Exogenous Regulators Enhance Physiological Recovery and Yield Compensation in Maize Following Mechanical Leaf Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Site
2.2. Experimental Design
2.3. Field Management
2.4. Methods
2.4.1. Meteorological Data Collection
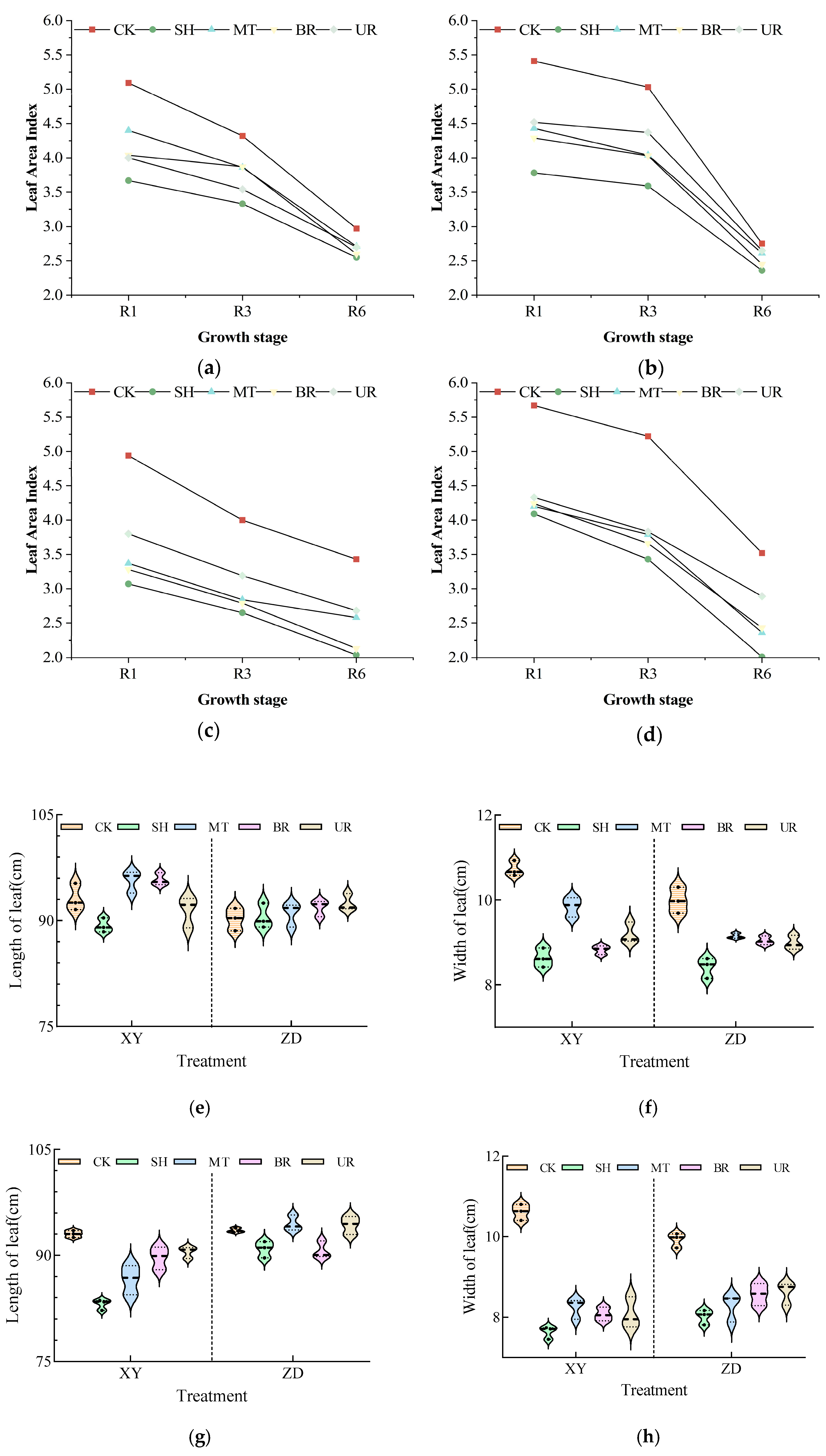
2.4.2. Morphological and Leaf Area Measurements
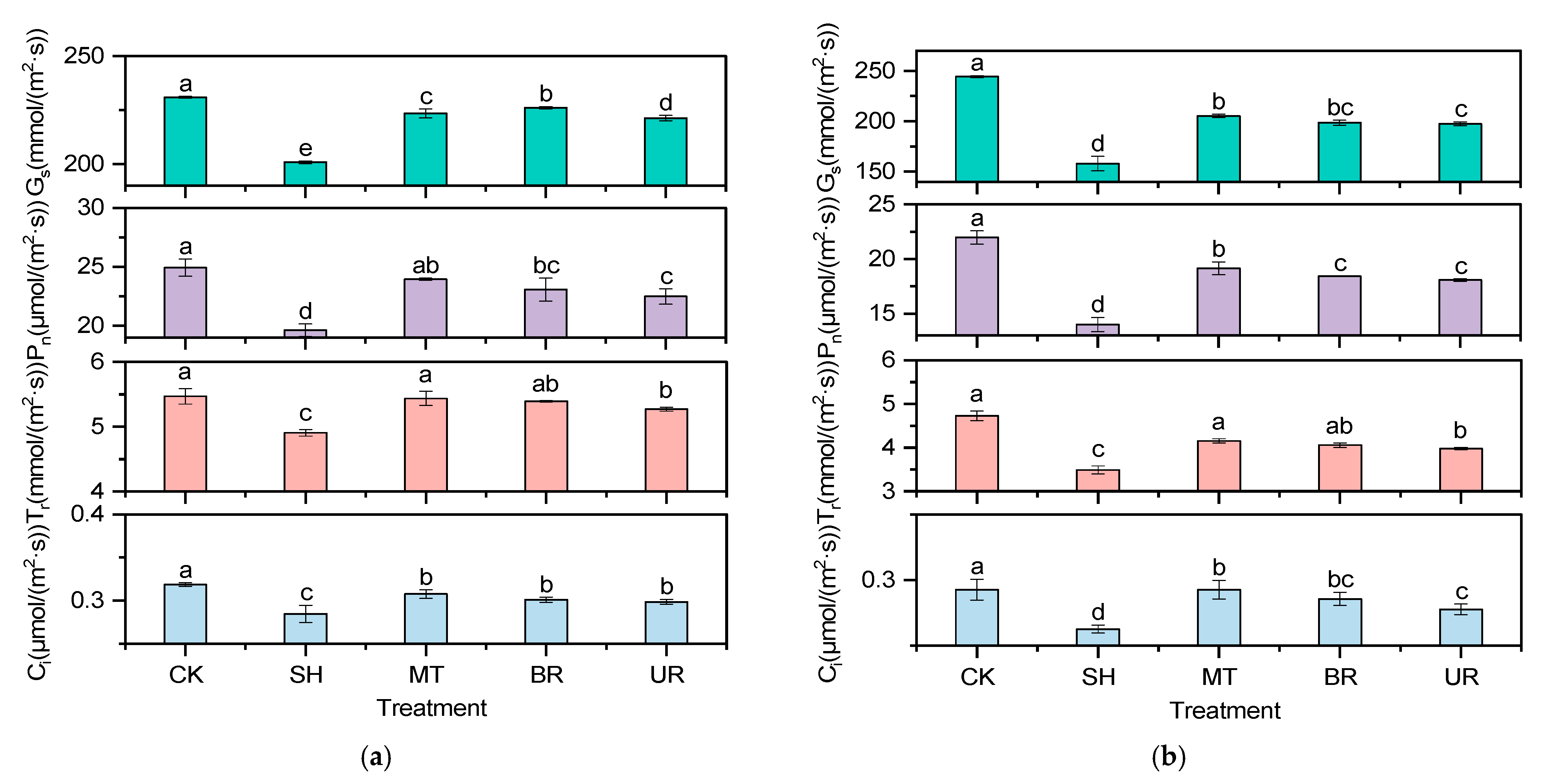
2.4.3. Photosynthetic Parameters
2.4.4. Dry Matter Accumulation and Translocation
2.4.5. Grain Filling Parameters
2.4.6. Yield Determination and Ear Trait Measurement
2.5. Data Processing and Analysis
3. Results
3.1. Effects of Exogenous Regulators on Yield and Its Components
3.2. Effects of Exogenous Regulators on Plant Traits
3.3. Restorative Effects of Exogenous Regulators on LAI
3.4. Effects of Exogenous Regulators on Photosynthetic Parameters
3.5. Effects of Exogenous Regulators on the Dynamic Changes in Post-Silking Dry Matter Accumulation
3.5.1. Recovery of Dry Matter and Kernel Allocation by Exogenous Regulators
3.5.2. Effects of Exogenous Regulators on Dry Matter Accumulation Dynamics
3.6. Effects of Exogenous Regulators on Grain Filling Parameters
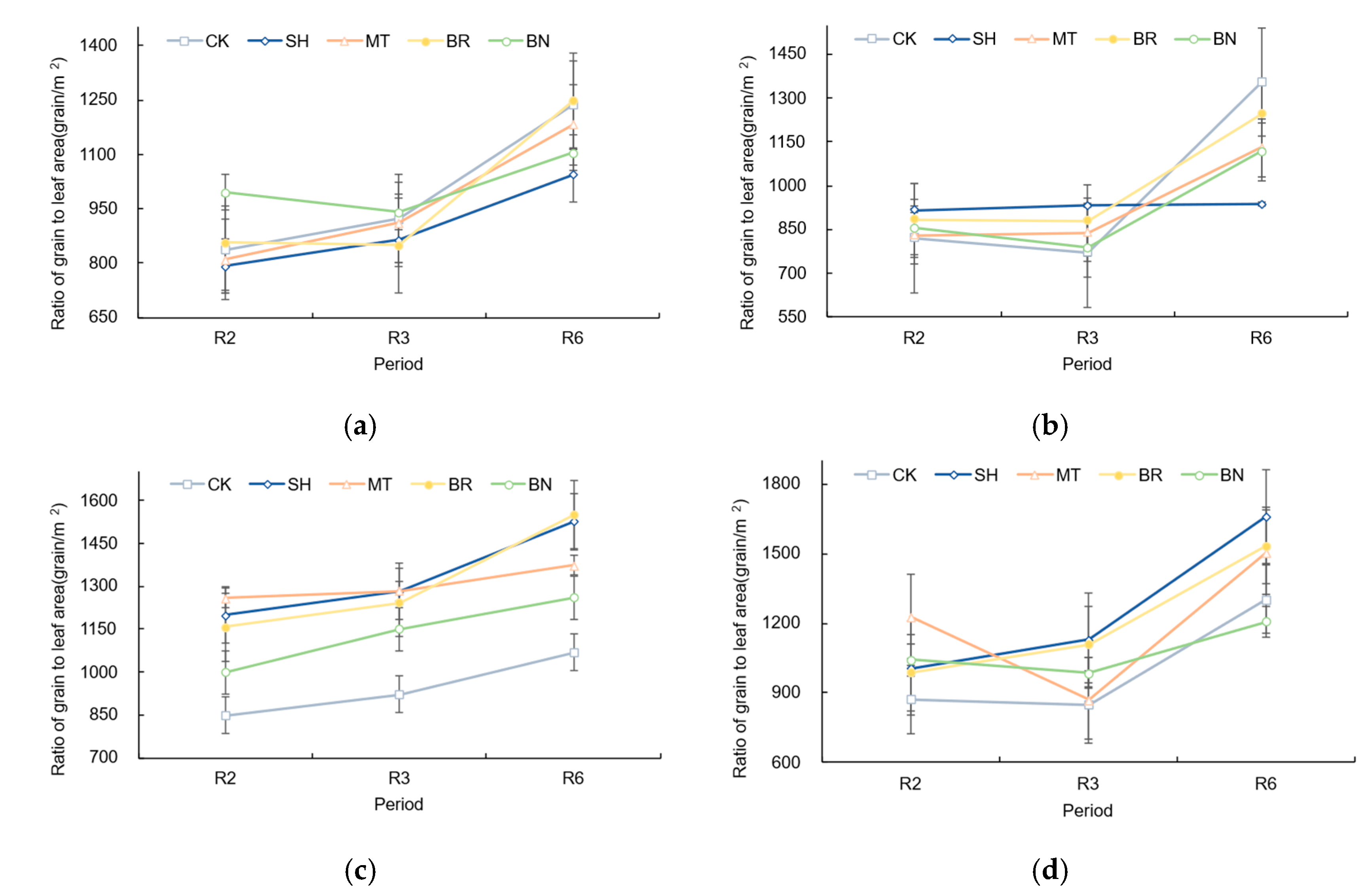
3.7. Effects of Exogenous Regulators on the Kernel-to-Leaf Ratio
3.8. Construction of the Structural Equation Model Among Traits
4. Discussion
4.1. Source–Sink Balance Restoration and Enhancement of Canopy Photosynthetic Efficiency: Core Pathways of Exogenous Regulators
4.2. Differential Mechanisms and Practical Implications of MT, BR, and UR
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SH | Simulated herbivory |
| MT | Melatonin |
| BR | Brassinolide |
| UR | Urea |
| Pn | Net photosynthetic rate |
| Gs | Stomatal conductance |
| Tr | Transpiration rate |
| Ci | Intercellular CO2 concentration |
| LAI | Leaf area index |
| DMA | Dry matter accumulation |
| HI | Harvest index |
References
- Lou, Y.; Feng, L.; Xing, W.; Hu, N.; Noellemeyer, E.; Le Cadre, E.; Minamikawa, K.; Muchaonyerwa, P.; AbdelRahman, M.A.E.; Pinheiro, É.F.M.; et al. Climate-smart agriculture: Insights and challenges. Clim. Smart Agric. 2024, 1, 100003. [Google Scholar] [CrossRef]
- Gatto, A.; Chepeliev, M. Global food loss and waste estimates show increasing nutritional and environmental pressures. Nat. Food 2024, 5, 136–147. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wright, I.J.; Zhu, S.; Onoda, Y.; Liu, H.; Li, R.; Liu, X.; Hua, L.; Oyanoghafo, O.O.; Ye, Q. Leaf mechanical strength and photosynthetic capacity vary independently across 57 subtropical forest species with contrasting light requirements. New Phytol. 2019, 223, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, R.L.; Messina, C.D.; Berning, D.; Haag, L.A.; Carter, P.; Hefley, T.J.; Prasad, P.V.V.; Ciampitti, I.A. Tiller biomass in low plant-density corn enhances transient C sink without direct harvest index detriment. Field Crops Res. 2023, 292, 108804. [Google Scholar] [CrossRef]
- Rahman, S.R.; Eng, N.E.; Ashraf, M.A.; Pang, W.L.; Tan, K.B.; Singh, A.K.; Chan, K.Y. Energy harvesting from living plant: A review on past research and way forward. Energy Rep. 2025, 14, 268–281. [Google Scholar] [CrossRef]
- Legé, K.E.; Cothren, J.T.; Morgan, P.W. Nitrogen fertility and leaf age effect on ethylene production of cotton in a controlled environment. Plant Growth Regul. 1997, 22, 23–28. [Google Scholar] [CrossRef]
