Pig Manure and Biochar Reduce Nitrogen Availability and Rice Yield Compared to Mineral Fertilization in a Three-Year Field Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Experimental Design
2.3. Plant Sampling and Analysis
2.4. Soil Sampling and Analysis
2.4.1. Analysis of Soil Properties
2.4.2. Method for Soil Gross N Turnover Rate
2.5. Data Processing and Statistical Analysis
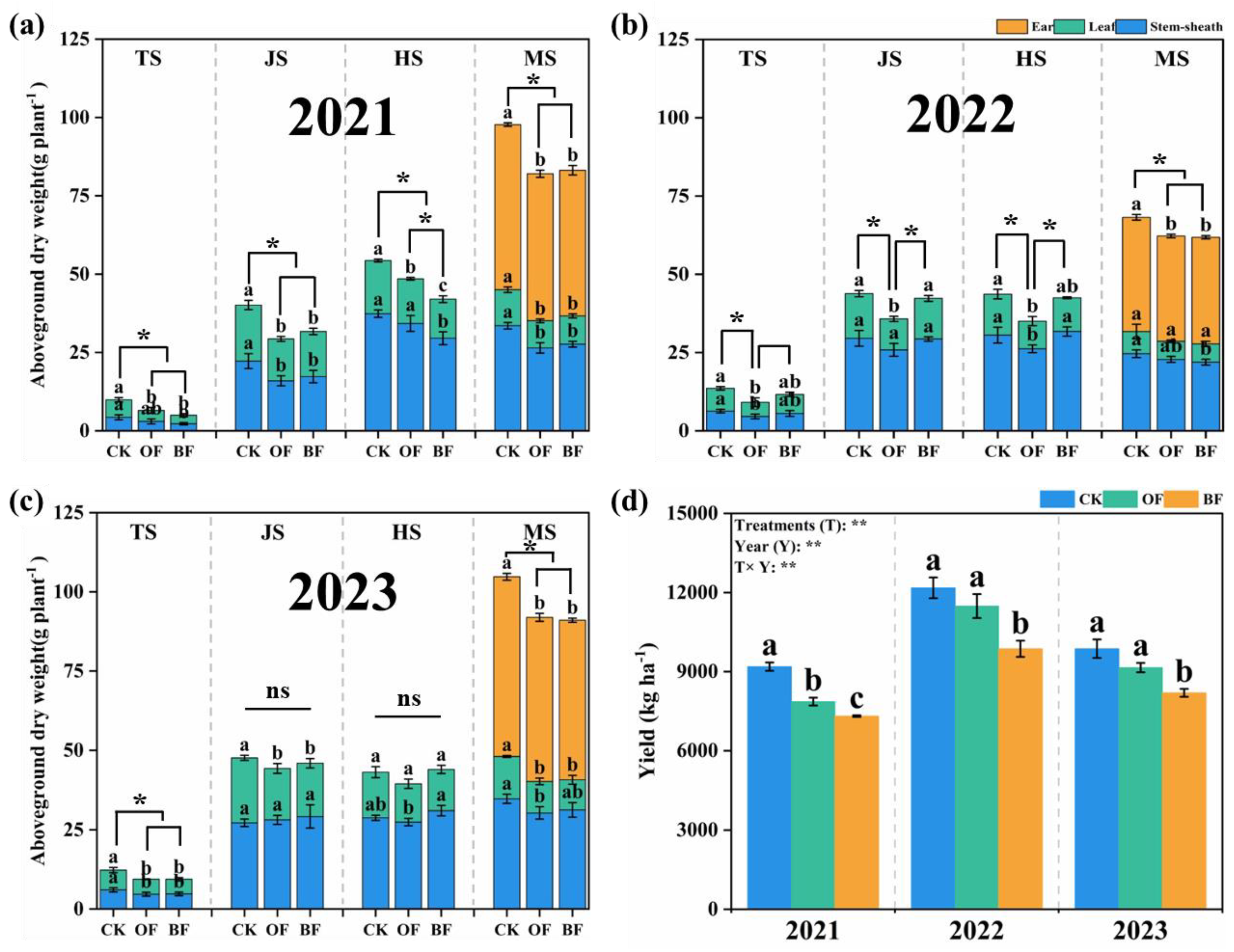
3. Results
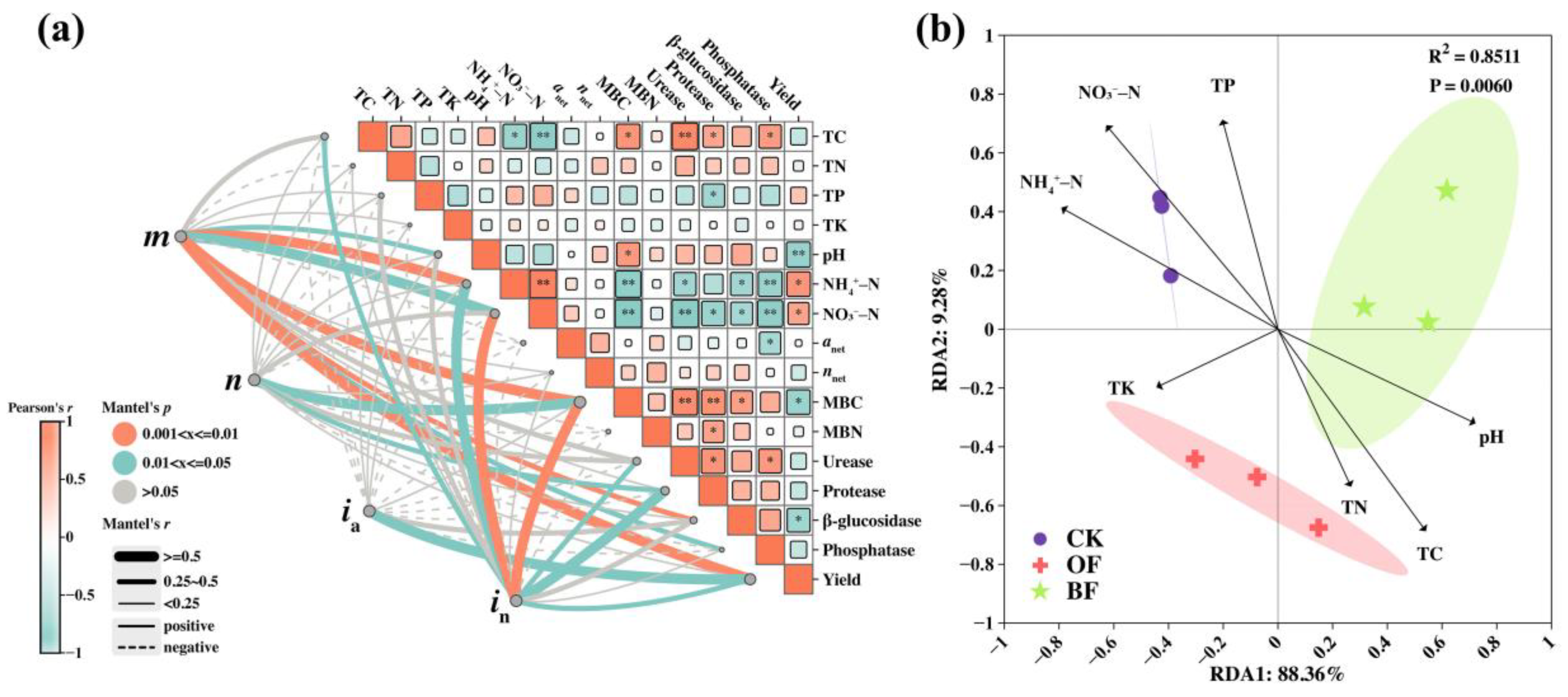
3.1. Soil Properties
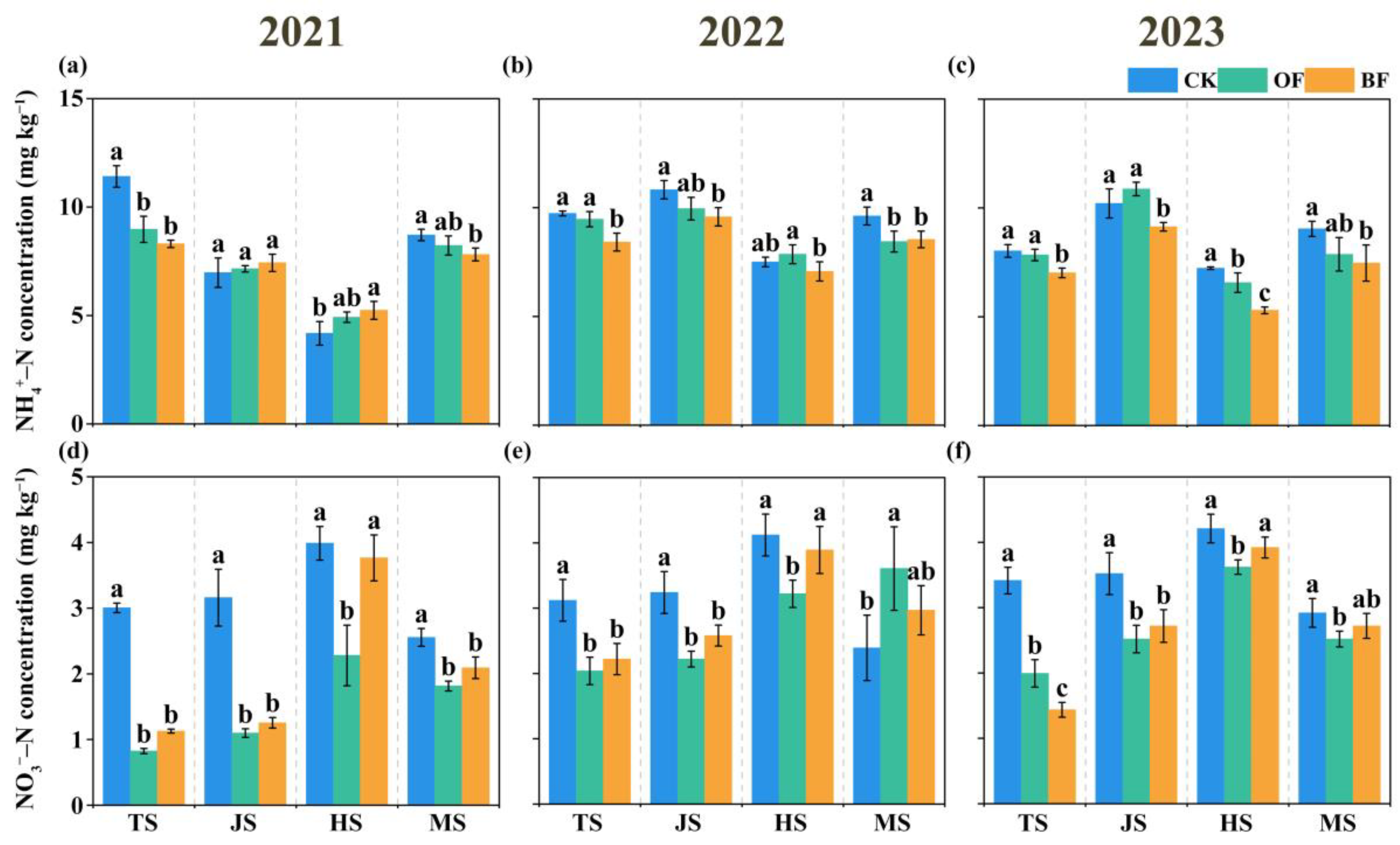
3.2. Soil Inorganic N Content
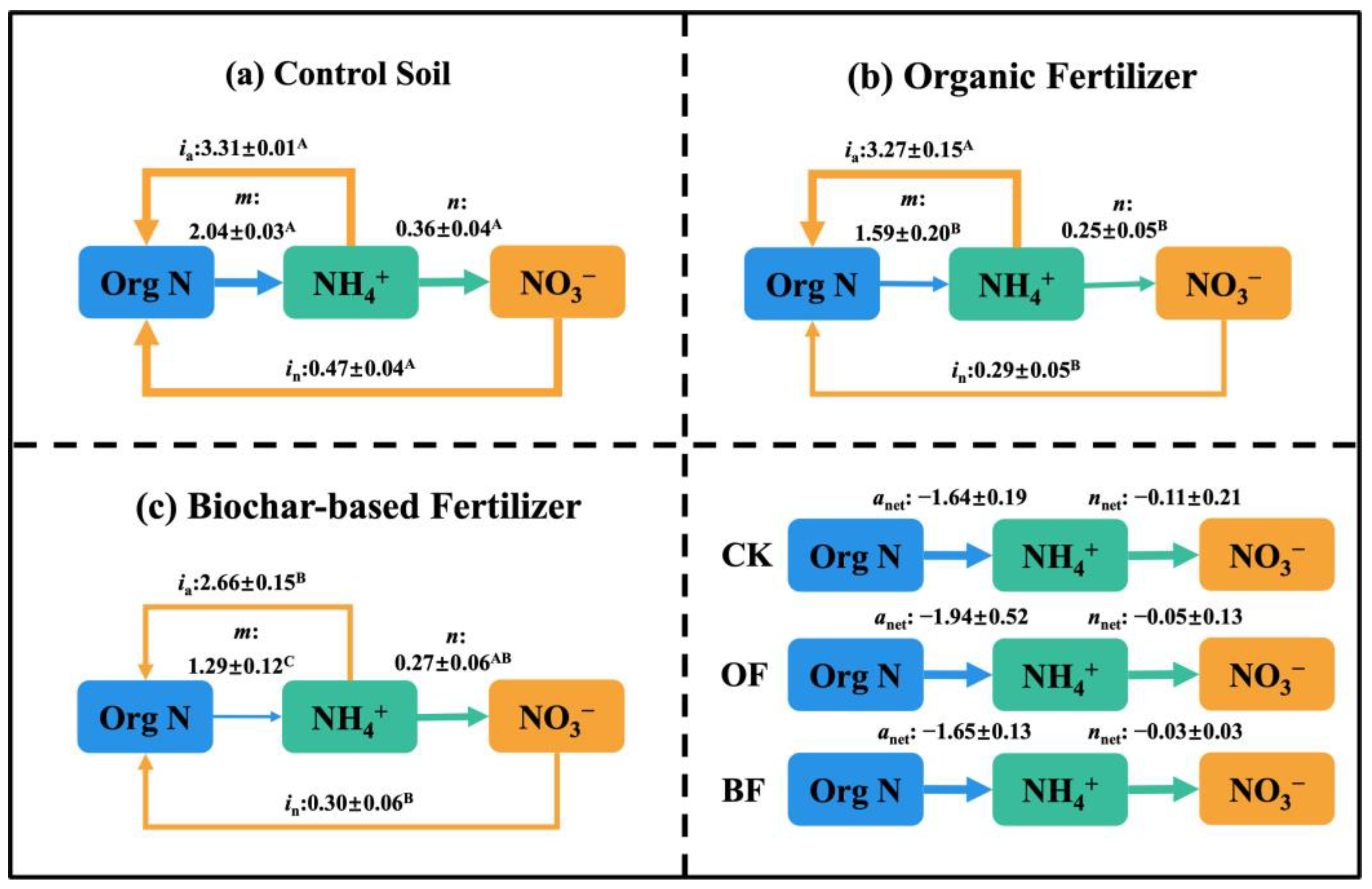
3.3. Effect of Different Fertilization on Soil N Transformation
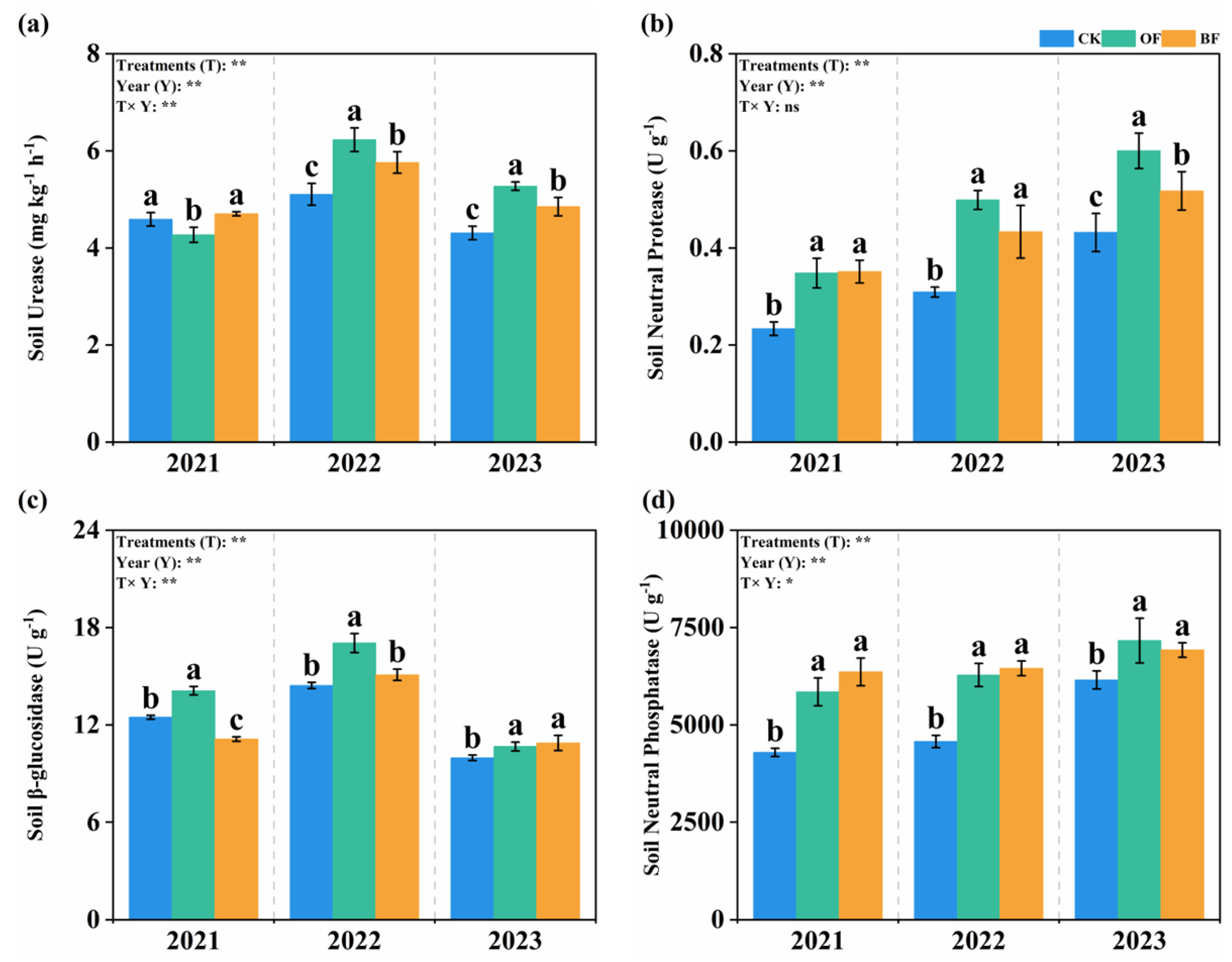
3.4. Effect of Different Fertilization on Soil Enzymatic Activity
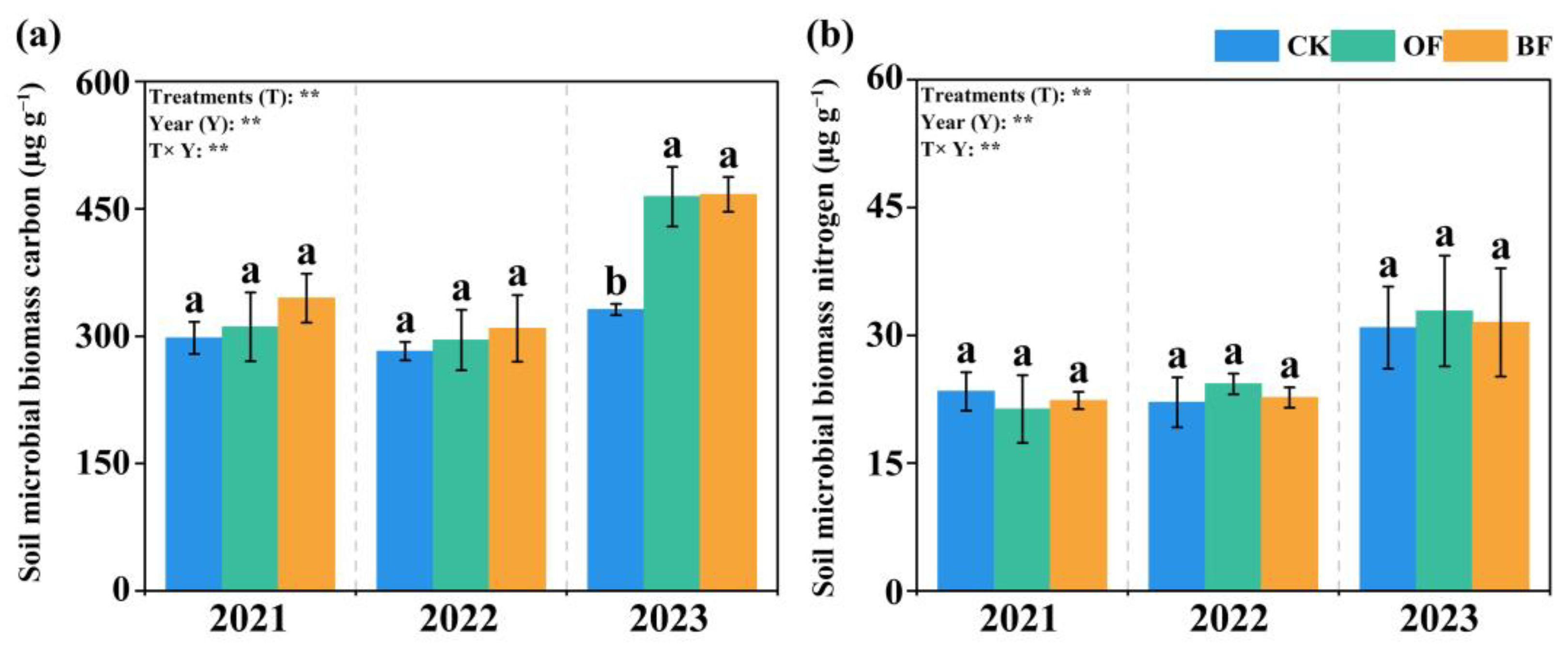
3.5. Soil Microbial Biomass Contents
3.6. Effect of Different Fertilization on Rice Growth
4. Discussion
4.1. Organic Substitution Decreased Soil Gross N Turnover Rate and N Availability
4.2. Decline in Nitrogen Content Directly Reduces Rice Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khoshnevisan, B.; Rafiee, S.; Pan, J.; Zhang, Y.; Liu, H. A multi-criteria evolutionary-based algorithm as a regional scale decision support system to optimize nitrogen consumption rate; a case study in North China plain. J. Clean. Prod. 2020, 256, 120213. [Google Scholar] [CrossRef]
- Ren, H.; Han, K.; Liu, Y.; Zhao, Y.; Zhang, L.; He, Q.; Li, Z.; Zhang, J.; Liu, P.; Wang, H.; et al. Improving smallholder farmers’ maize yields and economic benefits under sustainable crop intensification in the North China Plain. Sci. Total Environ. 2021, 763, 143035. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Huang, H.; Qian, Z.; Jiang, H.; Liu, G.; Xu, K.; Hu, Y.; Dai, Q.; Huo, Z. Effect of side deep placement of nitrogen on yield and nitrogen use efficiency of single season late japonica rice. J. Integr. Agr. 2021, 20, 1487–1502. [Google Scholar] [CrossRef]
- He, Z.; Ding, B.; Pei, S.; Cao, H.; Liang, J.; Li, Z. The impact of organic fertilizer replacement on greenhouse gas emissions and its influencing factors. Sci. Total Environ. 2023, 905, 166917. [Google Scholar] [CrossRef]
