (Nramp) family, has been identified as the primary transporter for Cd uptake in root cells [
105]. Iron-regulated transporters (IRT) such as IRT1, IRT2, and members of the zinc/iron-regulated transporter (ZIP) family such as ZIP1 in root cells also have Cd transport functions [
106]. Existing studies show that Se treatment can downregulate the expression of these genes, thereby restricting Cd entry into root cells. Cui et al. found that Se treatment significantly inhibited the expression of
OsNramp5,
OsIRT1, and
OsIRT2, directly reducing the Cd uptake rate of rice suspension cells [
98]. Meanwhile, Barman et al. also found that Se treatment significantly downregulated the expression of
OsZIP1, reducing Cd uptake in rice roots. In addition to inhibiting uptake, Se application can also stimulate the expression of the Cd efflux protein TM20 [
99]. In wheat roots, Se application significantly downregulated
TaNramp5 while also upregulating
TaTM20, which not only reduced Cd uptake but also promoted Cd efflux, further enhancing wheat tolerance to Cd [
103].
Second, Se can inhibit the translocation of HMs from root cells to aboveground edible parts. HM ATPase (HMA) family members HMA2 and HMA4, mainly located on the plasma membrane of xylem parenchyma cells in plant roots, are responsible for pumping metal ions such as Zn and Cd from root stele parenchyma cells into the xylem, initiating their long-distance transport to aboveground parts [
107]. In rapeseed (
Brassica napus), Se inhibits the expression of
HMA2 and
HMA4, reducing the efficiency of Cd translocation from the roots to aboveground parts [
108]—the same conclusion was found in rice [
100]. The low-affinity cation transporter (LCT1), located on the plasma membrane of the root phloem, is responsible for transporting Cd from cells to leaves and grains [
109]. In rice, Se treatment can also downregulate the expression of
OsLCT1, leading to a decrease in Cd content in inflorescences and grains [
101]. By inhibiting the expression of these key transport genes, Se effectively reduces the distribution of Cd from roots to stems, leaves, and grains, ensuring the safety of agricultural products.
5.2. Se Promotes Chelation and Compartmentalization of HM in Root Cells
For HMs that have entered the cytoplasm, plants initiate a “dual detoxification” strategy: first, chelating HMs with endogenous chelators to mitigate their toxic effects; second, transporting free HMs or their chelates into vacuoles for sequestration via tonoplast transporters. Se can markedly enhance the efficiency of both processes by upregulating the genes responsible for chelator biosynthesis and tonoplast transporter expression.
5.2.1. Se Enhances HM Chelation Capacity of Root Cells
Se treatment significantly improves the chelation capacity of plant cells for HM ions. HM chelators in plants mainly include phytochelatins (PCs) and MTs, which bind HM ions via abundant sulfhydryl groups (-SH) to form stable complexes, thereby reducing their biological activity [
110]. Se increases intracellular PC content by promoting the synthesis of the PC precursor glutathione (GSH) and activating the expression of the key PC synthase gene
PCS1, thus chelating HMs. In rice, Se treatment significantly increases GSH content and promotes
OsPCS1 expression in root cells, enhancing PC levels to form stable PCs–Cd complexes, which are then transported into vacuoles for sequestration [
99]. In tobacco, Se has also been shown to upregulate
PCS1 to chelate chromium (Cr) [
111].
5.2.2. Se Activates Vacuolar Membrane Transporters to Enhance Compartmentalization Efficiency
Vacuoles are the primary organelles for storing and sequestering toxic substances in plant cells; efficient transport and compartmentalization of HM ions into vacuoles is a core mechanism for plants to cope with HM stress [
112]. HM ATPase 3 (HMA3), a member of the HMA family, is a key transporter localized on the vacuolar membrane of root cells, specifically responsible for pumping HM into vacuoles [
113]. For example, in rice, Se treatment significantly activates
OsHMA3 expression, promoting Cd transport and sequestration into vacuoles of root cells [
102]. Additionally, in mustard (
Brassica juncea), Se treatment also activates genes of the ATP-binding cassette (ABC) transporter subfamily
ABCC, whose members are confirmed to participate in transporting stable PCs–Cd complexes into vacuoles [
104]. By activating these vacuolar membrane transporters, Se greatly enhances the ability of root cells to stabilize HM in the cytoplasm and safely sequester them in vacuoles.
6. Challenges and Future Perspectives
Although the efficacy of Se in inhibiting crop uptake of HMs has been widely verified, this technology faces critical challenges in practical farmland remediation. Long-term Se application risks excessive Se accumulation in crops via soil buildup, threatening human health through the food chain and impairing soil microbial community structure, enzyme activity, nutrient cycling, and ecological stability. Most existing studies focus on single-HM contamination, yet farmland soils are often co-contaminated with Cd–As and other HMs; the interaction between Se and combined HMs is regulated by soil pH, Eh, and HM speciation, with the underlying mechanisms remaining unclear. Additionally, discrepancies exist between lab/pot experiment results and field performance—field soil complexity (e.g., water content, pH, colloid composition) reduces Se-mediated remediation efficiency (e.g., root iron plaque formation and HM interception), and large-scale Se application lacks systematic verification regarding cost-effectiveness and long-term stability.