- Yan, H.; Fu, K.; Li, J.; Li, M.; Li, S.; Dai, Z.; Jin, X. Photosynthesis, Chlorophyll Fluorescence, and Hormone Regulation in Tomato Exposed to Mechanical Wounding. Plants 2024, 13, 2594. [Google Scholar] [CrossRef] [PubMed]
- Nabity, P.D.; Zavala, J.A.; DeLucia, E.H. Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Ann. Bot. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in plants: More studies are necessary. Plant Signal. Behav. 2007, 2, 381. [Google Scholar] [CrossRef]
- Gautam, H.; Fatma, M.; Sehar, Z.; Mir, I.R.; Khan, N.A. Hydrogen sulfide, ethylene, and nitric oxide regulate redox homeostasis and protect photosynthetic metabolism under high temperature stress in rice plants. Antioxidants 2022, 11, 1478. [Google Scholar] [CrossRef]
- Hassan, M.U.; Mahmood, A.; Awan, M.I.; Maqbool, R.; Aamer, M.; Alhaithloul, H.A.; Huang, G.; Skalicky, M.; Brestic, M.; Pandey, S.; et al. Melatonin-induced protection against plant abiotic stress: Mechanisms and prospects. Front. Plant Sci. 2022, 13, 902694. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, W.; Wang, Z.; Gao, S.; Zhang, R. Integrated metabolome, transcriptome and physiological analyses of melatonin-induced drought responses in maize roots and leaves. Plant Growth Regul. 2025, 105, 229–244. [Google Scholar] [CrossRef]
- Zhang, Y.W. Regulatory Effect of Exogenous Melatonin on Seed Priming, Seedling Growth and Antioxidant Defence System of Sophora alopecuroides under Compound Salt Stress and Compound Alkali Stress. Russ. J. Plant Physiol. 2025, 72, 137. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Y.; Liang, B.; Kumar, S.; Zhao, W.; Liu, T.; Li, Y.; Zhu, G. Melatonin-Induced Transcriptome Variation of Sweet Potato Under Heat Stress. Plants 2025, 14, 430. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Bai, T.; Ma, Y.; Zhao, Y.; Ci, J.; Ren, X.; Zang, Z.; Ma, C.; Xiong, R.; Song, X.; et al. Molecular Mechanisms Underlying Salt Tolerance in Maize: A Combined Transcriptome and Metabolome Analysis. Plants 2025, 14, 2031. [Google Scholar] [CrossRef]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.Y.; Muhammad, I.; Farooq, S.; Kamran, M.; Ahmad, I.; Zeeshan, M.; Javed, T.; Ullah, S.; Huang, J.H.; et al. Application of melatonin-mediated modulation of drought tolerance by regulating photosynthetic efficiency, chloroplast ultrastructure, and endogenous hormones in maize. Chem. Biol. Technol. Agric. 2022, 9, 5. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, J.; Sun, X.; Zhu, Y.; Li, Q.; Zhang, L.; Zhao, D.; Huang, L.; Zhang, C.; Liu, Q. Exogenous melatonin improves the quality performance of rice under high temperature during grain filling. Agronomy 2022, 12, 949. [Google Scholar] [CrossRef]
- Yang, X.; Ren, J.; Li, J.; Lin, X.; Xia, X.; Yan, W.; Zhang, Y.; Deng, X. Meta-analysis of the effect of melatonin application on abiotic stress tolerance in plants. Plant Biotechnol. Rep. 2023, 17, 39–52. [Google Scholar] [CrossRef]
- Cai, S.; Zhao, X.; Yan, X. Towards precise nitrogen fertilizer management for sustainable agriculture. Earth Crit. Zone 2025, 2, 100026. [Google Scholar] [CrossRef]