- Jin, N.; Jin, L.; Wang, S.; Li, J.; Liu, F.; Liu, Z.; Luo, S.; Wu, Y.; Lyu, J.; Yu, J. Reduced chemical fertilizer combined with bio-organic fertilizer affects the soil microbial community and yield and quality of lettuce. Front. Microbiol. 2022, 13, 863325. [Google Scholar] [CrossRef]
- Geisseler, D.; Linquist, B.A.; Lazicki, P.A. Effect of fertilization on soil microorganisms in paddy rice systems—A meta-analysis. Soil Biol. Biochem. 2017, 115, 452–460. [Google Scholar] [CrossRef]
- Hansen, S.; Berland Frøseth, R.; Stenberg, M.; Stalenga, J.; Olesen, J.E.; Krauss, M.; Radzikowski, P.; Doltra, J.; Nadeem, S.; Torp, T.; et al. Reviews and syntheses: Review of causes and sources of N2O emissions and NO3 leaching from organic arable crop rotations. Biogeosciences 2019, 16, 2795–2819. [Google Scholar] [CrossRef]
- Salam, M.A.; Sarker, M.N.I.; Sharmin, S. Do organic fertilizer impact on yield and efficiency of rice farms? Empirical evidence from Bangladesh. Heliyon 2021, 7, e07731. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, M.; Xing, Y.; Han, J.; Wang, X. Reduction of nitrogen fertilizer and simultaneously application of organic fertilizer optimizes yield, water productivity and nitrogen metabolism of spring maize by improving soil properties in the Loess Plateau of China. J. Agric. Food Res. 2025, 19, 101634. [Google Scholar] [CrossRef]
- Guan, G.; Tu, S.; Yang, J.; Zhang, J.; Yang, L. A field study on effects of nitrogen fertilization modes on nutrient uptake, crop yield and soil biological properties in rice-wheat rotation system. Agr. Sci. China 2011, 10, 1254–1261. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, H.; Jiang, Z.; Dai, Y.; Liu, G.; Chen, L.; Luo, X.; Liu, M.; Wang, Z. Efficacies of biochar and biochar-based amendment on vegetable yield and nitrogen utilization in four consecutive planting seasons. Sci. Total Environ. 2017, 593–594, 124–133. [Google Scholar] [CrossRef]
- Xia, F.; Zhang, Z.; Zhang, Q.; Huang, H.; Zhao, X. Life cycle assessment of greenhouse gas emissions for various feedstocks-based biochars as soil amendment. Sci. Total Environ. 2024, 911, 168734. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Y.; Chen, C.; Xiang, Y.; Rezaei Rashti, M.; Li, Y.; Deng, Q.; Zhang, R. Effects of biochar application on soil nitrogen transformation, microbial functional genes, enzyme activity, and plant nitrogen uptake: A meta-analysis of field studies. GCB Bioenergy 2021, 13, 1859–1873. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; Sun, Y.; Yang, S.; Qin, Q.; Xue, Y. Integrated enzyme activities and untargeted metabolome to reveal the mechanism that allow long-term biochar-based fertilizer substitution improves soil quality and maize yield. Environ. Res. 2025, 270, 120935. [Google Scholar] [CrossRef]