Future research should prioritize the cross-interaction mechanisms among Se, HMs, plant roots, and rhizosphere microorganisms, especially clarifying molecular-level signal pathways and gene co-regulation patterns. By targeting soil type variations and combined HM contamination, efforts should focus on optimizing Se application forms, dosages, and timing to establish precision schemes tailored to the “soil–crop–HM type” system. Beyond conventional Se(IV), SeNPs (low-toxic, stable and non-accumulative) and slow-release organic Se are promising Se fertilizer alternatives. Soil incorporation suits long-term remediation, while foliar spraying is ideal for mild contamination or emergency use; Se application timing should be crop-specific (e.g., rice tillering stage for maximum efficacy). Synergistic application of Se and functional microorganisms requires further optimization of mixing ratios, activation conditions, and field management to clarify synergistic mechanisms. Meanwhile, long-term Se application impacts on soil ecological functions and agricultural product quality must be evaluated to mitigate environmental and food safety risks. Finally, accelerating the translation of lab findings into field practices and formulating standardized protocols balancing HM control and Se biofortification will support food security and public health.
7. Conclusions
Soil HM pollution poses a severe threat to ecological security and food safety, and Se-mediated regulation of HM bioavailability has emerged as a promising “remediation-nutrition” dual-purpose technology. This review systematically constructs a multi-dimensional, synergistic regulatory network of Se in the rhizosphere system, which is composed of four interconnected lines of defense: geochemical immobilization (source passivation), rhizosphere microbial interception (biological regulation), root structure and function regulation (plant self-defense), and intracellular transport regulation (intracellular detoxification). Geochemically, Se reduces HM mobility at the source by regulating rhizosphere Eh and pH, inducing precipitation of metal selenides (e.g., CdSe, HgSe), and enhancing soil colloid adsorption. In terms of microbial synergy, Se reshapes the rhizosphere microbial community, indirectly immobilizing HMs by regulating Se speciation transformation and directly participating in HM sequestration via microbial adsorption and chelation. At the root structure and function level, Se induces root iron plaque formation, reshapes root morphology (reduces lateral root proportion, extends primary root), and strengthens root cell wall components (promotes lignin and polysaccharide synthesis), enhancing physical interception and adsorption of HM. Intracellularly, Se inhibits the expression of HM uptake and transport genes, promotes phytochelatin synthesis, and facilitates vacuolar compartmentalization of HM complexes, sequestering HMs in roots. This multi-level network not only effectively blocks HM migration to edible parts but also provides a theoretical basis for producing Se-enriched agricultural products.
Author Contributions
Writing—original draft preparation, Q.G.; Conceptualization, methodology, supervision, H.C. and L.L.; software, X.Z.; validation, formal analysis, S.J.; data curation, Y.N.; project administration, investigation, funding acquisition, S.C.; resources, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Major Program of Hubei Province, grant number 2025DJB079 and the Horizontal science and technology of Enshi Se-De Bioengineering Co., Ltd., grant number Se2-202307.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors extend their gratitude to Enshi Se-De Biotechnology Co., Ltd. for their financial support in the realm of this research endeavor.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Omotayo, A.O.; Omotayo, O.P. Potentials of microbe-plant assisted bioremediation in reclaiming heavy metal polluted soil environments for sustainable agriculture. Environ. Sustain. Indic. 2024, 22, 100396. [Google Scholar] [CrossRef]
- Hou, D.; Jia, X.; Wang, L.; McGrath, S.P.; Zhu, Y.; Hu, Q.; Zhao, F.; Bank, M.S.; O’Connor, D.; Nriagu, J. Global soil pollution by toxic metals threatens agriculture and human health. Science 2025, 388, 316–321. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, L.; Hao, S.; Zhou, X. Application of selenium to reduce heavy metal(loid)s in plants based on meta-analysis. Chemosphere 2024, 364, 143150. [Google Scholar] [CrossRef]