- Noor, H.; Ding, P.; Ren, A.; Sun, M.; Gao, Z. Effects of nitrogen fertilizer on photosynthetic characteristics and yield. Agronomy 2023, 13, 1550. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Zhao, H.; Li, Y.; Wei, J.; Ma, L.; Zheng, F.; Tan, D. Enhancing maize yield and nutrient utilization through improved soil quality under reduced fertilizer use: The efficacy of organic–inorganic compound fertilizer. Agriculture 2024, 14, 1482. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Guo, D.; Wang, R.; Chen, C.; Yin, B.; Ding, Z.; Wang, X.; Zhao, M.; Zhou, B. Nitrogen supply mitigates heat stress on photosynthesis of maize (Zea mays L.) during early grain filling by improving nitrogen assimilation. J. Agron. Crop Sci. 2024, 210, e12750. [Google Scholar] [CrossRef]
- Brodowska, M.S.; Wyszkowski, M.; Karsznia, M. Application of urea and ammonium nitrate solution with potassium thiosulfate as a factor determining macroelement contents in plants. Agronomy 2024, 14, 1097. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, D.; Xing, Z.; Ni, C.; Ye, M.; Zhang, Z. Optimizing controlled-release urea and urea combinations for sustainable rice-wheat production under nitrogen reduction. Front. Plant Sci. 2025, 16, 1576049. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W.; Wang, W.; Yu, N.; Liu, P.; Zhao, B.; Zhang, J.; Ren, B. Dual film-controlled model urea improves summer maize yields, N fertilizer use efficiency and reduces greenhouse gas emissions. Soil Tillage Res. 2025, 252, 106565. [Google Scholar] [CrossRef]
- Januszkiewicz, R.; Kulczycki, G.; Sacała, E.; Kabala, C. Effect of Nutrient Forms in Foliar Fertilizers on the Growth and Biofortification of Maize on Different Soil Types. Agronomy 2025, 15, 1482. [Google Scholar] [CrossRef]
- Li, G.; Gu, R.; Xu, K.; Guo, B.; Dai, Q.; Huo, Z.; Wei, H. Split application of a mixture of controlled-release and common urea for improving quality and agronomic and economic performance in wheat production. Crop Sci. 2021, 61, 4402–4415. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Biju, S.; Bhardwaj, R. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: A review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef]
- Jin, H.; Do, J.; Shin, S.-J.; Choi, J.W.; Choi, Y.I.; Kim, W.; Kwon, M. Exogenously applied 24-epi brassinolide reduces lignification and alters cell wall carbohydrate biosynthesis in the secondary xylem of Liriodendron tulipifera. Phytochemistry 2014, 101, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yao, X.; Liu, X.; Qiao, Z.; Liu, Y.; Li, X.; Jiang, X. Brassinolide can improve drought tolerance of maize seedlings under drought stress: By inducing the photosynthetic performance, antioxidant capacity and ZmMYB gene expression of maize seedlings. J. Soil Sci. Plant Nutr. 2022, 22, 2092–2104. [Google Scholar] [CrossRef]
- Fan, J.; Tang, X.; Cai, J.; Tan, R.; Gao, X. Effects of exogenous EBR on the physiology of cold resistance and the expression of the VcCBF3 gene in blueberries during low-temperature stress. PLoS ONE 2025, 20, e0313194. [Google Scholar] [CrossRef]
- Ahmad Lone, W.; Majeed, N.; Yaqoob, U.; Riffatjohn, S. Exogenous brassinosteroid and jasmonic acid improve drought tolerance in Brassica rapa L. genotypes by modulating osmolytes, antioxidants and photosynthetic system. Plant Cell Rep. 2022, 41, 603–617. [Google Scholar] [CrossRef]
- Wu, J.S.; Mu, D.W.; Feng, N.J.; Zheng, D.F.; Sun, Z.Y.; Khan, A.; Zhou, H.; Song, Y.W.; Liu, J.X.; Luo, J.Q. Integrated Analyses Reveal the Physiological and Molecular Mechanisms of Brassinolide in Modulating Salt Tolerance in Rice. Plants 2025, 14, 1555. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Z.; Hou, J.; Gao, J.; Li, X.; Zhang, Y.; Zhao, Q. 2,4-Epibrassinolide Mitigates Cd Stress by Enhancing Chloroplast Structural Remodeling and Chlorophyll Metabolism in Vigna angularis Leaves. Biology 2025, 14, 674. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, D.; Tang, Z.; Zhang, Y.; Zhang, K.; Dong, J.; Wang, F. Exogenous brassinosteroids promotes root growth, enhances stress tolerance, and increases yield in maize. Plant Signal. Behav. 2022, 17, 2095139. [Google Scholar] [CrossRef]