- Gu, W.; Wang, Y.; Sun, Y.; Liu, Z.; Wang, W.; Wu, D.; Zhang, Y.; Sun, W.; Wang, X.; Feng, Z.; et al. Assessing the formation and stability of paddy soil aggregate driven by organic carbon and Fe/Al oxides in rice straw cyclic utilization strategies: Insight from a six-year field trial. Sci. Total Environ. 2024, 951, 175607. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Wallace, H.M.; Xu, C.-Y.; Xu, Z.; Farrar, M.B.; Joseph, S.; Van Zwieten, L.; Bai, S.H. Short-term effects of organo-mineral biochar and organic fertilisers on nitrogen cycling, plant photosynthesis, and nitrogen use efficiency. J. Soil Sediment 2017, 17, 2763–2774. [Google Scholar] [CrossRef]
- Gelardi, D.L.; Lazicki, P.A.; Rath, D.; Leinfelder-Miles, M.M.; Scow, K.M.; Geisseler, D.J.; Parikh, S.J. Three-year field trials with seven biochars reveal minor changes in soil chemical properties but no impact on crop yield. Field Crop Res. 2025, 325, 109807. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: A meta-analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Craswell, E.T.; Chalk, P.M.; Kaudal, B.B. Role of 15N in tracing biologically driven nitrogen dynamics in soils amended with biochar: A review. Soil Biol. Biochem. 2021, 162, 108416. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Yang, J. Improving nitrogen use efficiency of rice crop through an optimized root system and agronomic practices. Crop Environ. 2023, 2, 192–201. [Google Scholar] [CrossRef]
- Barraclough, D. The use of mean pool abundances to interpret 15N tracer experiments: I. Theory. Plant Soil 1991, 131, 89–96. [Google Scholar] [CrossRef]
- Braun, J.; Mooshammer, M.; Wanek, W.; Prommer, J.; Walker, T.W.N.; Rütting, T.; Richter, A. Full 15N tracer accounting to revisit major assumptions of 15N isotope pool dilution approaches for gross nitrogen mineralization. Soil Biol. Biochem. 2018, 117, 16–26. [Google Scholar] [CrossRef]
- National Agricultural Technology Extension Service Center. Specification for Soil Analysis Techniques; China Agricultural Press: Beijing, China, 2006. [Google Scholar]