- Sorrentino, M.C.; Capozzi, F.; Amitrano, C.; Giordano, S.; Arena, C.; Spagnuolo, V. Performance of three cardoon cultivars in an industrial heavy metal-contaminated soil: Effects on morphology, cytology and photosynthesis. J. Hazard. Mater. 2018, 351, 131–137. [Google Scholar] [CrossRef]
- Pang, X.; Chen, C.; Sun, J.; Zhan, H.; Xiao, Y.; Cai, J.; Yu, X.; Liu, Y.; Long, L.; Yang, G. Effects of complex pollution by microplastics and heavy metals on soil physicochemical properties and microbial communities under alternate wetting and drying conditions. J. Hazard. Mater. 2023, 458, 131989. [Google Scholar] [CrossRef]
- Ngo, H.T.T.; Hang, N.T.T.; Nguyen, X.C.; Nguyen, N.T.M.; Truong, H.B.; Liu, C.; La, D.D.; Kim, S.S.; Nguyen, D.D. Toxic metals in rice among Asian countries: A review of occurrence and potential human health risks. Food Chem. 2024, 460, 140479. [Google Scholar] [CrossRef]
- Pan, Z.; Gong, T.; Liang, P. Heavy metal exposure and cardiovascular disease. Circ. Res. 2024, 134, 1160–1178. [Google Scholar] [CrossRef]
- Mei, Z.; Yang, J.; Zhao, Y.; Li, W.; Li, R.; Liu, D.; Lu, H.; He, Z.; Gu, S. Comparative neurotoxic effects and mechanism of cadmium chloride and cadmium sulfate in neuronal cells. Environ. Int. 2025, 203, 109749. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hong, Y.; Duan, X.; Zhou, Q.; Chen, J.; Liu, S.; Su, J.; Han, L.; Zhang, J.; Niu, B. Unveiling the metal mutation nexus: Exploring the genomic impacts of heavy metal exposure in lung adenocarcinoma and colorectal cancer. J. Hazard. Mater. 2024, 461, 132590. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Lin, Y.; Lin, R.; Liu, J.; Wang, H.; Hu, W.; Chen, B.; Chen, T. Traditional Chinese medicine active ingredients-based selenium nanoparticles regulate antioxidant selenoproteins for spinal cord injury treatment. J. Nanobiotechnology 2022, 20, 278. [Google Scholar] [CrossRef]
- Angulo-Elizari, E.; Raza, A.; Encío, I.; Sharma, A.K.; Sanmartín, C.; Plano, D. Seleno-warfare against cancer: Decoding antitumor activity of novel acylselenoureas and Se-acylisoselenoureas. Pharmaceutics 2024, 16, 272. [Google Scholar] [CrossRef]
- Rocca, C.; Pasqua, T.; Boukhzar, L.; Anouar, Y.; Angelone, T. Progress in the emerging role of selenoproteins in cardiovascular disease: Focus on endoplasmic reticulum-resident selenoproteins. Cell. Mol. Life Sci. 2019, 76, 3969–3985. [Google Scholar] [CrossRef]
- Ullah, H.; Liu, G.; Yousaf, B.; Ali, M.U.; Irshad, S.; Abbas, Q.; Ahmad, R. A comprehensive review on environmental transformation of selenium: Recent advances and research perspectives. Environ. Geochem. Health 2019, 41, 1003–1035. [Google Scholar] [CrossRef]
- Song, T.J.; Cui, G.; Su, X.S.; He, J.; Tong, S.Z.; Liu, Y. The origin of soil selenium in a typical agricultural area in Hamatong River Basin, Sanjiang Plain, China. Catena 2020, 185, 104355. [Google Scholar] [CrossRef]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef]
- Tang, C.; Li, S.; Zhang, K.; Li, J.; Han, Y.; Zhan, T.; Zhao, Q.; Guo, X.; Zhang, J. Selenium deficiency-induced redox imbalance leads to metabolic reprogramming and inflammation in the liver. Redox Biol. 2020, 36, 101519. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, Y.; Zhu, N.; Jin, H. Foliar application of biosynthetic nano-selenium alleviates the toxicity of Cd, Pb, and Hg in Brassica chinensis by inhibiting heavy metal adsorption and improving antioxidant system in plant. Ecotoxicol. Environ. Saf. 2022, 240, 113681. [Google Scholar] [CrossRef] [PubMed]
- Solomon, W.; Janda, T.; Molnár, Z. Unveiling the significance of rhizosphere: Implications for plant growth, stress response, and sustainable agriculture. Plant Physiol. Biochem. 2024, 206, 108290. [Google Scholar] [CrossRef] [PubMed]
- Gallego, J.L.; Olivero-Verbel, J. Cytogenetic toxicity from pesticide and trace element mixtures in soils used for conventional and organic crops of Allium cepa L. Environ. Pollut. 2021, 276, 116558. [Google Scholar] [CrossRef]
- Huang, Q.; Xu, Y.; Liu, Y.; Qin, X.; Huang, R.; Liang, X. Selenium application alters soil cadmium bioavailability and reduces its accumulation in rice grown in Cd-contaminated soil. Environ. Sci. Pollut. Res. 2018, 25, 31175–31182. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, J.; Cao, K.; Liu, Y.; Wang, B.; Wang, X.; Wang, Y.; Jiang, D.; Cao, B.; Zhang, Y. Foliar application of selenium and gibberellins reduce cadmium accumulation in soybean by regulating interplay among rhizosphere soil metabolites, bacteria community and cadmium speciation. J. Hazard. Mater. 2024, 476, 134868. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, J.; Zeng, J.; Chen, L.; Korpelainen, H.; Li, C. Selenium species transforming along soil–plant continuum and their beneficial roles for horticultural crops. Hortic. Res. 2023, 10, uhac270. [Google Scholar] [CrossRef]
