Towards Site-Specific Management: UAV- and Ground-Based Assessment of Intra-Field Variability in SHD Almond Orchards †
Abstract
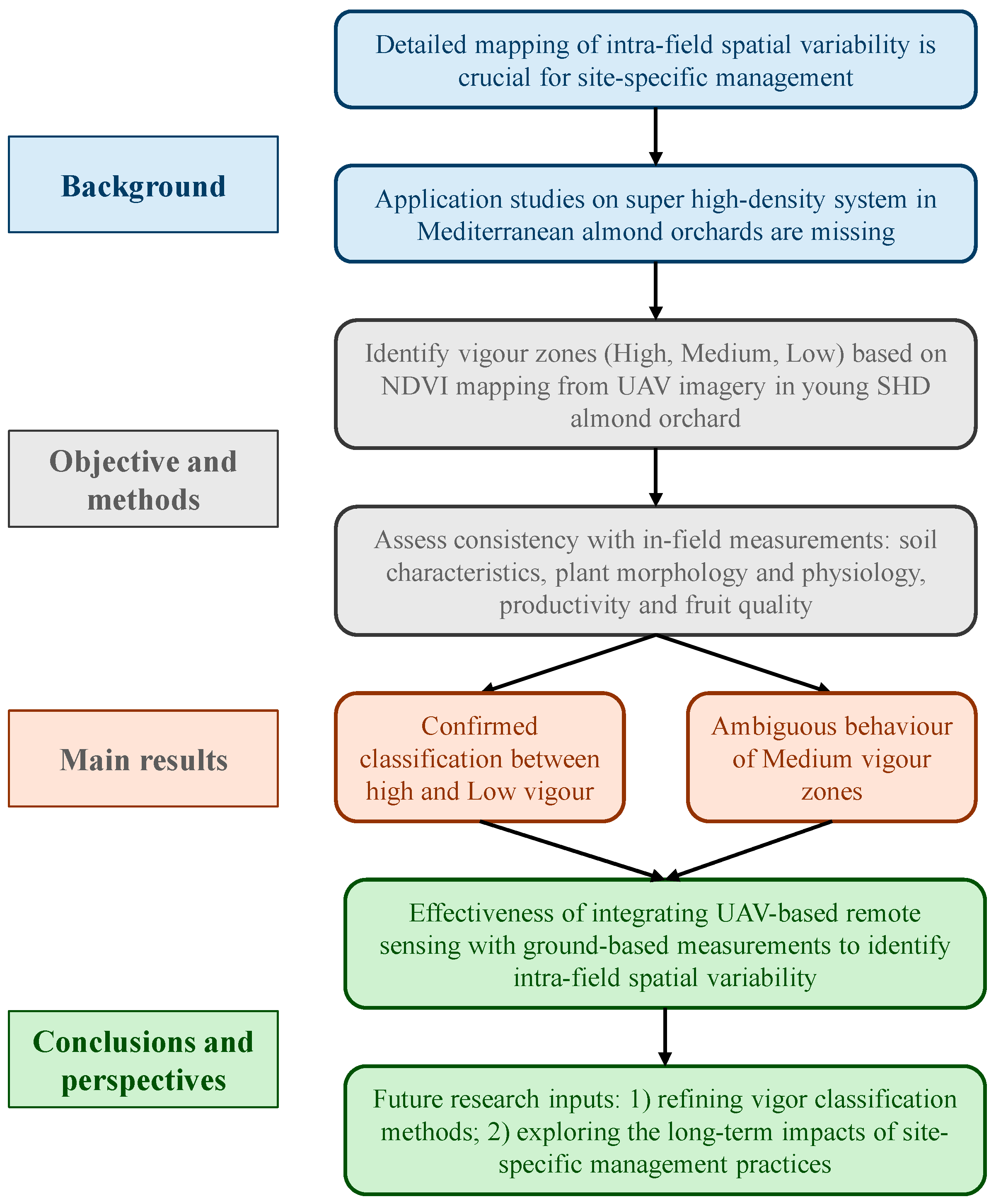
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. UAV Setup
2.3. Image Processing and Index Calculations
2.4. Aerial Survey Procedure
- Low Vigor Area: Exhibited a moderately lower canopy volume and NDVI than the rest of the field.
- Medium Vigor Area: Showed a canopy volume and NDVI consistent with the majority of the field.
- High Vigor Area: Exhibited moderately a higher canopy volume and NDVI than the rest of the field.