- Bao, S. Soil Agricultural Chemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Zhao, R.; Liu, J.; Xu, N.; He, T.; Meng, J.; Liu, Z. Urea hydrolysis in different farmland soils as affected by long-term biochar application. Front. Environ. Sci. 2022, 10, 950482. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, J.; Wen, T. Stable Isotope Tracing Technology and Mass Spectrometry Analysis: Application in Soil, Ecology and Environment Research; Science Press: Beijing, China, 2018. [Google Scholar]
- Zhang, P.; Wen, T.; Zhang, J.; Cai, Z. On improving the diffusion method for determination of δ15N–NH4+ and δ15N–NO3- in soil extracts. Acta Pedol. Sin. 2017, 54, 948–957. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, N.; Cao, T.; An, Z.; Yang, X.; He, T.; Yang, T.; Meng, J. Maize straw is more effective than maize straw biochar at improving soil N availability and gross N transformation rate. Eur. J. Soil Sci. 2023, 74, e13403. [Google Scholar] [CrossRef]
- Kirkham, D.; Bartholomew, W.V. Equations for following nutrient transformations in soil, utilizing tracer data. Soil Sci. Soc. Am. J. 1954, 18, 33–34. [Google Scholar] [CrossRef]
- Hart, S.C.; Nason, G.E.; Myrold, D.D.; Perry, D.A. Dynamics of gross nitrogen transformations in an old-growth forest: The carbon connection. Ecology 1994, 75, 880–891. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, P.; Chi, Y.; Wang, D.; Wang, P.; Liu, N.; Cai, D.; Wu, Z.; Zhong, N. Controlling the hydrolysis and loss of nitrogen fertilizer (urea) by using a nanocomposite favors plant growth. ChemSusChem 2017, 10, 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Nasraoui-Hajaji, A.; Gouia, H. Photosynthesis sensitivity to NH4+–N change with nitrogen fertilizer type. Plant Soil Environ. 2014, 60, 274–279. [Google Scholar] [CrossRef]
- Jiang, P.; Xie, X.; Huang, M.; Zhou, X.; Zhang, R.; Chen, J.; Wu, D.; Xia, B.; Xiong, H.; Xu, F.; et al. Characterizing N uptake and use efficiency in rice as influenced by environments. Plant Prod. Sci. 2016, 19, 96–104. [Google Scholar] [CrossRef]
- Atere, C.T.; Ge, T.; Zhu, Z.; Tong, C.; Jones, D.L.; Shibistova, O.; Guggenberger, G.; Wu, J. Rice rhizodeposition and carbon stabilisation in paddy soil are regulated via drying-rewetting cycles and nitrogen fertilisation. Biol. Fertil. Soils. 2017, 53, 407–417. [Google Scholar] [CrossRef]