- Goldberg, S. Modeling Selenate Adsorption Behavior on Oxides, Clay Minerals, and Soils Using the Triple Layer Model. Soil Sci. 2014, 179, 568–576. [Google Scholar] [CrossRef]
- Chan, Y.T.; Kuan, W.H.; Chen, T.Y.; Wang, M.K. Adsorption mechanism of selenate and selenite on the binary oxide systems. Water Res. 2009, 43, 4412–4420. [Google Scholar] [CrossRef]
- Li, Z.; Liang, D.L.; Peng, Q.; Cui, Z.W.; Huang, J.; Lin, Z.Q. Interaction between selenium and soil organic matter and its impact on soil selenium bioavailability: A review. Geoderma 2017, 295, 69–79. [Google Scholar] [CrossRef]
- Tolu, J.; Thiry, Y.; Bueno, M.; Jolivet, C.; Potin-Gautier, M.; Le Hécho, I. Distribution and speciation of ambient selenium in contrasted soils, from mineral to organic rich. Sci. Total Environ. 2014, 479–480, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Peak, D. Adsorption mechanisms of selenium oxyanions at the aluminum oxide/water interface. J. Colloid Interface Sci. 2006, 303, 337–345. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Lai, F. Effects of different water management on absorption and accumulation of selenium in rice. Saudi J. Biol. Sci. 2018, 25, 1178–1182. [Google Scholar] [CrossRef]
- Vermeiren, C.; Ceulemans, J.; Thiry, Y.; Smolders, E. Increased soil pH and enhanced microbial activity stimulate the gradual immobilisation of selenate added to soils. Soil Biol. Biochem. 2025, 202, 109688. [Google Scholar] [CrossRef]
- Stroud, J.L.; McGrath, S.P.; Zhao, F.J. Selenium speciation in soil extracts using LC-ICP-MS. Int. J. Environ. Anal. Chem. 2012, 92, 222–236. [Google Scholar] [CrossRef]
- Araujo, A.M.; Lessa, J.H.D.; Chanavat, L.G.; Curi, N.; Guilherme, L.R.G.; Lopes, G. How sulfate content and soil depth affect the adsorption/desorption of selenate and selenite in tropical soils? Rev. Bras. Cienc. Solo 2020, 44, e0200087. [Google Scholar] [CrossRef]
- Li, H.; Lombi, E.; Stroud, J.L.; McGrath, S.P.; Zhao, F. Selenium speciation in soil and rice: Influence of water management and Se fertilization. J. Agric. Food Chem. 2010, 58, 11837–11843. [Google Scholar] [CrossRef]
- Nakamaru, Y.M.; Altansuvd, J. Speciation and bioavailability of selenium and antimony in non-flooded and wetland soils: A review. Chemosphere 2014, 111, 366–371. [Google Scholar] [CrossRef]
- Deng, G.; Fan, Z.; Wang, Z.; Peng, M. Dynamic role of selenium in soil–plant-microbe systems: Mechanisms, biofortification, and environmental remediation. Plant Soil 2025, 515, 1085–1105. [Google Scholar] [CrossRef]
- Mal, J.; Sinharoy, A.; Lens, P.N.L. Simultaneous removal of lead and selenium through biomineralization as lead selenide by anaerobic granular sludge. J. Hazard. Mater. 2021, 420, 126663. [Google Scholar] [CrossRef]
- Wan, Y.; Camara, A.Y.; Huang, Q.; Yu, Y.; Wang, Q.; Li, H. Arsenic uptake and accumulation in rice (Oryza sativa L.) with selenite fertilization and water management. Ecotoxicol. Environ. Saf. 2018, 156, 67–74. [Google Scholar] [CrossRef]
- Shen, B.; Wang, X.; Zhang, Y.; Zhang, M.; Wang, K.; Xie, P.; Ji, H. The optimum pH and Eh for simultaneously minimizing bioavailable cadmium and arsenic contents in soils under the organic fertilizer application. Sci. Total Environ. 2020, 711, 135229. [Google Scholar] [CrossRef]
- Pokhrel, G.R.; Wang, K.T.; Zhuang, H.M.; Wu, Y.C.; Chen, W.; Lan, Y.; Zhu, X.; Li, Z.; Fu, F.F.; Yang, G.D. Effect of selenium in soil on the toxicity and uptake of arsenic in rice plant. Chemosphere 2020, 239, 124712. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wang, Y.; Lan, Y.; An, L.; Wang, G.; Li, M.; Zheng, S. Microbial oxidation of organic and elemental selenium to selenite. Sci. Total Environ. 2022, 833, 155203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yuan, L.; Qi, S.; Yin, X. The threshold effect between the soil bioavailable molar Se:Cd ratio and the accumulation of Cd in corn (Zea mays L.) from natural Se-Cd rich soils. Sci. Total Environ. 2019, 688, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dang, F.; Evans, R.D.; Zhong, H.; Zhao, J.; Zhou, D. Mechanistic understanding of MeHg-Se antagonism in soil-rice systems: The key role of antagonism in soil. Sci. Rep. 2016, 6, 19477. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Jin, Z.; Chen, G.; Tong, L. Transformation of Selenium-Containing Phases in Copper Anode Slimes During Leaching. Jom 2017, 69, 1932–1938. [Google Scholar] [CrossRef]