- Castañeda-Murillo, C.C.; Rojas-Ortiz, J.G.; Sánchez-Reinoso, A.D.; Chávez-Arias, C.C.; Restrepo-Díaz, H. Foliar brassinosteroid analogue (DI-31) sprays increase drought tolerance by improving plant growth and photosynthetic efficiency in lulo plants. Heliyon 2022, 8, e08977. [Google Scholar] [CrossRef]
- Liang, Z.; Cao, X.; Gao, R.; Guo, N.; Tang, Y.; Nangia, V.; Liu, Y. Brassinosteroids alleviate wheat floret degeneration under low nitrogen stress by promoting the redistribution of sucrose from stems to spikes. J. Integr. Agric. 2025, 24, 497–516. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Brassinosteroids in plant tolerance to abiotic stress. J. Plant Growth Regul. 2020, 39, 1451–1464. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, M.; Wu, X.; Wang, Y.; Zhang, R. Physiological and transcriptomic analyses of the effects of exogenous melatonin on drought tolerance in maize (Zea mays L.). Plant Physiol. Biochem. 2021, 168, 128–142. [Google Scholar] [CrossRef]
- Sun, Y.; He, Y.; Irfan, A.R.; Liu, X.; Yu, Q.; Zhang, Q.; Yang, D. Exogenous brassinolide enhances the growth and cold resistance of maize (Zea mays L.) seedlings under chilling stress. Agronomy 2020, 10, 488. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, M.; Liu, Z.; Zhou, H.; Lu, H.; Zhang, W.; Yan, Y.; Li, C.; Chen, B. Combining controlled-release urea and normal urea to improve the nitrogen use efficiency and yield under wheat-maize double cropping system. Field Crops Res. 2016, 197, 52–62. [Google Scholar] [CrossRef]
- Zhai, J.; Zhang, Y.; Zhang, G.; Tian, M.; Xie, R.; Ming, B.; Hou, P.; Wang, K.; Xue, J.; Li, S. Effects of nitrogen fertilizer management on stalk lodging resistance traits in summer maize. Agriculture 2022, 12, 162. [Google Scholar] [CrossRef]
- Wu, C.; Cui, K.; Tang, S.; Li, G.; Wang, S.; Fahad, S.; Nie, L.; Huang, J.; Peng, S.; Ding, Y. Intensified pollination and fertilization ameliorate heat injury in rice (Oryza sativa L.) during the flowering stage. Field Crops Res. 2020, 252, 107795. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, X.Y.; Ren, H.; Zhang, J.W.; Zhao, B.; Ren, B.Z.; Liu, P. Deep nitrogen fertilizer placement improves the yield of summer maize (Zea mays L.) by enhancing its photosynthetic performance after silking. BMC Plant Biol. 2025, 25, 172. [Google Scholar] [CrossRef] [PubMed]
- Baethgen, W.E.; Christianson, C.B.; Lamothe, A.G. Nitrogen fertilizer effects on growth, grain yield, and yield components of malting barley. Field Crops Res. 1995, 43, 87–99. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. The physiological function of melatonin in plants. Plant Signal. Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef]
- Murch, S.J.; Saxena, P.K. Melatonin: A potential regulator of plant growth and development? Vitr. Cell. Dev. Biol.-Plant 2002, 38, 531–536. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin promotes adventitious-and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J. Pineal Res. 2007, 42, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Pouryousef, M.; Tavakoli, A.; Mohseni Fard, E. Improvement in photosynthesis, seed yield and protein content of common bean (Phaseolus vulgaris) by foliar application of 24-epibrassinolide under drought stress. Crop Pasture Sci. 2019, 70, 535–545. [Google Scholar] [CrossRef]