- Jones, D.L.; Kemmitt, S.J.; Wright, D.; Cuttle, S.P.; Bol, R.; Edwards, A.C. Rapid intrinsic rates of amino acid biodegradation in soils are unaffected by agricultural management strategy. Soil Biol. Biochem. 2005, 37, 1267–1275. [Google Scholar] [CrossRef]
- Shaw, M.R.; Harte, J. Response of nitrogen cycling to simulated climate change: Differential responses along a subal pine ecotone. Global Change Biol. 2001, 7, 193–210. [Google Scholar] [CrossRef]
- Dai, S.; Wang, J.; Cheng, Y.; Zhang, J.; Cai, Z. Effects of long-term fertilization on soil gross N transformation rates and their implications. J. Integr. Agr. 2017, 16, 2863–2870. [Google Scholar] [CrossRef]
- Tan, C.; Du, Y.; Hu, X.; Li, X.; Wang, Y.; Yan, T.; Zhang, J.; Niu, W.; Gu, X.; Müller, C.; et al. Aerated irrigation improves soil gross nitrogen transformations in greenhouse tomato: Insights from a 15N-tracing study. Soil Till. Res. 2024, 242, 106140. [Google Scholar] [CrossRef]
- Yu, Q.; Ye, J.; Sun, W.; Lin, H.; Wang, Q.; Ma, J. Influences of organic material application on the physically separated soil organic carbon and nitrogen fractions in rice fields. J. Soil Sediment 2021, 21, 1079–1088. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, A.; Zhang, Y.; McLaughlin, N.; Zhang, S.; Chen, X.; Zheng, H.; Fan, R. Dynamics of microbial biomass, nitrogen mineralization and crop uptake in response to placement of maize residue returned to Chinese Mollisols over the maize growing season. Atmosphere 2021, 12, 1166. [Google Scholar] [CrossRef]
- Yuan, C.; Wei, K.; Wang, J.; Wang, Y.; Zhu, B. Effect of different proportions of mineral nitrogen fertilizer substitutions with organic manure on soil phosphorus fractions distribution in an Entisol profile. J. Soil Sci. Plant Nutr. 2024, 24, 3238–3248. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.; Tian, Q.; Yan, C.; Zhang, Y. Soil nitrogen dynamics and crop residues. A review. Agron. Sustain. Dev. 2014, 34, 429–442. [Google Scholar] [CrossRef]