- Wan, Y.; Camara, A.Y.; Yu, Y.; Wang, Q.; Guo, T.; Zhu, L.; Li, H. Cadmium dynamics in soil pore water and uptake by rice: Influences of soil-applied selenite with different water managements. Environ. Pollut. 2018, 240, 523–533. [Google Scholar] [CrossRef]
- Huang, G.; Ding, C.; Guo, F.; Li, X.; Zhang, T.; Wang, X. Underlying mechanisms and effects of hydrated lime and selenium application on cadmium uptake by rice (Oryza sativa L.) seedlings. Environ. Sci. Pollut. Res. 2017, 24, 18926–18935. [Google Scholar] [CrossRef]
- Nie, M.; Wu, C.; Tang, Y.; Shi, G.; Wang, X.; Hu, C.; Cao, J.; Zhao, X. Selenium and Bacillus proteolyticus SES synergistically enhanced ryegrass to remediate Cu–Cd–Cr contaminated soil. Environ. Pollut. 2023, 323, 121272. [Google Scholar] [CrossRef]
- Ni, G.; Shi, G.; Hu, C.X.; Wang, X.; Nie, M.; Cai, M.; Cheng, Q.; Zhao, X.H. Selenium improved the combined remediation efficiency of Pseudomonas aeruginosa and ryegrass on cadmium-nonylphenol co-contaminated soil. Environ. Pollut. 2021, 287, 117552. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Ma, J.; Wu, F.; Wang, L.; Sun, L.; Zhang, P.; Wang, W.; Xu, J. Comparative physiological and soil microbial community structural analysis revealed that selenium alleviates cadmium stress in Perilla frutescens. Front. Plant Sci. 2022, 13, 1022935. [Google Scholar] [CrossRef]
- Hauptmann, A.L.; Johansen, J.; Stæger, F.F.; Nielsen, D.S.; Mulvad, G.; Hanghøj, K.; Rasmussen, S.; Hansen, T.; Albrechtsen, A. Gut heavy metal and antibiotic resistome of humans living in the high Arctic. Front. Microbiol. 2024, 15, 1493803. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.E.; James, B.R.; Santelli, C.M. Persistent bacterial and fungal community shifts exhibited in selenium-contaminated reclaimed mine soils. Appl. Environ. Microbiol. 2018, 84, e01394-18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, D.; Hu, C.; Du, X.; Liang, L.; Wang, X.; Shi, G.; Han, C.; Tang, Y.; Lei, Z.; et al. Bacteria from the rhizosphere of a selenium hyperaccumulator plant can improve the selenium uptake of a non-hyperaccumulator plant. Biol. Fertil. Soils 2024, 60, 987–1008. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.; Sun, W.; Chen, Q. Bioavailable metal(loid)s and physicochemical features co-mediating microbial communities at combined metal(loid) pollution sites. Chemosphere 2020, 260, 127619. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C.; Wu, Y.; An, Q.; Zhang, J.; Fang, Y.; Li, J.; Pan, C. Nanoselenium integrates soil-pepper plant homeostasis by recruiting rhizosphere-beneficial microbiomes and allocating signaling molecule levels under Cd stress. J. Hazard. Mater. 2022, 432, 128763. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Hunter, W.J.; Manter, D.K. Reduction of selenite to elemental red selenium by Pseudomonas sp. Strain CA5. Curr. Microbiol. 2009, 58, 493–498. [Google Scholar] [CrossRef]
- Hunter, W.J.; Kuykendall, L.D.; Manter, D.K. Rhizobium selenireducens sp. nov.: A selenite-reducing alpha-Proteobacteria isolated from a bioreactor. Curr. Microbiol. 2007, 55, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Khoei, N.S.; Lampis, S.; Zonaro, E.; Yrjälä, K.; Bernardi, P.; Vallini, G. Insights into selenite reduction and biogenesis of elemental selenium nanoparticles by two environmental isolates of Burkholderia fungorum. New Biotechnol. 2017, 34, 1–11. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, F.; Zhu, X.; Zheng, S.; Wang, R.; Wang, G. Draft genomic sequence of a selenite-reducing bacterium, Paenirhodobacter enshiensis DW2-9T. Stand. Genom. Sci. 2015, 10, 38. [Google Scholar] [CrossRef]
- Zheng, S.; Su, J.; Wang, L.; Yao, R.; Wang, D.; Deng, Y.; Wang, R.; Wang, G.; Rensing, C. Selenite reduction by the obligate aerobic bacterium Comamonas testosteroni S44 isolated from a metal-contaminated soil. BMC Microbiol. 2014, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.R.; Prajapati, S.; Das, J.; Dangar, T.K.; Das, N.; Thatoi, H. Reduction of selenite to red elemental selenium by moderately halotolerant Bacillus megaterium strains isolated from Bhitarkanika mangrove soil and characterization of reduced product. Chemosphere 2011, 84, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yao, R.; Wang, R.; Wang, D.; Wang, G.; Zheng, S. Reduction of selenite to Se(0) nanoparticles by filamentous bacterium Streptomyces sp. ES2-5 isolated from a selenium mining soil. Microb. Cell Factories 2016, 15, 157. [Google Scholar] [CrossRef]