- Soylemez, S.; Kaya, C.; Dikilitas, S.K. Promotive effects of epibrassinolide on plant growth, fruit yield, antioxidant, and mineral nutrition of saline stressed tomato plants. Pak. J. Bot 2017, 49, 1655–1661. [Google Scholar]
- Cao, Y.; Hu, R.; Huang, F.; Hou, J. One-time root-zone application of controlled-release urea increases maize yield and nitrogen use efficiency. J. Plant Nutr. Fertil. 2025, 31, 1455–1466. [Google Scholar]
- Johnson, R.R. Growth and yield of maize as affected by early-season defoliation 1. Agron. J. 1978, 70, 995–998. [Google Scholar] [CrossRef]
- Maddonni, G.A.; Otegui, M.E.; Cirilo, A.G. Plant population density, row spacing and hybrid effects on maize canopy architecture and light attenuation. Field Crops Res. 2001, 71, 183–193. [Google Scholar] [CrossRef]
- Hou, Y.; Xu, X.; Kong, L.; Zhang, L.; Zhang, Y.; Liu, Z. Improving nitrogen contribution in maize post-tasseling using optimum management under mulch drip irrigation in the semiarid region of Northeast China. Front. Plant Sci. 2022, 13, 1095314. [Google Scholar] [CrossRef]
- Yan, Y.; Hou, P.; Duan, F.; Niu, L.; Dai, T.; Wang, K.; Zhao, M.; Li, S.; Zhou, W. Improving photosynthesis to increase grain yield potential: An analysis of maize hybrids released in different years in China. Photosynth. Res. 2021, 150, 295–311. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.C.; Niu, X.G.; Ma, F.; Wei, N.; Hao, X.Y.; Dong, L.B.; Guo, L.P. Effects of elevated atmospheric CO2 concentration and nitrogen fertilizer on the yield of summer maize and carbon and nitrogen metabolism after flowering. Sci. Agric. Sin. 2021, 54, 3647–3665. [Google Scholar]
- Bhattacharya, A. Dry matter production, partitioning, and seed yield under soil water deficit: A review. Soil Water Deficit Physiol. Issues Plants 2021, 585–702. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, M. Improving maize grain yield by formulating plant growth regulator strategies in North China. J. Integr. Agric. 2021, 20, 622–632. [Google Scholar] [CrossRef]
- Bolaños, J.; Edmeades, G.O. The importance of the anthesis-silking interval in breeding for drought tolerance in tropical maize. Field Crops Res. 1996, 48, 65–80. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Yang, Z.; Liu, Y.; Yang, S.; Shi, Y.; Jiang, C.; Qin, F. Manipulating ZmEXPA4 expression ameliorates the drought-induced prolonged anthesis and silking interval in maize. Plant Cell 2021, 33, 2058–2071. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Muhammad, I.; Wang, G.Y.; Zeeshan, M.; Yang, L.; Ali, I.; Zhou, X.B. Ameliorative effect of melatonin improves drought tolerance by regulating growth, photosynthetic traits and leaf ultrastructure of maize seedlings. BMC Plant Biol. 2021, 21, 368. [Google Scholar] [CrossRef] [PubMed]