- Kheirfam, H.; Sadeghi, S.H.; Homaee, M.; Zarei Darki, B. Quality improvement of an erosion-prone soil through microbial enrichment. Soil Till. Res. 2017, 165, 230–238. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Cheng, H.; Ren, L.; Jin, X.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Cao, A. Organic fertilizers activate soil enzyme activities and promote the recovery of soil beneficial microorganisms after dazomet fumigation. J. Environ. Manag. 2022, 309, 114666. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Y.; Wang, Y.; Rong, X.; Peng, J.; Fei, J.; Luo, G. Biochar amendments combined with organic fertilizer improve maize productivity and mitigate nutrient loss by regulating the C–N–P stoichiometry of soil, microbiome, and enzymes. Chemosphere 2023, 324, 138293. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, W.; Dai, X.; Schaeffer, S.; Yang, F.; Radosevich, M.; Xu, L.; Liu, X.; Sun, X. Responses of absolute and specific soil enzyme activities to long term additions of organic and mineral fertilizer. Sci. Total Environ. 2015, 536, 59–67. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, Z.; Abdalla, K.; Ge, T.; Wu, X.; Kuzyakov, Y.; Pausch, J. Microbial response to long-term fertilization of paddy soils: Apparent and real priming effects. Geoderma 2024, 445, 116884. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Liu, Z.; Chang, Q.; Wang, B.; Zhang, Y.; Bai, E. Intensified Aridity Hinders Soil Microbes From Improving Their Nitrogen Use Efficiency. Global Change Biol. 2025, 31, e70453. [Google Scholar] [CrossRef]
- Chen, W.; Meng, J.; Han, X.; Lan, Y.; Zhang, W. Past, present, and future of biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Xie, Z.; Xu, Y.; Liu, G.; Liu, Q.; Zhu, J.; Tu, C.; Amonette, J.E.; Cadisch, G.; Yong, J.W.H.; Hu, S. Impact of biochar application on nitrogen nutrition of rice, greenhouse-gas emissions and soil organic carbon dynamics in two paddy soils of China. Plant Soil. 2013, 370, 527–540. [Google Scholar] [CrossRef]
- Ghani Achakzai, A.; Buriro, A.H.; Gul, S.; Ziad, T.; Bano, A.; Ghori, S.A.; Ponya, Z.; Ismail, T. Evaluation of the effect of biochar-based organic fertilizer on the growth performance of fennel and cumin plants for three years. Environ. Pollut. Bioavail. 2022, 34, 374–384. [Google Scholar] [CrossRef]
- Wiedner, K.; Schimpf, C.; Polifka, S.; Glaser, B. Effect of biochar fertilizers on amino acid variability of Secale cereale and Lupinus angustifolius. Biochar 2019, 1, 187–201. [Google Scholar] [CrossRef]