- Eswayah Abdurrahman, S.; Smith Thomas, J.; Gardiner Philip, H.E. Microbial transformations of selenium species of relevance to bioremediation. Appl. Environ. Microbiol. 2016, 82, 4848–4859. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Luo, X.; Zhang, Q.; Duan, X.; Yuan, Y.; Zheng, S. Contributions of selenium-oxidizing bacteria to selenium biofortification and cadmium bioremediation in a native seleniferous Cd-polluted sandy loam soil. Ecotoxicol. Environ. Saf. 2024, 272, 116081. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Guo, C.; Lu, G.; Yi, X.; Zhu, L.; Dang, Z. Bioaccumulation characterization of cadmium by growing Bacillus cereus RC-1 and its mechanism. Chemosphere 2014, 109, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Nie, Z.; Tao, Z.; Peng, H.; Shi, H.; Zhao, P.; Liu, H. Combined application of arbuscular mycorrhizal fungi and selenium fertilizer increased wheat biomass under cadmium stress and shapes rhizosphere soil microbial communities. BMC Plant Biol. 2024, 24, 359. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, W.; Pang, F. Selenium in soil–plant-microbe: A review. Bull. Environ. Contam. Toxicol. 2022, 108, 167–181. [Google Scholar] [CrossRef]
- Sakr, E.A.E.; Khater, D.Z.; El-khatib, K.M. Electroactive Brevundimonas diminuta consortium mediated selenite bioreduction, biogenesis of selenium nanoparticles and bio-electricity generation. J. Nanobiotechnology 2024, 22, 352. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.; Tian, X. Nano-selenium modulates heavy metal transport and toxicity in soil-plant systems. J. Environ. Chem. Eng. 2025, 13, 118164. [Google Scholar] [CrossRef]
- Di, X.; Jing, R.; Qin, X.; Liang, X.; Wang, L.; Xu, Y.; Sun, Y.; Huang, Q. The role and transcriptomic mechanism of cell wall in the mutual antagonized effects between selenium nanoparticles and cadmium in wheat. J. Hazard. Mater. 2024, 472, 134549. [Google Scholar] [CrossRef]
- Nikam, P.B.; Salunkhe, J.D.; Minkina, T.; Rajput, V.D.; Kim, B.S.; Patil, S.V. A review on green synthesis and recent applications of red nano Selenium. Results Chem. 2022, 4, 100581. [Google Scholar] [CrossRef]
- Qu, L.; Xu, J.; Dai, Z.; Elyamine, A.M.; Huang, W.; Han, D.; Dang, B.; Xu, Z.; Jia, W. Selenium in soil-plant system: Transport, detoxification and bioremediation. J. Hazard. Mater. 2023, 452, 131272. [Google Scholar] [CrossRef]
- Tolu, J.; Bouchet, S.; Helfenstein, J.; Hausheer, O.; Chékifi, S.; Frossard, E.; Tamburini, F.; Chadwick, O.A.; Winkel, L.H.E. Understanding soil selenium accumulation and bioavailability through size resolved and elemental characterization of soil extracts. Nat. Commun. 2022, 13, 6974. [Google Scholar] [CrossRef]
- Fu, R.; Zhu, M.; Zhang, Y.; Li, J.; Feng, H. Harnessing the rhizosphere microbiome for selenium biofortification in plants: Mechanisms, applications and future perspectives. Microorganisms 2025, 13, 1234. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Zhou, C.; Zhao, L.; Wei, A.; Wang, Y.; Cui, H.; Zheng, S. Selenium-oxidizing Agrobacterium sp. T3F4 decreases arsenic uptake by Brassica rapa L. under a native polluted soil. J. Environ. Sci. 2024, 138, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Belimov, A.A.; Zinovkina, N.Y.; Safronova, V.I.; Litvinsky, V.A.; Nosikov, V.V.; Zavalin, A.A.; Tikhonovich, I.A. Rhizobial ACC deaminase contributes to efficient symbiosis with pea (Pisum sativum L.) under single and combined cadmium and water deficit stress. Environ. Exp. Bot. 2019, 167, 103859. [Google Scholar] [CrossRef]
- Guo, Q.; Xiao, Y.; Zhu, Y.; Korpelainen, H.; Li, C. Selenium availability in tea: Unraveling the role of microbiota assembly and functions. Sci. Total Environ. 2024, 952, 175995. [Google Scholar] [CrossRef]
- Szuba, A.; Karliński, L.; Krzesłowska, M.; Hazubska-Przybył, T. Inoculation with a Pb-tolerant strain of Paxillus involutus improves growth and Pb tolerance of Populus × canescens under in vitro conditions. Plant Soil 2017, 412, 253–266. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Lv, J.; Li, G.; Zhang, Z.; Zhang, J.; et al. Chromium immobilization by extra- and intraradical fungal structures of arbuscular mycorrhizal symbioses. J. Hazard. Mater. 2016, 316, 34–42. [Google Scholar] [CrossRef]