2.5. Soil Analysis, Plant Based Assessments, and Yield Analysis
2.6. Validation and Statistical Analysis
3. Results
3.1. Meteorological Conditions
3.2. NDVI-Based Vigor and Canopy Assessment
3.3. Soil Characteristics
3.4. Physiological Parameters
3.5. Yield Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sottile, F.; Massaglia, S.; Peano, C. Ecological and Economic Indicators for the Evaluation of Almond (Prunus dulcis L.) Orchard Renewal in Sicily. Agriculture 2020, 10, 301. [Google Scholar] [CrossRef]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, İ.; et al. Almonds (Prunus dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef]
- Petersen, B.; Snapp, S. What Is Sustainable Intensification? Views from Experts. Land Use Policy 2015, 46, 1–10. [Google Scholar] [CrossRef]
- Connor, D.J.; Gómez-del-Campo, M.; Rousseaux, M.C.; Searles, P.S. Structure, Management and Productivity of Hedgerow Olive Orchards: A Review. Sci. Hortic. 2014, 169, 71–93. [Google Scholar] [CrossRef]
- Barbagallo, M.G.; Vesco, G.; Di Lorenzo, R.; Lo Bianco, R.; Pisciotta, A. Soil and Regulated Deficit Irrigation Affect Growth, Yield and Quality of ‘Nero d’Avola’ Grapes in a Semi-Arid Environment. Plants 2021, 10, 641. [Google Scholar] [CrossRef]
- Maldera, F.; Vivaldi, G.A.; Iglesias-Castellarnau, I.; Camposeo, S. Row Orientation and Canopy Position Affect Bud Differentiation, Leaf Area Index and Some Agronomical Traits of a Super High-Density Almond Orchard. Agronomy 2021, 11, 251. [Google Scholar] [CrossRef]
- Trentacoste, E.R.; Connor, D.J.; Gómez-del-Campo, M. Row Orientation: Applications to Productivity and Design of Hedgerows in Horticultural and Olive Orchards. Sci. Hortic. 2015, 187, 15–29. [Google Scholar] [CrossRef]
- Pica, A.L.; Silvestri, C.; Cristofori, V. Evaluation of Phenological and Agronomical Traits of Different Almond Grafting Combinations under Testing in Central Italy. Agriculture 2021, 11, 1252. [Google Scholar] [CrossRef]
- Sperling, O.; Karunakaran, R.; Yermiyahu, U. Precise Fertilization by a Mass-Balance of the Seasonal Changes in Nutrient Uptake by Almond Trees. Agronomy 2020, 10, 1277. [Google Scholar] [CrossRef]
- Fernandes de Oliveira, A.; Mameli, M.G.; Lo Cascio, M.; Sirca, C.; Satta, D. An Index for User-Friendly Proximal Detection of Water Requirements to Optimized Irrigation Management in Vineyards. Agronomy 2021, 11, 323. [Google Scholar] [CrossRef]
- Cocco, M.; Mercenaro, L.; Lo Cascio, M.; Nieddu, G. Effects of Vine Water Status and Exogenous Abscisic Acid on Berry Composition of Three Red Wine Grapes Grown under Mediterranean Climate. Horticulturae 2020, 6, 12. [Google Scholar] [CrossRef]
- Ferrise, R.; Moriondo, M.; Trombi, G.; Miglietta, F.; Bindi, M. Climate Change Impacts on Typical Mediterranean Crops and Evaluation of Adaptation Strategies to Cope With. In Regional Assessment of Climate Change in the Mediterranean; Springer: Dordrecht, The Netherlands, 2013; pp. 49–70. [Google Scholar]
- del Pozo, A.; Brunel-Saldias, N.; Engler, A.; Ortega-Farias, S.; Acevedo-Opazo, C.; Lobos, G.A.; Jara-Rojas, R.; Molina-Montenegro, M.A. Climate Change Impacts and Adaptation Strategies of Agriculture in Mediterranean-Climate Regions (MCRs). Sustainability 2019, 11, 2769. [Google Scholar] [CrossRef]
- Freitas, T.R.; Santos, J.A.; Silva, A.P.; Fraga, H. Reviewing the Adverse Climate Change Impacts and Adaptation Measures on Almond Trees (Prunus dulcis). Agriculture 2023, 13, 1423. [Google Scholar] [CrossRef]
- Fernandes de Oliveira, A.; Mameli, M.G.; De Pau, L.; Satta, D. Almond Tree Adaptation to Water Stress: Differences in Physiological Performance and Yield Responses among Four Cultivar Grown in Mediterranean Environment. Plants 2023, 12, 1131. [Google Scholar] [CrossRef]
- Fridman, E. Consequences of Hybridization and Heterozygosity on Plant Vigor and Phenotypic Stability. Plant Sci. 2015, 232, 35–40. [Google Scholar] [CrossRef]