- Gillani, S.F.A.; Zhuang, Z.; Rasheed, A.; Ul Haq, I.; Abbasi, A.; Ahmed, S.; Wang, Y.; Khan, M.T.; Sardar, R.; Peng, Y. Brassinosteroids induced drought resistance of contrasting drought-responsive genotypes of maize at physiological and transcriptomic levels. Front. Plant Sci. 2022, 13, 961680. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Guo, H.; Wang, J.; Wang, Y.; Zhang, R. Melatonin enhances drought tolerance by regulating leaf stomatal behavior, carbon and nitrogen metabolism, and related gene expression in maize plants. Front. Plant Sci. 2021, 12, 779382. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Sattar, A.; Ijaz, A.S.; Baig, A.; Naz, I.; Almaghasla, M.I.; Hamed, L.M.M.; Ramadan, K.M.; El-Mogy, M.M. Exogenous Application of Silicon and Brassinosteroids Alleviate the Adversities of Drought Stress on Maize through Up-Regulation of Photosynthetic Efficiency, Antioxidants Defense System and Osmotic Adjustment. Russ. J. Plant Physiol. 2025, 72, 84. [Google Scholar] [CrossRef]




| Sowing Date | Hybrid | Treatment | Precipitation (mm) | Sunshine Duration (h) | Daily Mean Temperature (°C) | Evapotranspiration (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sd-V6 | V6-R1 | R1-HT | Sd-V6 | V6-R1 | R1-HT | Sd-V6 | V6-R1 | R1-HT | Sd-V6 | V6-R1 | R1-HT | |||
| S1 | XY | CK | 135.1 | 175.6 | 201.9 | 176.4 | 133.5 | 273.1 | 787.6 | 658.6 | 1317.8 | 79.7 | 72.0 | 181.5 |
| SH | 169.2 | 141.5 | 201.7 | 185.2 | 142.0 | 272.2 | 841.7 | 685.4 | 1288.3 | 84.4 | 76.7 | 184.1 | ||
| Re | 135.4 | 175.3 | 202.0 | 180.8 | 146.4 | 272.2 | 814.7 | 712.4 | 1288.3 | 82.0 | 79.1 | 184.1 | ||
| ZD | CK | 135.1 | 175.6 | 202.0 | 176.4 | 139.5 | 272.6 | 787.6 | 684.4 | 1313.0 | 79.7 | 75.0 | 183.1 | |
| SH | 169.2 | 141.5 | 202.0 | 185.2 | 148.7 | 271.0 | 841.7 | 713.9 | 1275.5 | 84.4 | 80.3 | 183.8 | ||
| Re | 135.4 | 175.3 | 202.0 | 180.8 | 153.0 | 271.0 | 814.7 | 740.9 | 1275.5 | 82.0 | 82.7 | 183.8 | ||
| S2 | XY | CK | 153.6 | 169.0 | 147.9 | 147.7 | 123.3 | 260.6 | 762.9 | 641.2 | 1190.9 | 76.6 | 74.9 | 182.6 |
| SH | 153.6 | 169.0 | 143.4 | 165.3 | 115.1 | 259.3 | 843.8 | 610.5 | 1158.7 | 86.7 | 70.8 | 181.5 | ||
| Re | 153.6 | 173.5 | 143.4 | 154.7 | 125.7 | 259.3 | 790.9 | 663.5 | 1158.7 | 80.4 | 77.1 | 181.5 | ||
| ZD | CK | 153.6 | 169.0 | 147.9 | 147.7 | 129.9 | 257.7 | 762.9 | 666.0 | 1174.2 | 76.6 | 78.3 | 181.5 | |
| SH | 153.6 | 174.2 | 142.7 | 165.3 | 117.3 | 261.9 | 843.8 | 637.4 | 1143.5 | 86.7 | 73.4 | 181.6 | ||
| Re | 153.6 | 169.0 | 143.4 | 154.7 | 125.7 | 259.3 | 790.9 | 663.5 | 1158.7 | 80.4 | 77.1 | 181.5 | ||
| Sowing Date | Hybrid | Treatment | Sowing | Emergence | V6 | V12 | R1 | R3 | R6 |
|---|---|---|---|---|---|---|---|---|---|
| S1 | XY | CK | 6.4 | 6.10 | 7.3 | 7.16 | 7.28 | 8.22 | 9.21 |
| SH | 6.4 | 6.10 | 7.5 | 7.22 | 7.31 | 8.25 | 9.24 | ||
| Re | 6.4 | 6.10 | 7.4 | 7.19 | 7.31 | 8.25 | 9.24 | ||
| ZD | CK | 6.4 | 6.10 | 7.3 | 7.14 | 7.29 | 8.23 | 9.22 | |
| SH | 6.4 | 6.10 | 7.5 | 7.20 | 8.1 | 8.26 | 9.25 | ||
| Re | 6.4 | 6.10 | 7.4 | 7.17 | 8.1 | 8.26 | 9.25 | ||
| S2 | XY | CK | 6.20 | 6.25 | 7.17 | 7.30 | 8.10 | 9.4 | 10.4 |
| SH | 6.20 | 6.25 | 7.20 | 8.2 | 8.12 | 9.6 | 10.6 | ||
| Re | 6.20 | 6.25 | 7.18 | 7.31 | 8.12 | 9.6 | 10.6 | ||
| ZD | CK | 6.20 | 6.26 | 7.17 | 7.29 | 8.11 | 9.5 | 10.5 | |
| SH | 6.20 | 6.26 | 7.20 | 8.2 | 8.13 | 9.7 | 10.7 | ||
| Re | 6.20 | 6.26 | 7.18 | 7.31 | 8.12 | 9.6 | 10.6 |
| Variety | Treatment | 100-Grain Weight (g) | Kernel Numbers Per Ear | Ear Density (104 hm−2) | Yield (kg·hm−2) | Empty Bar Rate (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | ||
| XY | CK | 37.5 a | 34 ab | 544.2 a | 542.5 a | 6.49 b | 6.53 b | 13.23 a | 12.04 a | 3.9 b | 3.3 b |
| SH | 34.0 c | 32.0 b | 394.8 c | 461.2 c | 6.32 c | 6.38 c | 8.48 c | 9.41 b | 6.4 a | 5.4 a | |