- Huang, G.; Ding, C.; Li, Y.; Zhang, T.; Wang, X. Selenium enhances iron plaque formation by elevating the radial oxygen loss of roots to reduce cadmium accumulation in rice (Oryza sativa L.). J. Hazard. Mater. 2020, 398, 122860. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, X.; Shen, H. Root iron plaque alleviates cadmium toxicity to rice (Oryza sativa) seedlings. Ecotoxicol. Environ. Saf. 2018, 161, 534–541. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, S.; Wan, N.; Feng, B.; Wang, Q.; Huang, K.; Fang, Y.; Bao, Z.; Xu, F. Iron plaque effects on selenium and cadmium stabilization in Cd-contaminated seleniferous rice seedlings. Environ. Sci. Pollut. Res. 2023, 30, 22772–22786. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y. Effect of iron plaque and selenium on mercury uptake and translocation in rice seedlings grown in solution culture. Environ. Sci. Pollut. Res. 2019, 26, 13795–13803. [Google Scholar] [CrossRef]
- Huang, G.; Ding, C.; Guo, F.; Zhang, T.; Wang, X. The optimum Se application time for reducing Cd uptake by rice (Oryza sativa L.) and its mechanism. Plant Soil 2018, 431, 231–243. [Google Scholar] [CrossRef]
- Feng, R.; Wang, L.; Yang, J.; Zhao, P.; Zhu, Y.; Li, Y.; Yu, Y.; Liu, H.; Rensing, C.; Wu, Z.; et al. Underlying mechanisms responsible for restriction of uptake and translocation of heavy metals (metalloids) by selenium via root application in plants. J. Hazard. Mater. 2021, 402, 123570. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Zhang, C.; Zhao, L.; Kong, L.; Wang, Q.; Li, H.; Wan, Y. Selenite and selenate showed contrasting impacts on the fate of arsenic in rice (Oryza sativa L.) regardless of the formation of iron plaque. Environ. Pollut. 2022, 312, 120039. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Yang, N.; Li, Y.; Liu, B.; Rensing, C.; Dai, J.; Fekih, I.B.; Wang, L.; Mazhar, S.H.; et al. Roles of root cell wall components and root plaques in regulating elemental uptake in rice subjected to selenite and different speciation of antimony. Environ. Exp. Bot. 2019, 163, 36–44. [Google Scholar] [CrossRef]
- Huang, L.; Li, W.C.; Tam, N.F.Y.; Ye, Z. Effects of root morphology and anatomy on cadmium uptake and translocation in rice (Oryza sativa L.). J. Environ. Sci. 2019, 75, 296–306. [Google Scholar] [CrossRef]
- Huang, B.; Xin, J.; Dai, H.; Liu, A.; Zhou, W.; Yi, Y.; Liao, K. Root morphological responses of three hot pepper cultivars to Cd exposure and their correlations with Cd accumulation. Environ. Sci. Pollut. Res. 2015, 22, 1151–1159. [Google Scholar] [CrossRef]
- Malheiros, R.S.P.; Costa, L.C.; Ávila, R.T.; Pimenta, T.M.; Teixeira, L.S.; Brito, F.A.L.; Zsögön, A.; Araújo, W.L.; Ribeiro, D.M. Selenium downregulates auxin and ethylene biosynthesis in rice seedlings to modify primary metabolism and root architecture. Planta 2019, 250, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, C.; Wang, X.; Qing, X.; Wang, P.; Zhang, Y.; Zhang, X.; Zhao, X. Selenium alleviated chromium stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis) by regulating root morphology and metal element uptake. Ecotoxicol. Environ. Saf. 2019, 173, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Feng, R.; Wang, R.; Guo, J.; Zheng, X. A dual effect of Se on Cd toxicity: Evidence from plant growth, root morphology and responses of the antioxidative systems of paddy rice. Plant Soil 2014, 375, 289–301. [Google Scholar] [CrossRef]
- Wang, L.; Wu, K.; Liu, Z.; Li, Z.; Shen, J.; Wu, Z.; Liu, H.; You, L.; Yang, G.; Rensing, C.; et al. Selenite reduced uptake/translocation of cadmium via regulation of assembles and interactions of pectins, hemicelluloses, lignins, callose and Casparian strips in rice roots. J. Hazard. Mater. 2023, 448, 130812. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhou, C.; Ma, J.; Wu, Y.; Kang, L.; An, Q.; Zhang, J.; Deng, K.; Li, J.; Pan, C. Nanoselenium transformation and inhibition of cadmium accumulation by regulating the lignin biosynthetic pathway and plant hormone signal transduction in pepper plants. J. Nanobiotechnology 2021, 19, 316. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lipton, A.S.; Munson, C.R.; Ma, Y.; Johnson, K.L.; Murray, D.T.; Scheller, H.V.; Mortimer, J.C. Elongated galactan side chains mediate cellulose-pectin interactions in engineered Arabidopsis secondary cell walls. Plant J. 2023, 115, 529–545. [Google Scholar] [CrossRef]
- Loix, C.; Huybrechts, M.; Vangronsveld, J.; Gielen, M.; Keunen, E.; Cuypers, A. Reciprocal Interactions between cadmium-induced cell wall responses and oxidative stress in plants. Front. Plant Sci. 2017, 8, 1867. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, Z. Mechanisms of cadmium phytoremediation and detoxification in plants. Crop J. 2021, 9, 521–529. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, C.; Tang, F.; Zhou, Y.; Zhu, C.; Ding, Y. The cell wall functions in plant heavy metal response. Ecotoxicol. Environ. Saf. 2025, 299, 118326. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Wu, Z.; Liu, X.; Cai, M.; Jia, W.; Zhao, X. Selenium reduces cadmium accumulation in seed by increasing cadmium retention in root of oilseed rape (Brassica napus L.). Environ. Exp. Bot. 2019, 158, 161–170. [Google Scholar] [CrossRef]