- Dobbertin, M. Tree Growth as Indicator of Tree Vitality and of Tree Reaction to Environmental Stress: A Review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Dhakal Poudel, P.; Cowan, M.; Shaw, L.; De Faveri, J.; Topp, B.; Alam, M. Macadamia Breeding for Reduced Plant Vigor: Progress and Prospects for Profitable and Sustainable Orchard Systems. Sustainability 2023, 15, 14506. [Google Scholar] [CrossRef]
- Musacchi, S.; Iglesias, I.; Neri, D. Training Systems and Sustainable Orchard Management for European Pear (Pyrus communis L.) in the Mediterranean Area: A Review. Agronomy 2021, 11, 1765. [Google Scholar] [CrossRef]
- Prey, L.; von Bloh, M.; Schmidhalter, U. Evaluating RGB Imaging and Multispectral Active and Hyperspectral Passive Sensing for Assessing Early Plant Vigor in Winter Wheat. Sensors 2018, 18, 2931. [Google Scholar] [CrossRef]
- Xue, J.; Fan, Y.; Su, B.; Fuentes, S. Assessment of Canopy Vigor Information from Kiwifruit Plants Based on a Digital Surface Model from Unmanned Aerial Vehicle Imagery. Int. J. Agric. Biol. Eng. 2019, 12, 165–171. [Google Scholar] [CrossRef]
- Van de Peer, T.; Mereu, S.; Verheyen, K.; María Costa Saura, J.; Morillas, L.; Roales, J.; Lo Cascio, M.; Spano, D.; Paquette, A.; Muys, B. Tree Seedling Vitality Improves with Functional Diversity in a Mediterranean Common Garden Experiment. For. Ecol. Manag. 2018, 409, 614–633. [Google Scholar] [CrossRef]
- Taylor, J.A. Precision Agriculture. In Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 710–725. [Google Scholar]
- Prgomet, I.; Pascual-Seva, N.; Morais, M.C.; Aires, A.; Barreales, D.; Castro Ribeiro, A.; Silva, A.P.; Barros, A.I.R.N.A.; Gonçalves, B. Physiological and Biochemical Performance of Almond Trees under Deficit Irrigation. Sci. Hortic. 2020, 261, 108990. [Google Scholar] [CrossRef]
- Bakhtiari, E.S.; Mousavi, A.; Yadegari, M.; Haghighati, B.; Martínez-García, P.J. Physiological and Biochemical Responses of Almond (Prunus dulcis) Cultivars to Drought Stress in Semi-Arid Conditions in Iran. Plants 2025, 14, 734. [Google Scholar] [CrossRef]
- Candiago, S.; Remondino, F.; De Giglio, M.; Dubbini, M.; Gattelli, M. Evaluating Multispectral Images and Vegetation Indices for Precision Farming Applications from UAV Images. Remote Sens. 2015, 7, 4026–4047. [Google Scholar] [CrossRef]
- Maes, W.H.; Steppe, K. Perspectives for Remote Sensing with Unmanned Aerial Vehicles in Precision Agriculture. Trends Plant Sci. 2019, 24, 152–164. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Ju, C.; Son, H.I. Unmanned Aerial Vehicles in Agriculture: A Review of Perspective of Platform, Control, and Applications. IEEE Access 2019, 7, 105100–105115. [Google Scholar] [CrossRef]
- Barbedo, J.G.A. A Review on the Use of Unmanned Aerial Vehicles and Imaging Sensors for Monitoring and Assessing Plant Stresses. Drones 2019, 3, 40. [Google Scholar] [CrossRef]
- Guimarães, N.; Sousa, J.J.; Pádua, L.; Bento, A.; Couto, P. Remote Sensing Applications in Almond Orchards: A Comprehensive Systematic Review of Current Insights, Research Gaps, and Future Prospects. Appl. Sci. 2024, 14, 1749. [Google Scholar] [CrossRef]
- de Castro, A.I.; Shi, Y.; Maja, J.M.; Peña, J.M. UAVs for Vegetation Monitoring: Overview and Recent Scientific Contributions. Remote Sens. 2021, 13, 2139. [Google Scholar] [CrossRef]
- Matese, A.; Di Gennaro, S.F. Beyond the Traditional NDVI Index as a Key Factor to Mainstream the Use of UAV in Precision Viticulture. Sci. Rep. 2021, 11, 2721. [Google Scholar] [CrossRef]
- Noun, G.; Lo Cascio, M.; Spano, D.; Marras, S.; Sirca, C. Plant-Based Methodologies and Approaches for Estimating Plant Water Status of Mediterranean Tree Species: A Semi-Systematic Review. Agronomy 2022, 12, 2127. [Google Scholar] [CrossRef]
- Squeri, C.; Diti, I.; Rodschinka, I.P.; Poni, S.; Dosso, P.; Scotti, C.; Gatti, M. The High-Yielding Lambrusco (Vitis vinifera L.) Grapevine District Can Benefit from Precision Viticulture. Am. J. Enol. Vitic. 2021, 72, 267–278. [Google Scholar] [CrossRef]