- Zheng, Y.; Bolan, N.; Jenkins, S.N.; Mickan, B.S. Organic particles and high pH in food waste anaerobic digestate enhanced NH4+ adsorption on wood-derived biochar. Sci. Total Environ. 2024, 946, 174458. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Xu, C.-Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Li, H.; Zhang, A.; Rengel, Z. Long-term biochar application promotes rice productivity by regulating root dynamic development and reducing nitrogen leaching. GCB Bioenergy 2021, 13, 257–268. [Google Scholar] [CrossRef]
- He, L.; Shan, J.; Zhao, X.; Wang, S.; Yan, X. Variable responses of nitrification and denitrification in a paddy soil to long-term biochar amendment and short-term biochar addition. Chemosphere 2019, 234, 558–567. [Google Scholar] [CrossRef]
- Lan, X.; Shan, J.; Huang, Y.; Liu, X.; Lv, Z.; Ji, J.; Hou, H.; Xia, W.; Liu, Y. Effects of long-term manure substitution regimes on soil organic carbon composition in a red paddy soil of southern China. Soil Till. Res. 2022, 221, 105395. [Google Scholar] [CrossRef]
- Ren, F.; Zhang, X.; Liu, J.; Sun, N.; Sun, Z.; Wu, L.; Xu, M. A synthetic analysis of livestock manure substitution effects on organic carbon changes in China’s arable topsoil. CATENA 2018, 171, 1–10. [Google Scholar] [CrossRef]
- Wei, Z.; Hoffland, E.; Zhuang, M.; Hellegers, P.; Cui, Z. Organic inputs to reduce nitrogen export via leaching and runoff: A global meta-analysis. Environ. Pollut. 2021, 291, 118176. [Google Scholar] [CrossRef]
- Fageria, N.K. Yield physiology of rice. J. Plant Nutr. 2007, 30, 843–879. [Google Scholar] [CrossRef]
- Zhao, S.; Jang, S.; Lee, Y.K.; Kim, D.-G.; Jin, Z.; Koh, H.-J. Genetic basis of tiller dynamics of rice revealed by genome-wide association studies. Plants 2020, 9, 1695. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, G.; Hong, Y.; Ma, Y.; Guo, S.; Yan, P.; Mi, W. Biochar amendment was less effective for rice yield improvement but more effective for soil quality relative to inorganic fertilization in a low fertility paddy soil. J. Soil Sci. Plant Nutr. 2024, 24, 4918–4928. [Google Scholar] [CrossRef]

| TC (g kg−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | CEC (cmol kg−1) | pH | |
|---|---|---|---|---|---|---|
| Soil | 14.94 | 1.21 | 0.49 | 24.53 | 65.79 | 6.84 |
| Organic fertilizer | 74.69 | 10.54 | 7.62 | 28.48 | 66.97 | / |
| Biochar-based fertilizer | 83.85 | 9.69 | 7.10 | 28.78 | 83.80 | / |