| MT | 36.1 b | 33.4 c | 473.4 b | 523.4 ab | 6.36 c | 6.50 b | 10.87 b | 11.36 a | 5.8 a | 3.8 b | |
| BR | 36.2 b | 34.5 a | 479.8 b | 487.8 bc | 6.49 b | 6.51 b | 11.28 b | 10.95 a | 3.9 b | 3.6 b | |
| UR | 36.5 b | 33.2 b | 441.1 bc | 497.1 abc | 6.66 a | 6.64 a | 10.72 b | 10.96 a | 1.3 c | 1.7 c | |
| ZD | CK | 32.2 a | 31.7 a | 496.4 a | 611.4 a | 6.35 a | 6.19 b | 10.16 a | 12.01 a | 6.0 d | 8.3 c |
| SH | 30.9 c | 26.4 d | 294.9 c | 445.8 c | 5.70 d | 6.00 d | 5.19 c | 7.04 d | 15.6 a | 11.2 a | |
| MT | 31.2 bc | 27.6 c | 393.7 b | 472.9 bc | 6.05 c | 6.13 c | 7.42 b | 7.98 c | 10.4 b | 9.2 b | |
| BR | 31.5 b | 28.9 b | 406.5 b | 494.9 b | 6.16 d | 6.16 bc | 7.91 b | 8.81 b | 8.7 c | 8.8 bc | |
| UR | 31.4 bc | 28.9 b | 394.6 b | 465.3 bc | 6.39 a | 6.24 a | 7.91 b | 8.38 bc | 5.4 d | 7.5 d | |
| Sowing | Independent Variable | Simple Correlation Coefficient with Yield | Direct Path Coefficient | Indirect Path Coefficient | Total | ||
|---|---|---|---|---|---|---|---|
| Kernel Numbers Per Row | 100-Grain Weight | Ear Density | |||||
| S1 | Kernel numbers per row | 0.817 | 0.647 | - | 0.70 | 0.63 | 1.330 |
| 100-grain weight | 0.954 | 0.365 | 0.60 | - | 0.59 | 1.190 | |
| Ear density | 0.866 | 0.078 | 0.60 | 0.66 | - | 1.260 | |
| S2 | Kernel numbers per row | 0.717 | 0.443 | - | 0.16 | 0.79 | 0.950 |
| 100-grain weight | 0.804 | 0.571 | 0.14 | - | 0.38 | 0.520 | |
| Ear density | 0.880 | 0.208 | 0.65 | 0.35 | - | 1.000 | |
| Sowing | Variety | Treatment | Post-Silking DM Accumulation (g/Plant) | Before-Silking DM Accumulation Rate (%) | Post-Silking DM Accumulation Rate (%) | Post DM/Grain (%) |
|---|---|---|---|---|---|---|
| S1 | XY | CK | 263.14 a | 35.69 a | 64.31 c | 135.03 b |
| SH | 230.17 b | 29.98 c | 70.02 a | 137.52 b | ||
| MT | 265.99 a | 30.53 bc | 69.47 ab | 140.58 ab | ||
| BR | 253.69 a | 29.75 c | 70.25 a | 147.73 a | ||
| UR | 234.13 b | 31.91 b | 68.09 b | 138.76 b | ||
| ZD | CK | 250.03 a | 33.84 b | 66.16 d | 124.25 a | |
| SH | 194.31 c | 28.58 e | 71.42 a | 123.62 a | ||
| MT | 200.79 c | 35.45 a | 64.55 e | 121.98 a | ||
| BR | 220.23 b | 30.06 d | 69.94 b | 123.37 a | ||
| UR | 201.14 c | 31.67 c | 68.33 c | 123.48 a | ||
| S2 | XY | CK | 214.67 a | 39.45 a | 60.55 b | 123.24 ab |
| SH | 159.05 c | 36.54 b | 63.46 a | 106.34 d | ||
| MT | 208.13 a | 34.12 b | 65.88 a | 125.42 a | ||
| BR | 175.71 b | 35.49 b | 64.51 a | 116.78 c | ||
| UR | 206.23 a | 34.12 b | 65.88 a | 120.34 bc | ||
| ZD | CK | 191.09 a | 38.78 ab | 61.22 bc | 115.09 a | |
| SH | 131.07 c | 36.05 bc | 63.95 ab | 101.76 b | ||
| MT | 139.70 c | 39.17 a | 60.83 c | 101.77 b | ||
| BR | 135.39 c | 38.21 ab | 61.79 bc | 101.43 b | ||
| UR | 176.07 b | 34.76 c | 65.24 a | 113.24 a |
| Sowing Date | Variety | Treatment | Equation Parameters | Grain-Filling Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | Gmax (g·d−1) | Gmean (g·d−1) | Wmax (g) | Tmax (d) | P(d) | Coefficient of Determination (R2) | |||
| S1 | XY | CK | 39.04 | 41.46 | 0.14 | 1.37 | 1.20 | 19.52 | 25.72 | 44.03 | 0.988 |
| SH | 34.71 | 14.69 | 0.11 | 0.95 | 0.84 | 17.35 | 26.93 | 56.69 | 0.987 | ||
| MT | 39.19 | 16.20 | 0.11 | 1.08 | 0.94 | 19.59 | 25.02 | 55.88 | 0.988 | ||
| BR | 39.62 | 14.60 | 0.10 | 0.99 | 0.87 | 19.81 | 24.96 | 58.77 | 0.991 | ||
| UR | 39.20 | 16.99 | 0.11 | 1.08 | 0.95 | 19.60 | 25.04 | 54.81 | 0.989 | ||
| ZD | CK | 37.15 | 33.17 | 0.13 | 1.21 | 1.06 | 18.58 | 25.57 | 47.28 | 0.973 | |
| SH | 31.34 | 16.36 | 0.11 | 0.86 | 0.76 | 15.67 | 27.97 | 52.72 | 0.971 | ||
| MT | 37.40 | 14.87 | 0.10 | 0.94 | 0.82 | 18.70 | 25.16 | 59.61 | 0.976 | ||
| BR | 35.89 | 16.11 | 0.11 | 0.99 | 0.87 | 17.94 | 25.95 | 55.25 | 0.986 | ||
| UR | 37.70 | 16.12 | 0.10 | 0.94 | 0.83 | 18.85 | 25.05 | 58.00 | 0.980 | ||
| S2 | XY | CK | 39.65 | 84.73 | 0.14 | 1.39 | 1.22 | 19.82 | 25.37 | 41.52 | 0.988 |
| SH | 34.04 | 27.01 | 0.11 | 0.94 | 0.82 | 17.02 | 26.47 | 53.00 | 0.997 | ||
| MT | 39.67 | 21.56 | 0.10 | 0.99 | 0.87 | 19.84 | 25.35 | 58.32 | 0.999 | ||
| BR | 39.90 | 21.59 | 0.10 | 1.00 | 0.87 | 19.94 | 25.33 | 57.54 | 0.992 | ||
| UR | 40.63 | 19.33 | 0.10 | 1.02 | 0.89 | 20.32 | 25.27 | 60.45 | 0.997 | ||
| ZD | CK | 40.28 | 70.41 | 0.13 | 1.31 | 1.15 | 20.14 | 25.30 | 46.22 | 0.987 | |
| SH | 34.47 | 18.29 | 0.09 | 0.78 | 0.68 | 17.24 | 27.27 | 63.94 | 0.986 | ||
| MT | 40.63 | 13.48 | 0.08 | 0.81 | 0.71 | 20.31 | 25.10 | 73.20 | 0.987 | ||
| BR | 40.31 | 15.47 | 0.09 | 0.91 | 0.80 | 20.16 | 25.18 | 69.13 | 0.980 | ||
| UR | 39.19 | 19.78 | 0.10 | 0.98 | 0.86 | 19.59 | 25.33 | 62.40 | 0.984 | ||
| Correlation coefficient (r) | -- | -- | -- | 0.715 ** | 0.712 ** | 0.807 ** | −0.821 ** | −0.353 | |||
| Sowing Date | Variety | Treatment | LAI_Recovery_R1 (%) | LAI_Recovery_R3 (%) | ∆Tmax (Days) | Yield_Loss (%) | Yield_Recovery (%) |
|---|---|---|---|---|---|---|---|
| S1 | XY | CK | 100.0 | 100.0 | 1.2 | 35.9 | 100.0 |
| SH | 0.0 | 0.0 | 0.0 | 35.9 | 0.0 | ||
| MT | 51.4 | 53.5 | 1.9 | 35.9 | 50.3 | ||
| BR | 26.1 | 54.6 | 2.0 | 35.9 | 59.0 | ||
| UR | 23.2 | 21.2 | 1.9 | 35.9 | 47.2 | ||
| ZD | CK | 100.0 | 100.0 | 2.4 | 48.9 | 100.0 | |
| SH | 0.0 | 0.0 | 0.0 | 48.9 | 0.0 | ||
| MT | 39.9 | 31.3 | 2.8 | 48.9 | 44.9 | ||
| BR | 31.3 | 30.6 | 2.0 | 48.9 | 54.7 | ||
| UR | 45.4 | 54.2 | 2.9 | 48.9 | 54.7 | ||
| S2 | XY | CK | 100.0 | 100.0 | 1.1 | 21.8 | 100.0 |
| SH | 0.0 | 0.0 | 0.0 | 21.8 | 0.0 | ||
| MT | 16.0 | 14.1 | 1.1 | 21.8 | 74.1 | ||
| BR | 11.2 | 10.4 | 1.1 | 21.8 | 58.6 | ||
| UR | 39.0 | 40.0 | 1.2 | 21.8 | 58.9 | ||
| ZD | CK | 100.0 | 100.0 | 2.0 | 41.4 | 100.0 | |
| SH | 0.0 | 0.0 | 0.0 | 41.4 | 0.0 | ||
| MT | 7.0 | 20.1 | 2.2 | 41.4 | 18.9 | ||
| BR | 9.5 | 12.9 | 2.1 | 41.4 | 35.6 | ||
| UR | 15.2 | 22.4 | 1.9 | 41.4 | 27.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, A.; Bian, D.; Chen, X.; Yang, Q.; Wei, Z.; Du, X.; Gao, Z.; Liu, G.; Cui, Y. Exogenous Regulators Enhance Physiological Recovery and Yield Compensation in Maize Following Mechanical Leaf Damage. Agronomy 2025, 15, 2234. https://doi.org/10.3390/agronomy15092234
Jiang A, Bian D, Chen X, Yang Q, Wei Z, Du X, Gao Z, Liu G, Cui Y. Exogenous Regulators Enhance Physiological Recovery and Yield Compensation in Maize Following Mechanical Leaf Damage. Agronomy. 2025; 15(9):2234. https://doi.org/10.3390/agronomy15092234
Chicago/Turabian StyleJiang, Aonan, Dahong Bian, Xushuang Chen, Qifan Yang, Zhongbo Wei, Xiong Du, Zhen Gao, Guangzhou Liu, and Yanhong Cui. 2025. "Exogenous Regulators Enhance Physiological Recovery and Yield Compensation in Maize Following Mechanical Leaf Damage" Agronomy 15, no. 9: 2234. https://doi.org/10.3390/agronomy15092234
APA StyleJiang, A., Bian, D., Chen, X., Yang, Q., Wei, Z., Du, X., Gao, Z., Liu, G., & Cui, Y. (2025). Exogenous Regulators Enhance Physiological Recovery and Yield Compensation in Maize Following Mechanical Leaf Damage. Agronomy, 15(9), 2234. https://doi.org/10.3390/agronomy15092234