- Pinter, P.J.; Hatfield, J.L.; Schepers, J.S.; Barnes, E.M.; Moran, M.S.; Daughtry, C.S.T.; Upchurch, D.R. Remote Sensing for Crop Management; American Society for Photogrammetry and Remote Sensing: Baton Rouge, LA, USA, 2003. [Google Scholar]
- González-Gómez, L.; Intrigliolo, D.S.; Rubio-Asensio, J.S.; Buesa, I.; Ramírez-Cuesta, J.M. Assessing Almond Response to Irrigation and Soil Management Practices Using Vegetation Indexes Time-Series and Plant Water Status Measurements. Agric. Ecosyst. Environ. 2022, 339, 108124. [Google Scholar] [CrossRef]
- Deidda, A.; Sassu, A.; Mercenaro, L.; Nieddu, G.; Fadda, C.; Deiana, P.F.; Gambella, F. A decision-supporting system for vineyard management: A multi-temporal approach with remote and proximal sensing. Prec. Agric. 2024, 25, 3001–3032. [Google Scholar] [CrossRef]
- Guimarães, N.; Fraga, H.; Sousa, J.J.; Pádua, L.; Bento, A.; Couto, P. Comparative Evaluation of Remote Sensing Platforms for Almond Yield Prediction. AgriEngineering 2024, 6, 240–258. [Google Scholar] [CrossRef]
- Espinel, R.; Herrera-Franco, G.; Rivadeneira García, J.L.; Escandón-Panchana, P. Artificial Intelligence in Agricultural Mapping: A Review. Agriculture 2024, 14, 1071. [Google Scholar] [CrossRef]
- Lucarini, A.; Lo Cascio, M.; Marras, S.; Sirca, C.; Spano, D. Artificial Intelligence and Eddy Covariance: A Review. Sci. Total Environ. 2024, 950, 175406. [Google Scholar] [CrossRef]
- Peddinti, S.R.; Kisekka, I. Advanced Monitoring of Almond Orchard Water Status Using Machine Learning and Remote Sensing. Sci. Hortic. 2025, 342, 114020. [Google Scholar] [CrossRef]
- Carella, A.; Bulacio Fischer, P.T.; Massenti, R.; Lo Bianco, R. Continuous Plant-Based and Remote Sensing for Determination of Fruit Tree Water Status. Horticulturae 2024, 10, 516. [Google Scholar] [CrossRef]
- Canu, S.; Rosati, L.; Fiori, M.; Motroni, A.; Filigheddu, R.; Farris, E. Bioclimate Map of Sardinia (Italy). J. Maps 2015, 11, 711–718. [Google Scholar] [CrossRef]
- Sakar, E.H.; El Yamani, M.; Boussakouran, A.; Rharrabti, Y. Codification and Description of Almond (Prunus dulcis) Vegetative and Reproductive Phenology According to the Extended BBCH Scale. Sci. Hortic. 2019, 247, 224–234. [Google Scholar] [CrossRef]
- Ghiani, L.; Sassu, A.; Lozano, V.; Brundu, G.; Piccirilli, D.; Gambella, F. Use of UAVs and Canopy Height Model Applied on a Time Scale in the Vineyard. In Innovative Biosystems Engineering for Sustainable Agriculture, Forestry and Food Production; Springer: Cham, Switzerland, 2020; pp. 837–844. [Google Scholar]
- Takeshige, R.; Htoo, K.K.; Onishi, M.; Rahman, F.M.; Hoshizaki, K.; Ida, H.; Ishihara, M.I.; Itoh, A.; Kaneko, T.; Katayama, A.; et al. High-resolution Digital Canopy Height Models, Terrain Models, Ortho-mosaic Photos, and Canopy Tree Crown Shapes Derived from UAV-borne LiDAR at 22 Tree Census Plots across Japanese Natural Forests. Ecol. Res. 2025, 40, 657–670. [Google Scholar] [CrossRef]
- Sassu, A.; Ghiani, L.; Salvati, L.; Mercenaro, L.; Deidda, A.; Gambella, F. Integrating Uavs and Canopy Height Models in Vineyard Management: A Time-Space Approach. Remote Sens. 2022, 14, 130. [Google Scholar] [CrossRef]
- Ministry of Agricultural and Forestry Policies. Official methods of chemical analysis of soils. In Supplemento Ordinario alla "Gazzetta Ufficiale,, n. 248 del 21 Ottobre 1999—Serie Generale; Gazzetta Ufficiale della Repubblica Italiana, 1999; Available online: https://www.gazzettaufficiale.it/eli/gu/1999/10/21/248/so/185/sg/pdf (accessed on 19 September 2025).
- Manzano, R.; Diquattro, S.; Roggero, P.P.; Pinna, M.V.; Garau, G.; Castaldi, P. Addition of Softwood Biochar to Contaminated Soils Decreases the Mobility, Leachability and Bioaccesibility of Potentially Toxic Elements. Sci. Total Environ. 2020, 739, 139946. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; ISBN 3-900051-07-0. Available online: http://www.r-project.org/ (accessed on 1 December 2023).
- Tang, M.; Sadowski, D.L.; Peng, C.; Vougioukas, S.G.; Klever, B.; Khalsa, S.D.S.; Brown, P.H.; Jin, Y. Tree-Level Almond Yield Estimation from High Resolution Aerial Imagery with Convolutional Neural Network. Front. Plant Sci. 2023, 14, 1070699. [Google Scholar] [CrossRef]
- Bellvert, J.; Adeline, K.; Baram, S.; Pierce, L.; Sanden, B.; Smart, D. Monitoring Crop Evapotranspiration and Crop Coefficients over an Almond and Pistachio Orchard Throughout Remote Sensing. Remote Sens. 2018, 10, 2001. [Google Scholar] [CrossRef]
- Vanella, D.; Peddinti, S.R.; Kisekka, I. Unravelling Soil Water Dynamics in Almond Orchards Characterized by Soil-Heterogeneity Using Electrical Resistivity Tomography. Agric. Water Manag. 2022, 269, 107652. [Google Scholar] [CrossRef]
- Sapkota, A.; Roby, M.; Peddinti, S.R.; Fulton, A.; Kisekka, I. Comparative Analysis of Evapotranspiration (ET), Crop Water Stress Index (CWSI), and Normalized Difference Vegetation Index (NDVI) to Delineate Site-Specific Irrigation Management Zones in Almond Orchards. Sci. Hortic. 2025, 339, 113860. [Google Scholar] [CrossRef]
- Sandonís-Pozo, L.; Oger, B.; Tisseyre, B.; Llorens, J.; Escolà, A.; Pascual, M.; Martínez-Casasnovas, J.A. Leafiness-LiDAR Index and NDVI for Identification of Temporal Patterns in Super-Intensive Almond Orchards as Response to Different Management Strategies. Eur. J. Agron. 2024, 159, 127278. [Google Scholar] [CrossRef]
- Casanova-Gascón, J.; Figueras-Panillo, M.; Iglesias-Castellarnau, I.; Martín-Ramos, P. Comparison of SHD and Open-Center Training Systems in Almond Tree Orchards Cv. ‘Soleta’. Agronomy 2019, 9, 874. [Google Scholar] [CrossRef]
- Ferro, M.V.; Catania, P.; Miccichè, D.; Pisciotta, A.; Vallone, M.; Orlando, S. Assessment of Vineyard Vigour and Yield Spatio-Temporal Variability Based on UAV High Resolution Multispectral Images. Biosyst. Eng. 2023, 231, 36–56. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. Nature and Properties of Soils, 15th ed.; Pearson Education: London, UK, 2017; ISBN 978-0133254488. [Google Scholar]
- Lo Cascio, M.; Morillas, L.; Ochoa-Hueso, R.; Delgado-Baquerizo, M.; Munzi, S.; Roales, J.; Spano, D.; Cruz, C.; Gallardo, A.; Manrique, E.; et al. Nitrogen Deposition Effects on Soil Properties, Microbial Abundance, and Litter Decomposition Across Three Shrublands Ecosystems From the Mediterranean Basin. Front. Environ. Sci. 2021, 9, 709391. [Google Scholar] [CrossRef]
- Hillel, D. Introduction to Environmental Soil Physics; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; Volume 56. [Google Scholar]
- Farquhar, G.D.; Sharkey, T.D. Stomatal Conductance and Photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Martinez-Ferri, E.; Balaguer, L.; Valladares, F.; Chico, J.M.; Manrique, E. Energy Dissipation in Drought-Avoiding and Drought-Tolerant Tree Species at Midday during the Mediterranean Summer. Tree Physiol. 2000, 20, 131–138. [Google Scholar] [CrossRef]
- Vivaldi, G.A.; Camposeo, S.; Romero-Trigueros, C.; Pedrero, F.; Caponio, G.; Lopriore, G.; Álvarez, S. Physiological Responses of Almond Trees under Regulated Deficit Irrigation Using Saline and Desalinated Reclaimed Water. Agric. Water Manag. 2021, 258, 107172. [Google Scholar] [CrossRef]
- Ben Yahmed, J.; Ghrab, M.; Ben Mimoun, M. Eco-Physiological Evaluation of Different Scion-Rootstock Combinations of Almond Grown in Mediterranean Conditions. Fruits 2016, 71, 185–193. [Google Scholar] [CrossRef]
- Ben Yahmed, J.; Ghrab, M.; Benmoussa, H.; Ben Mimoun, M. Physiological Behavior and Nutritional Status of Almond Scion-Rootstock Combinations in a High-Density Planting System under Warm Mediterranean Conditions. Sci. Hortic. 2022, 303, 111209. [Google Scholar] [CrossRef]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the Relationship between Leaf Chlorophyll Concentration and SPAD-502 Chlorophyll Meter Readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef]
- Pinochet, J. ‘Replantpac’ (Rootpac® R), a Plum–Almond Hybrid Rootstock for Replant Situations. HortScience 2010, 45, 299–301. [Google Scholar] [CrossRef]
- López-López, M.; Calderón, R.; González-Dugo, V.; Zarco-Tejada, P.; Fereres, E. Early Detection and Quantification of Almond Red Leaf Blotch Using High-Resolution Hyperspectral and Thermal Imagery. Remote Sens. 2016, 8, 276. [Google Scholar] [CrossRef]
- Mirsoleimani, A.; Najafi-Ghiri, M.; Heydari, H.; Farokhzadeh, S. Effect of Nitrogen, Phosphorus and Potassium Deficiencies on Some Morphological and Physiological Properties and Nutrient Uptake by Two Almond Rootstocks. Folia Hortic. 2021, 33, 235–247. [Google Scholar] [CrossRef]
- Mestre, L.; Reig, G.; Betrán, J.A.; Pinochet, J.; Moreno, M.Á. Influence of Peach–Almond Hybrids and Plum-Based Rootstocks on Mineral Nutrition and Yield Characteristics of ‘Big Top’ Nectarine in Replant and Heavy-Calcareous Soil Conditions. Sci. Hortic. 2015, 192, 475–481. [Google Scholar] [CrossRef]
- Gohari, S.; Imani, A.; Talaei, A.R.; Abdossi, V.; Asghari, M.R. Physiological Responses of Almond Genotypes to Drought Stress. Russ. J. Plant Physiol. 2023, 70, 141. [Google Scholar] [CrossRef]
- Ranjbarfordoei, A.; Samson, R.; Damme, P. Photosynthesis Performance in Sweet Almond [Prunus Dulcis (Mill) D. Webb] Exposed to Supplemental UV-B Radiation. Photosynthetica 2011, 49, 107–111. [Google Scholar] [CrossRef]
- Ranjbar, A.; Imani, A.; Piri, S.; Abdoosi, V. Drought Effects on Photosynthetic Parameters, Gas Exchanges and Water Use Efficiency in Almond Cultivars on Different Rootstocks. Plant Physiol. Rep. 2021, 26, 95–108. [Google Scholar] [CrossRef]
- Romero-Aroca, A.; Rovira, M.; Cristofori, V.; Silvestri, C. Hazelnut Kernel Size and Industrial Aptitude. Agriculture 2021, 11, 1115. [Google Scholar] [CrossRef]
- Maldera, F.; Vivaldi, G.A.; Iglesias-Castellarnau, I.; Camposeo, S. Two Almond Cultivars Trained in a Super-High Density Orchard Show Different Growth, Yield Efficiencies and Damages by Mechanical Harvesting. Agronomy 2021, 11, 1406. [Google Scholar] [CrossRef]
- Melis, R.; Morillas, L.; Roales, J.; Costa-Saura, J.M.; Lo Cascio, M.; Spano, D.; Mereu, S. Functional Traits Related to Competition for Light Influence Tree Diameter Increments in a Biodiversity Manipulation Experiment. Eur. J. For. Res. 2023, 142, 709–722. [Google Scholar] [CrossRef]
- Lo Cascio, M.; Deidda, A.; Sirca, C.; Nieddu, G.; Spano, D.; Deiana, P.; Gambella, F.; Mercenaro, L. UAV imagery to assess agronomic, physiological, and yield characteristics in a super-intensive almond orchard. Acta Hortic. 2024, 1406, 191–198. [Google Scholar] [CrossRef]
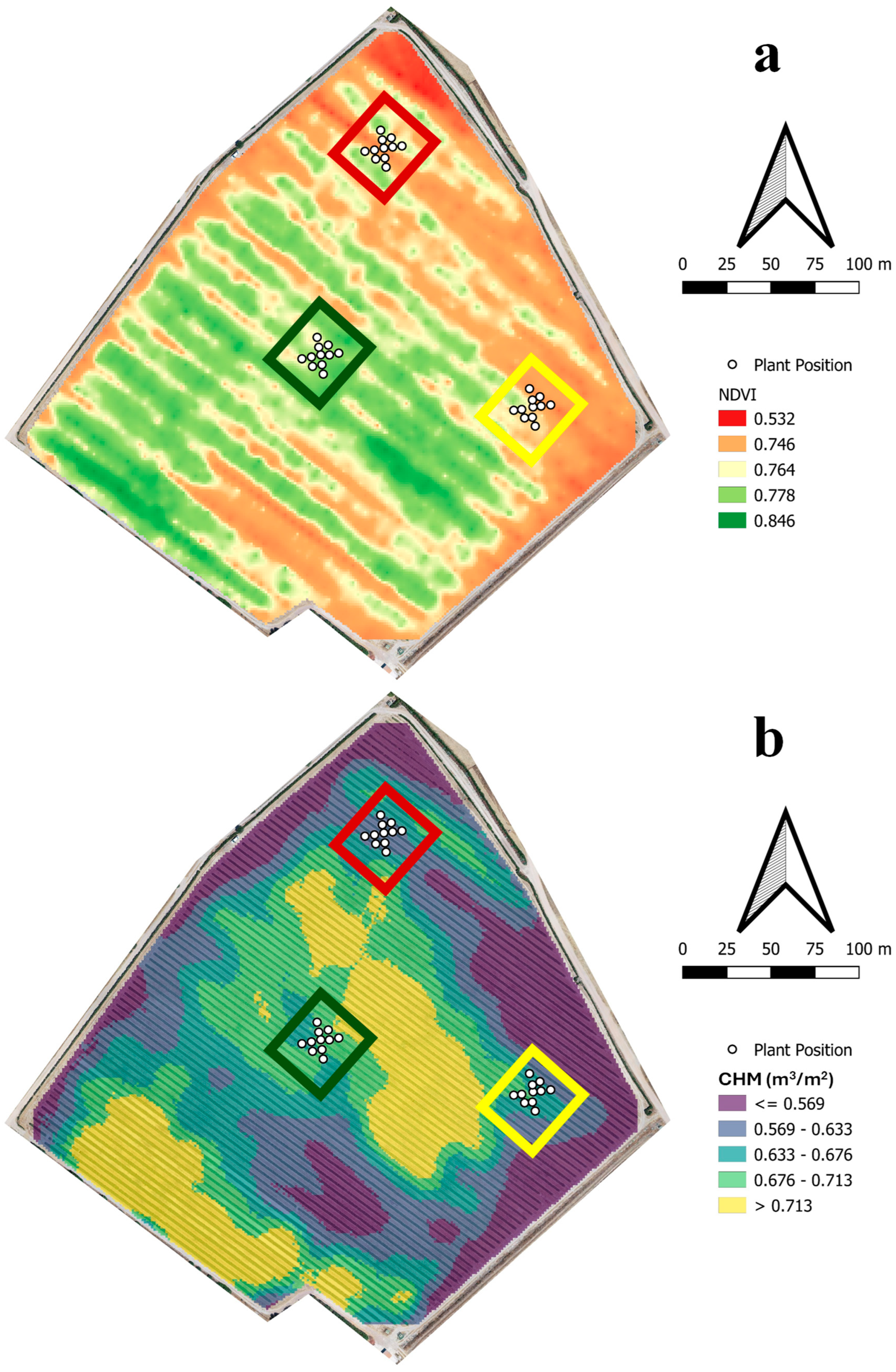

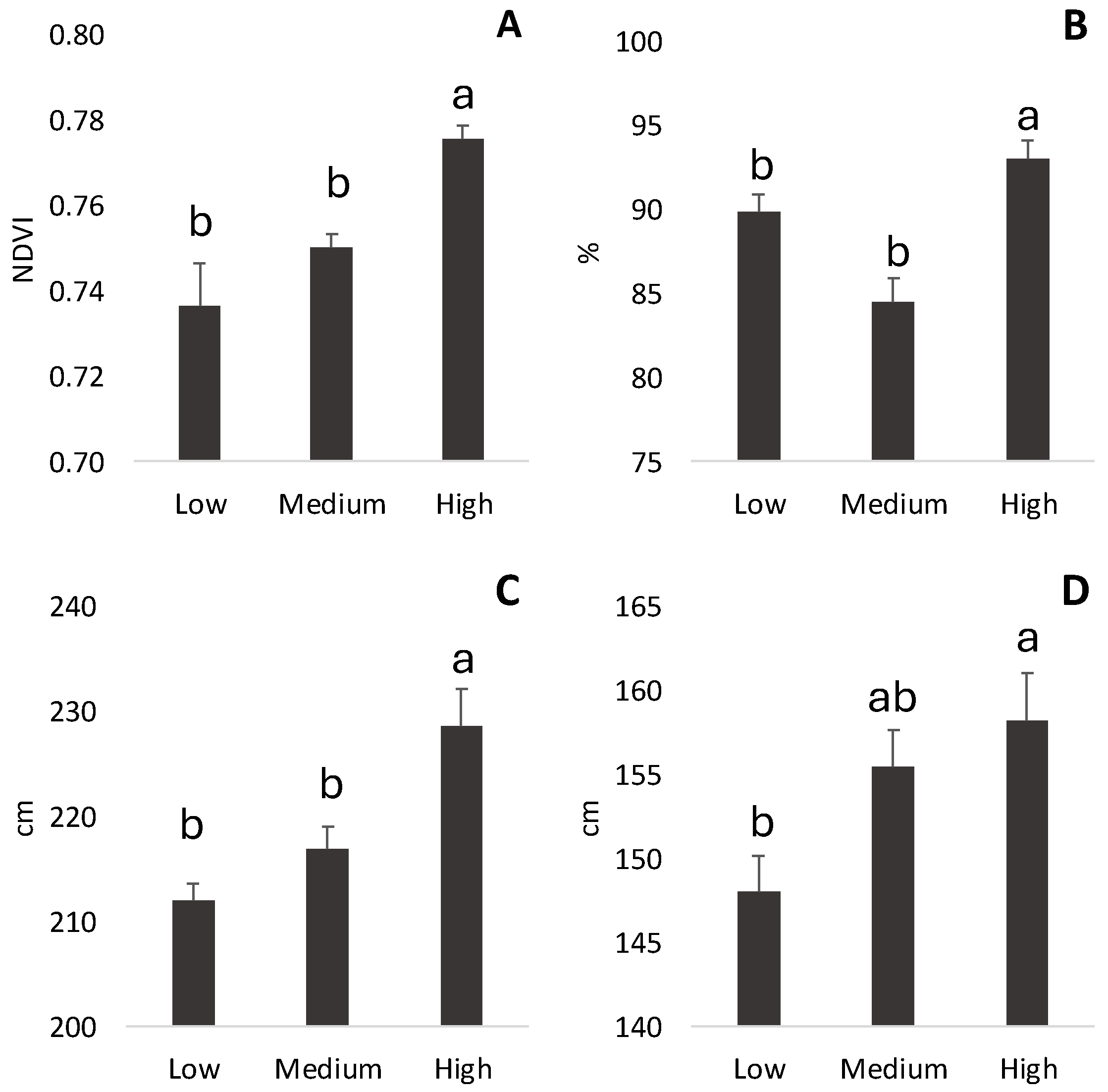

| Low Vigor | Medium Vigor | High Vigor | |
|---|---|---|---|
| Sand (%) | 43.37 | 41.41 | 37.33 |
| Silt (%) | 23.60 | 25.20 | 28.05 |
| Clay (%) | 33.03 | 33.39 | 34.62 |
| pH | 8.37 ± 0.02 a | 8.28 ± 0.03 b | 8.20 ± 0.08 c |
| Total C (%) | 4.87 ± 0.02 | 3.86 ± 1.36 | 4.72 ± 0.11 |
| Total N (%) | 0.035 ± 0.01 a | 0.041 ± 0.004 ab | 0.044 ± 0.004 b |
| C ing (%) | 0.35 ± 0.01 | 0.37 ± 0.06 | 0.28 ± 0.07 |
| DOC (mg g−1) | 0.128 ± 0.002 a | 0.125 ± 0.001 b | 0.120 ± 0.001 b |
| Organic Matter (%) | 5.22 ± 0.028 a | 5.65 ± 0.01 b | 5.03 ± 0.013 c |
| P available (mg kg−1) | 4.63 ± 0.03 a | 5.77 ± 0.09 b | 5.64 ± 0.05 c |
| Cation Exchange Capacity (cmol+ kg−1) | 24.70 ± 0.05 a | 26.39 ± 0.36 b | 24.01 ± 0.23 c |
| Year | Low | Medium | High | |
|---|---|---|---|---|
| Pn (μmol CO2 m−2 s−1) | 2022 | 12.68 ± 1.62 ab | 9.63 ± 0.79 b | 18.87 ± 2.01 a |
| 2023 | 11.85 ± 0.93 | 15.94 ± 0.93 | 15.31 ± 1.09 | |
| Gs (mol H2O m−2 s−1) | 2023 | 0.09 ± 0.01 b | 0.12 ± 0.02 b | 0.23 ± 0.01 a |
| Ψstem (Mpa) | 2022 | −2.35 ± 0.15 b | −2.40 ± 0.05 b | −1.76 ± 0.05 a |
| 2023 | −1.94 ± 0.10 b | −1.78 ± 0.07 ab | −1.60 ± 0.02 a | |
| SPAD | 2022 | 36.35 ± 0.53 | 39.05 ± 1.68 | 38.77 ± 1.44 |
| 2023 | 30.67 ± 0.74 b | 34.27 ± 1.15 a | 29.67 ± 0.79 b | |
| ϕPo (Fv/Fm) | 2022 | 0.75 ± 0.01 | 0.75 ± 0.02 | 0.77 ± 0.01 |
| 2023 | 0.65 ± 0.02 | 0.68 ± 0.02 | 0.69 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Cascio, M.; Deiana, P.; Deidda, A.; Sirca, C.; Nieddu, G.; Santona, M.; Spano, D.; Gambella, F.; Mercenaro, L. Towards Site-Specific Management: UAV- and Ground-Based Assessment of Intra-Field Variability in SHD Almond Orchards. Agronomy 2025, 15, 2241. https://doi.org/10.3390/agronomy15092241
Lo Cascio M, Deiana P, Deidda A, Sirca C, Nieddu G, Santona M, Spano D, Gambella F, Mercenaro L. Towards Site-Specific Management: UAV- and Ground-Based Assessment of Intra-Field Variability in SHD Almond Orchards. Agronomy. 2025; 15(9):2241. https://doi.org/10.3390/agronomy15092241
Chicago/Turabian StyleLo Cascio, Mauro, Pierfrancesco Deiana, Alessandro Deidda, Costantino Sirca, Giovanni Nieddu, Mario Santona, Donatella Spano, Filippo Gambella, and Luca Mercenaro. 2025. "Towards Site-Specific Management: UAV- and Ground-Based Assessment of Intra-Field Variability in SHD Almond Orchards" Agronomy 15, no. 9: 2241. https://doi.org/10.3390/agronomy15092241
APA StyleLo Cascio, M., Deiana, P., Deidda, A., Sirca, C., Nieddu, G., Santona, M., Spano, D., Gambella, F., & Mercenaro, L. (2025). Towards Site-Specific Management: UAV- and Ground-Based Assessment of Intra-Field Variability in SHD Almond Orchards. Agronomy, 15(9), 2241. https://doi.org/10.3390/agronomy15092241