| Year | Treatments | TC (g kg−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | pH |
|---|---|---|---|---|---|---|
| 2021 | CK | 15.06 ± 0.14 a | 1.28 ± 0.07 a | 0.51 ± 0.02 a | 24.87 ± 0.47 a | 6.83 ± 0.02 a |
| OF | 14.48 ± 0.08 b | 1.24 ± 0.07 a | 0.49 ± 0.02 a | 24.02 ± 0.45 a | 6.88 ± 0.03 a | |
| BF | 14.65 ± 0.14 b | 1.27 ± 0.04 a | 0.49 ± 0.03 a | 23.98 ± 0.48 a | 6.85 ± 0.01 a | |
| 2022 | CK | 14.88 ± 0.19 b | 1.28 ± 0.04 a | 0.53 ± 0.02 a | 24.65 ± 0.46 a | 6.86 ± 0.02 a |
| OF | 15.37 ± 0.20 a | 1.27 ± 0.01 a | 0.51 ± 0.03 a | 24.17 ± 0.49 a | 6.84 ± 0.02 a | |
| BF | 14.75 ± 0.17 b | 1.30 ± 0.03 a | 0.53 ± 0.02 a | 24.14 ± 0.53 a | 6.84 ± 0.02 a | |
| 2023 | CK | 14.65 ± 0.21 b | 1.30 ± 0.04 a | 0.53 ± 0.02 a | 24.68 ± 0.46 a | 6.80 ± 0.03 a |
| OF | 15.34 ± 0.18 a | 1.34 ± 0.03 a | 0.50 ± 0.01 a | 24.64 ± 0.39 a | 6.84 ± 0.03 a | |
| BF | 15.12 ± 0.18 a | 1.31 ± 0.04 a | 0.51 ± 0.01 a | 24.47 ± 0.31 a | 6.86 ± 0.04 a | |
| Source of variation | ||||||
| Treatment (T) | ** | ns | ns | * | ns | |
| Year (Y) | ** | ns | ns | ns | ns | |
| T × Y | * | ns | ns | ns | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, M.; Pan, M.; Yuan, H.; Sun, S.; Sun, Q.; He, T.; Meng, J.; Liu, Z.; Chen, W. Pig Manure and Biochar Reduce Nitrogen Availability and Rice Yield Compared to Mineral Fertilization in a Three-Year Field Experiment. Agronomy 2025, 15, 2242. https://doi.org/10.3390/agronomy15092242
Liu J, Zhang M, Pan M, Yuan H, Sun S, Sun Q, He T, Meng J, Liu Z, Chen W. Pig Manure and Biochar Reduce Nitrogen Availability and Rice Yield Compared to Mineral Fertilization in a Three-Year Field Experiment. Agronomy. 2025; 15(9):2242. https://doi.org/10.3390/agronomy15092242
Chicago/Turabian StyleLiu, Juying, Meiqi Zhang, Mingxia Pan, Hechong Yuan, Siwen Sun, Qiang Sun, Tianyi He, Jun Meng, Zunqi Liu, and Wenfu Chen. 2025. "Pig Manure and Biochar Reduce Nitrogen Availability and Rice Yield Compared to Mineral Fertilization in a Three-Year Field Experiment" Agronomy 15, no. 9: 2242. https://doi.org/10.3390/agronomy15092242
APA StyleLiu, J., Zhang, M., Pan, M., Yuan, H., Sun, S., Sun, Q., He, T., Meng, J., Liu, Z., & Chen, W. (2025). Pig Manure and Biochar Reduce Nitrogen Availability and Rice Yield Compared to Mineral Fertilization in a Three-Year Field Experiment. Agronomy, 15(9), 2242. https://doi.org/10.3390/agronomy15092242