- Cui, J.; Liu, T.; Li, Y.; Li, F. Selenium reduces cadmium uptake into rice suspension cells by regulating the expression of lignin synthesis and cadmium-related genes. Sci. Total Environ. 2018, 644, 602–610. [Google Scholar] [CrossRef]
- Barman, F.; Guha, T.; Kundu, R. Exogenous selenium supplements reduce cadmium accumulation and restore micronutrient content in rice grains. J. Soil Sci. Plant Nutr. 2025, 25, 2275–2293. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Rizwan, M.; Dai, Z.; Yuan, Y.; Hossain, M.M.; Cao, M.; Xiong, S.; Tu, S. Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants. J. Hazard. Mater. 2021, 401, 123393. [Google Scholar] [CrossRef]
- Wang, C.; Rong, H.; Zhang, X.; Shi, W.; Hong, X.; Liu, W.; Cao, T.; Yu, X.; Yu, Q. Effects and mechanisms of foliar application of silicon and selenium composite sols on diminishing cadmium and lead translocation and affiliated physiological and biochemical responses in hybrid rice (Oryza sativa L.) exposed to cadmium and lead. Chemosphere 2020, 251, 126347. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, L.; Zhou, Y.; Huang, J.; Wu, F.; Hu, Q.; Chang, N.; Qiu, T.; Zeng, Y.; He, H.; et al. Exogenous selenium promotes cadmium reduction and selenium enrichment in rice: Evidence, mechanisms, and perspectives. J. Hazard. Mater. 2024, 476, 135043. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Du, B.; Cui, H.; Fan, X.; Zhou, D.; Zhou, J. Soil and foliar applications of silicon and selenium effects on cadmium accumulation and plant growth by modulation of antioxidant system and Cd translocation: Comparison of soft vs. durum wheat varieties. J. Hazard. Mater. 2021, 402, 123546. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Wu, S.; Rao, S.; Li, L.; Cheng, S.; Cheng, H. Morphological and physiological indicators and transcriptome analyses reveal the mechanism of selenium multilevel mitigation of cadmium damage in Brassica juncea. Plants 2023, 12, 1583. [Google Scholar] [CrossRef]
- Cheng, Z.; Wei, J.; Zhu, B.; Gu, L.; Zeng, T.; Wang, H.; Du, X. Mutation of TaNRAMP5 impacts cadmium transport in wheat. Plant Physiol. Biochem. 2025, 223, 109879. [Google Scholar] [CrossRef]
- Liu, J.; Lv, Y.; Li, M.; Wu, Y.; Li, B.; Wang, C.; Tao, Q. Peroxidase in plant defense: Novel insights for cadmium accumulation in rice (Oryza sativa L.). J. Hazard. Mater. 2024, 474, 134826. [Google Scholar] [CrossRef]
- Zheng, P.; Cao, L.; Zhang, C.; Pan, W.; Wang, W.; Yu, X.; Li, Y.; Fan, T.; Miao, M.; Tang, X.; et al. MYB43 as a novel substrate for CRL4(PRL1) E3 ligases negatively regulates cadmium tolerance through transcriptional inhibition of HMAs in Arabidopsis. New Phytol. 2022, 234, 884–901. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M.A.; Elyamine, A.M.; Zhao, Y.; Moussa, M.G.; Rana, M.S.; Afzal, J.; Imran, M.; Zhao, X.; Hu, C. Can Selenium and Molybdenum Restrain Cadmium Toxicity to Pollen Grains in Brassica napus? Int. J. Mol. Sci. 2018, 19, 2163. [Google Scholar] [CrossRef]
- Xia, R.; Zhou, J.; Cui, H.; Liang, J.; Liu, Q.; Zhou, J. Nodes play a major role in cadmium (Cd) storage and redistribution in low-Cd-accumulating rice (Oryza sativa L.) cultivars. Sci. Total Environ. 2023, 859, 160436. [Google Scholar] [CrossRef]
- Bhat, B.A.; Rather, M.A.; Bilal, T.; Nazir, R.; Qadir, R.U.; Mir, R.A. Plant hyperaccumulators: A state-of-the-art review on mechanism of heavy metal transport and sequestration. Front. Plant Sci. 2025, 16, 1631378. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Jia, W.; Dai, Z.; Xu, Z.; Cai, M.; Huang, W.; Han, D.; Dang, B.; Ma, X.; Gao, Y.; et al. Selenium and molybdenum synergistically alleviate chromium toxicity by modulating Cr uptake and subcellular distribution in Nicotiana tabacum L. Ecotoxicol. Environ. Saf. 2022, 248, 114312. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Yamaji, N.; Ma, J.F. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J. Exp. Bot. 2014, 65, 6013–6021. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cai, X.; Huang, Y.; Feng, H.; Cai, L.; Luo, W.; Liu, G.; Tang, Y.; Sirguey, C.; Morel, J.L.; et al. Root Zn sequestration transporter heavy metal ATPase 3 from Odontarrhena chalcidica enhance Cd tolerance and accumulation in Arabidopsis thaliana. J. Hazard. Mater. 2024, 480, 135827. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |