Comparative UV-B Stress Responses in Maize and Sorghum Based on Biophoton Emission Measurements and Morphophysiological Traits
Abstract
1. Introduction
2. Materials and Methods
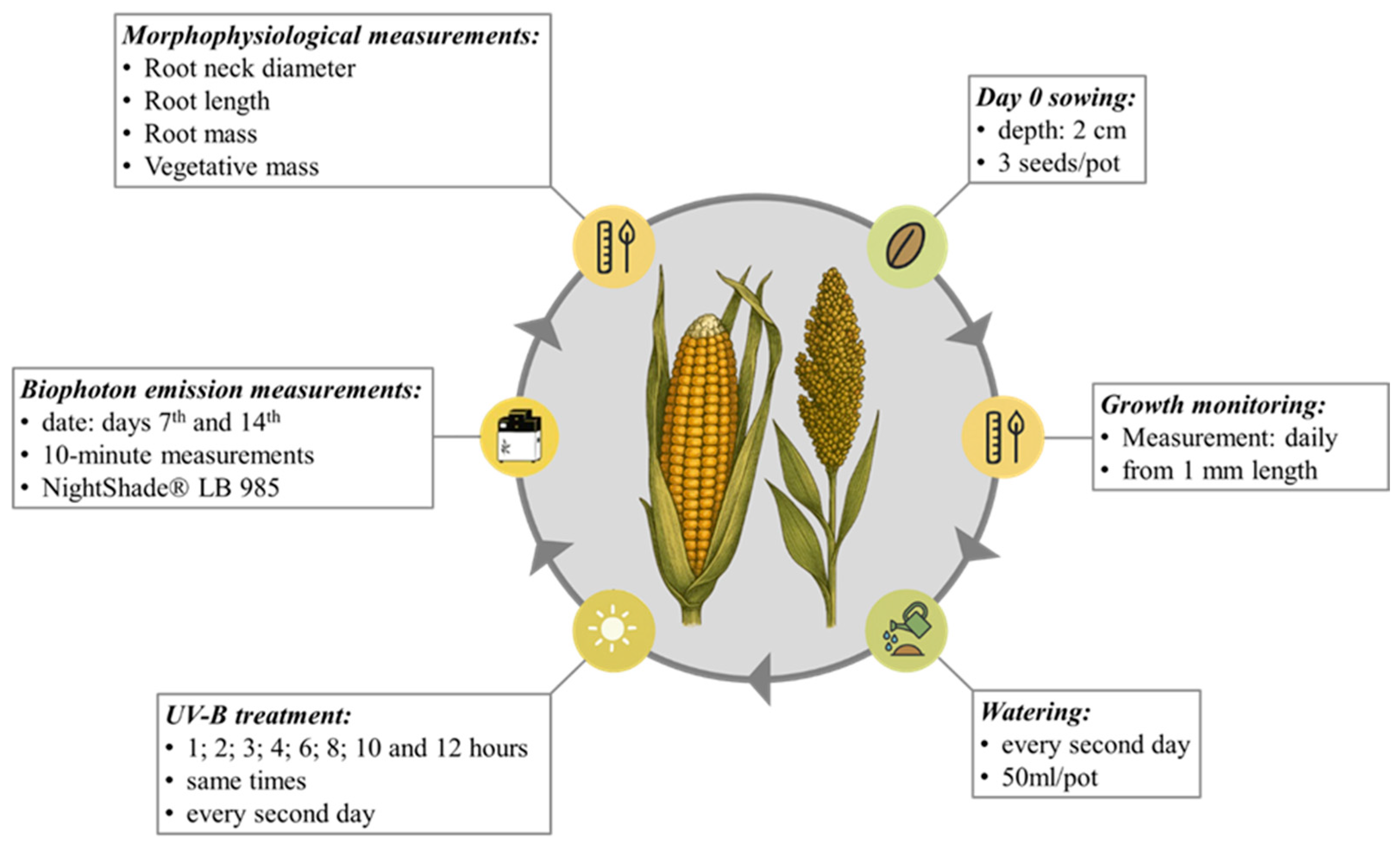
2.1. In Vivo Stress Testing Methods
2.2. Examining Stem and Root Growth
2.3. Statistical Methodologies
3. Results
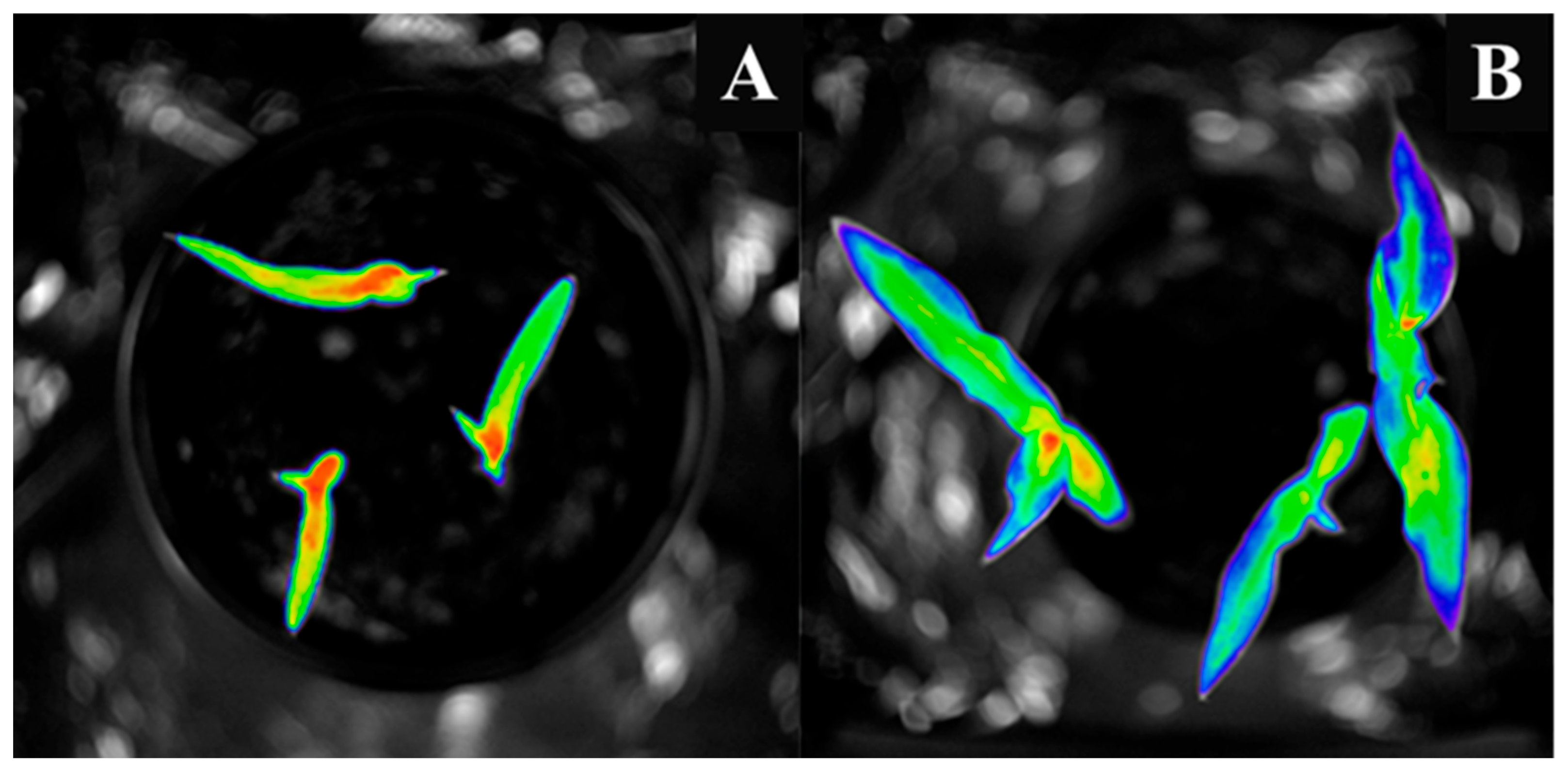
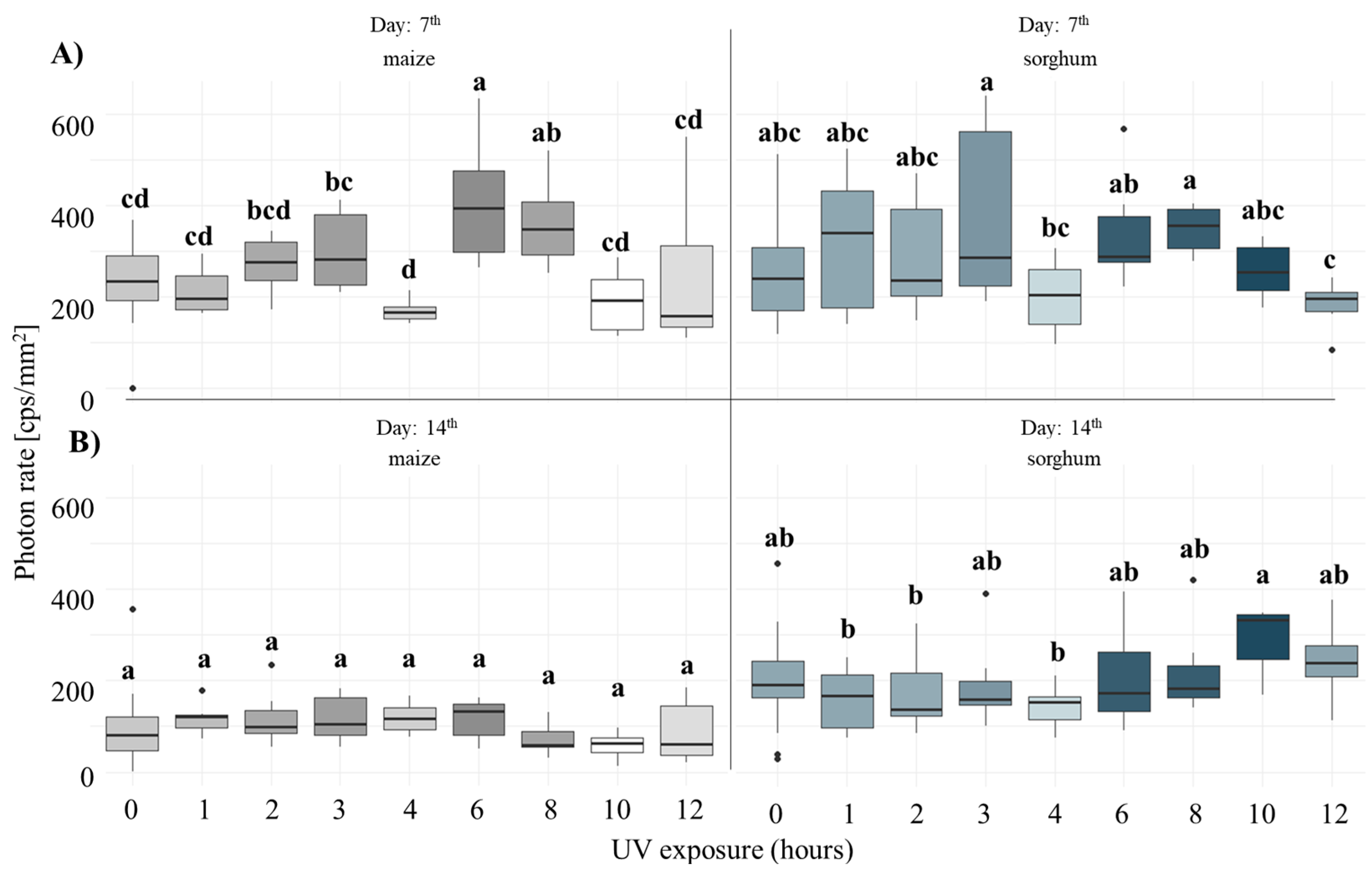
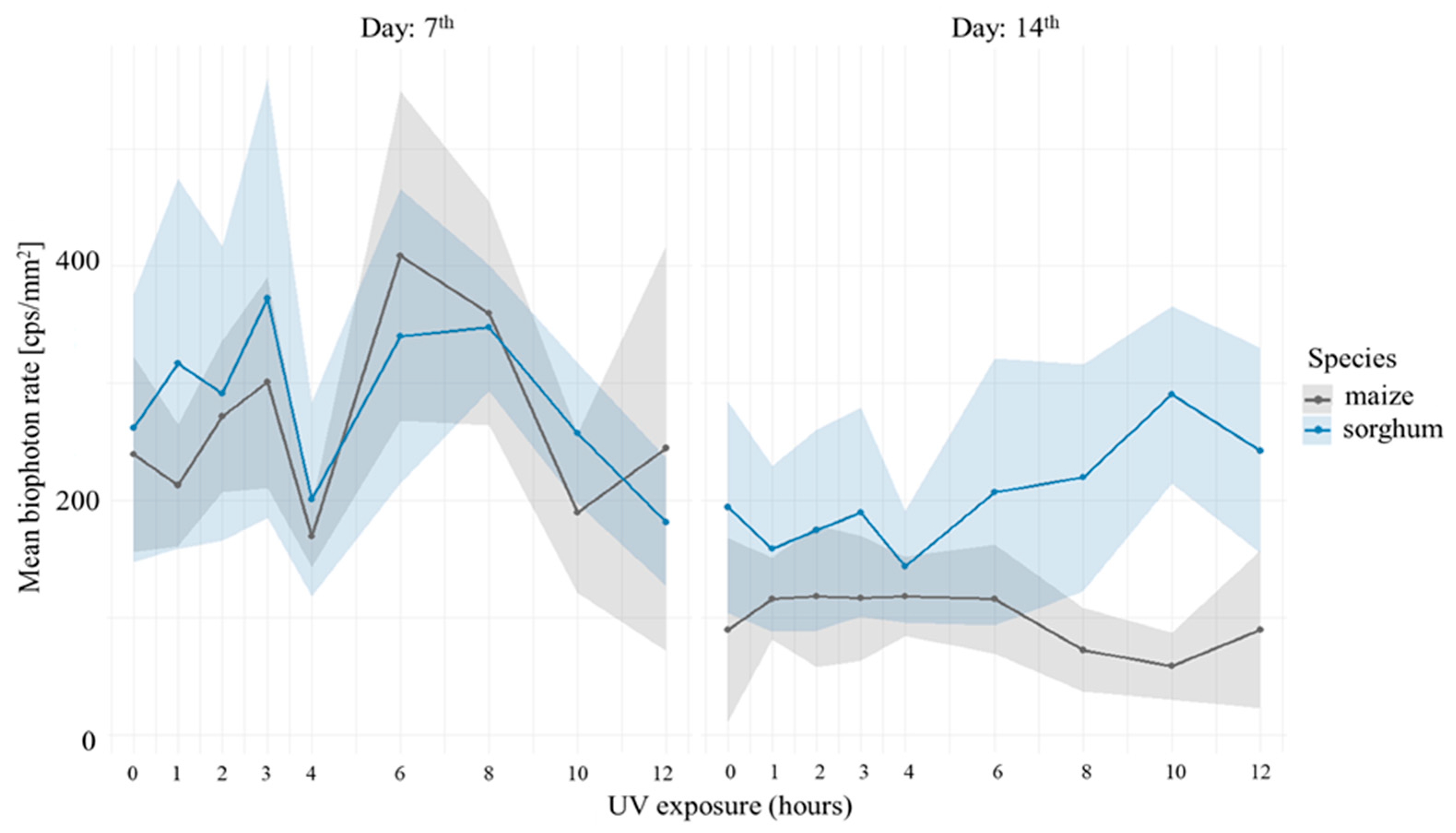
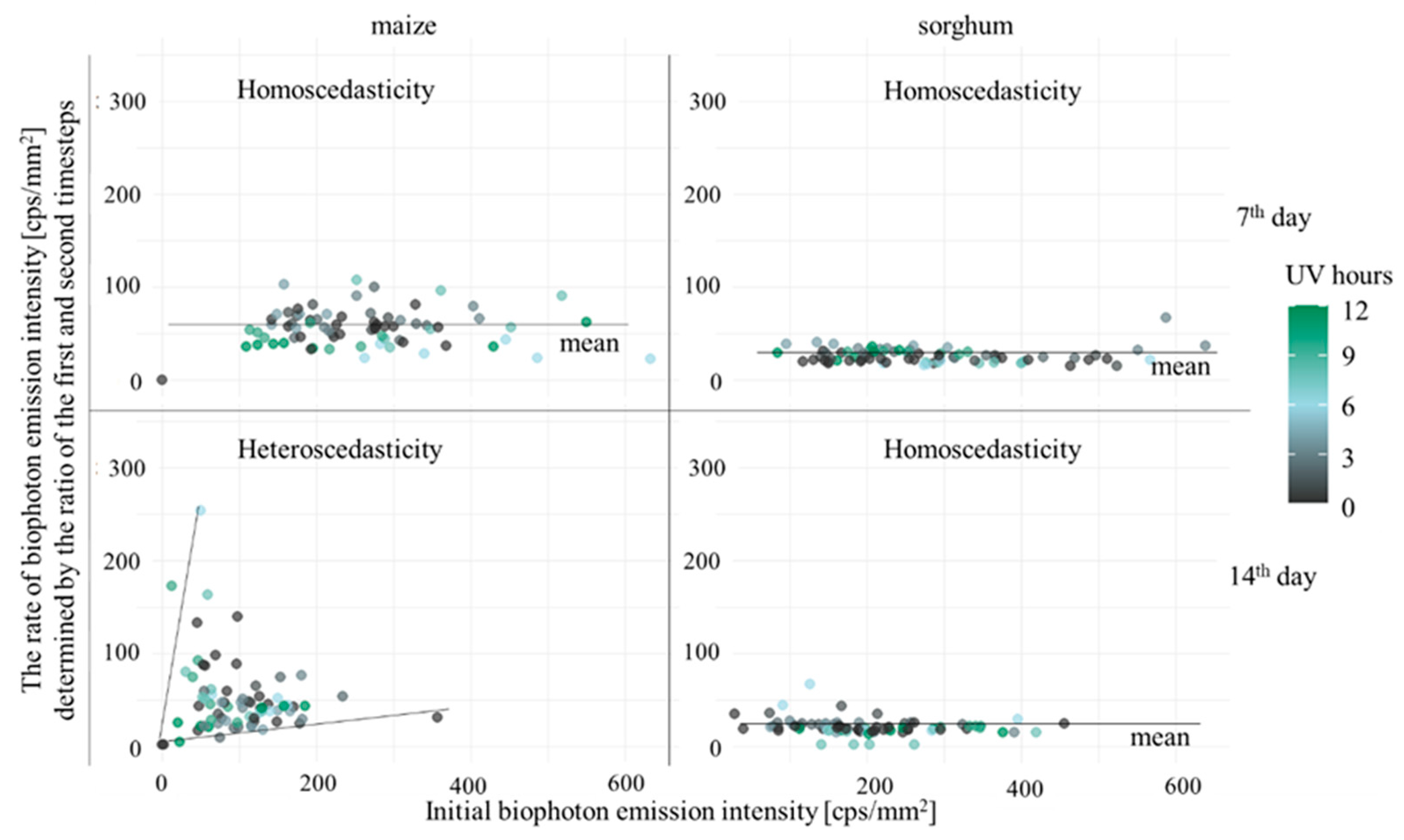
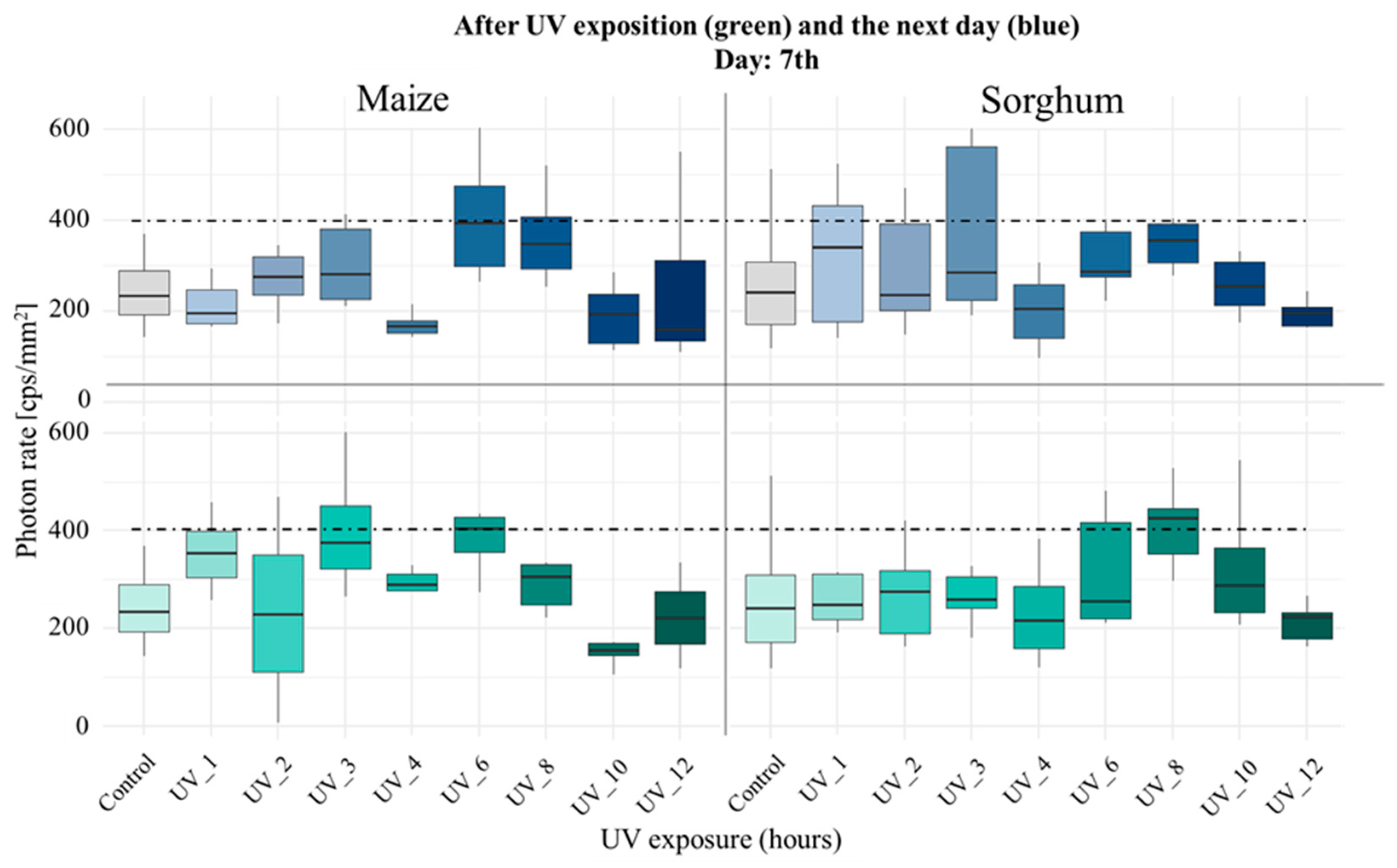
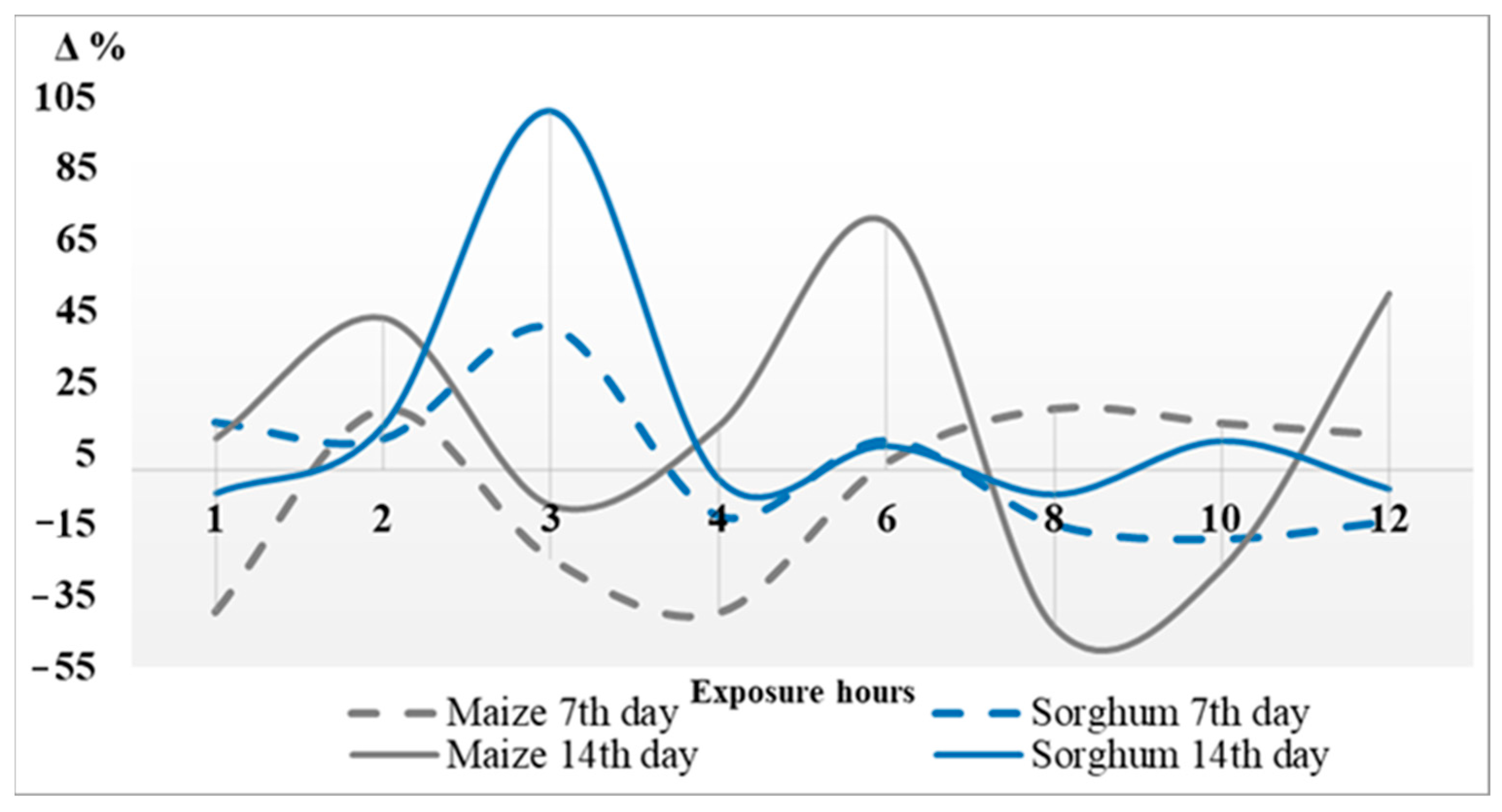
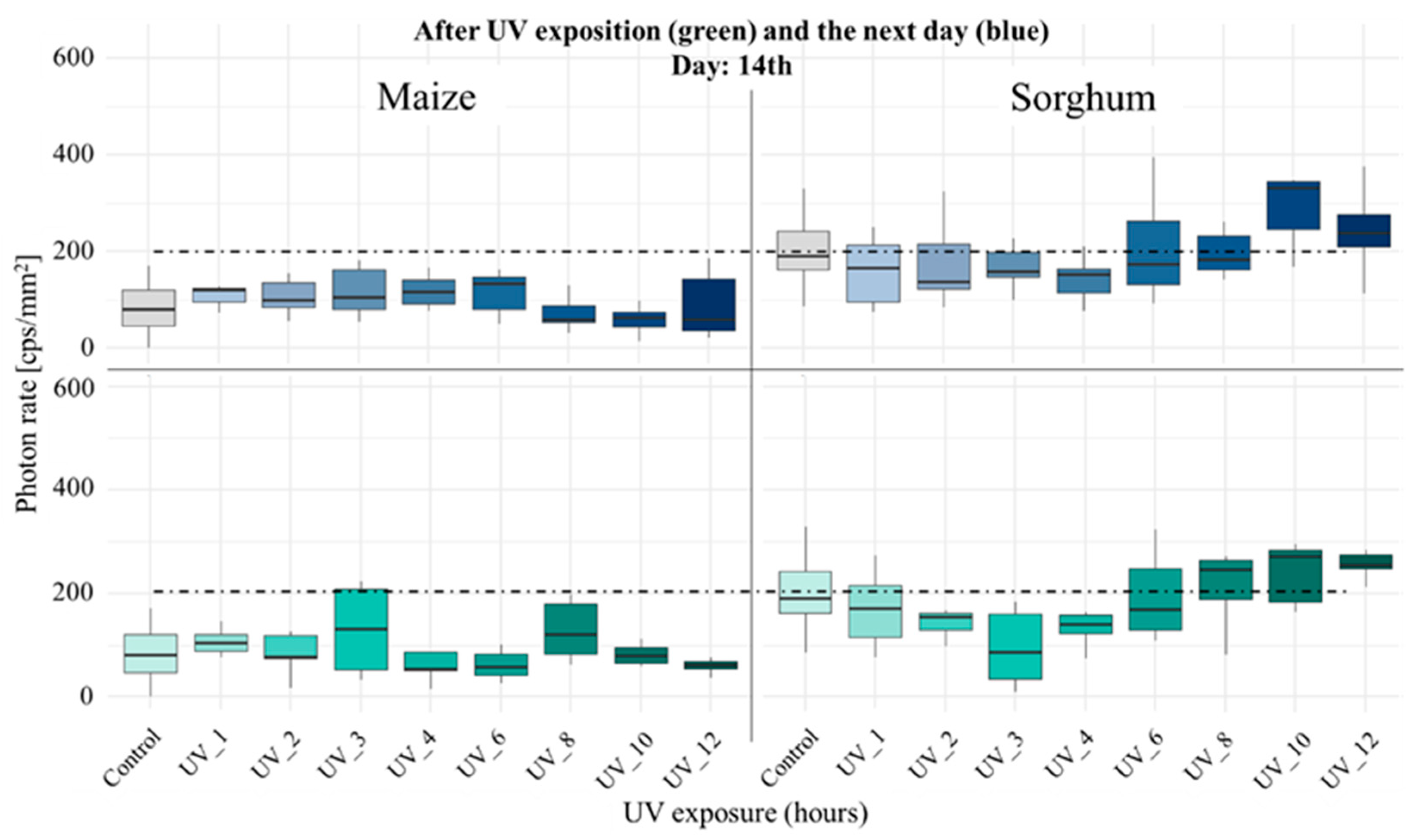
3.1. Comparison of Stress Tolerance of Maize and Sorghum
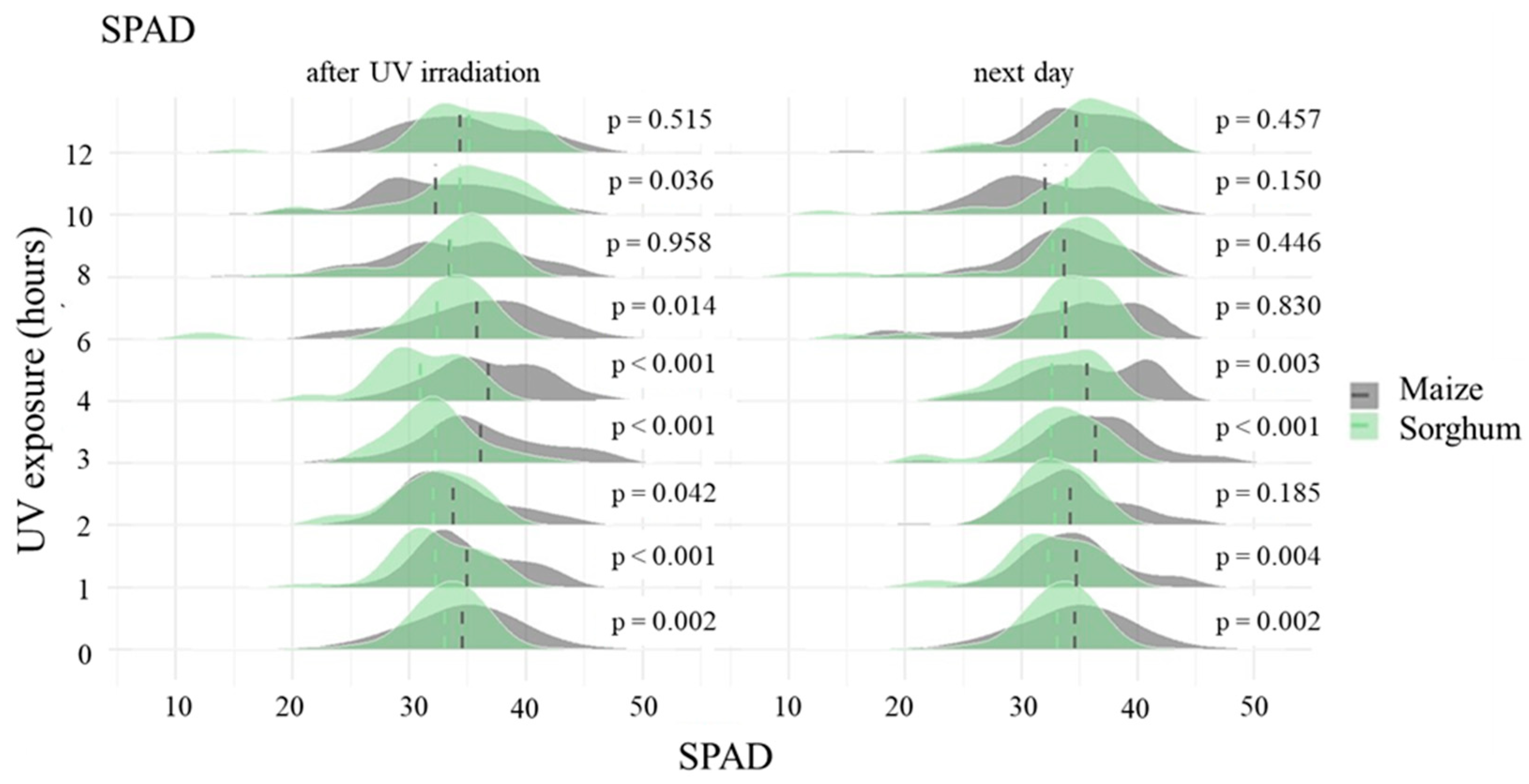
3.2. Comparison of the Leaf Chlorophyll Content of Maize and Sorghum as an Answer to UV-B Exposure
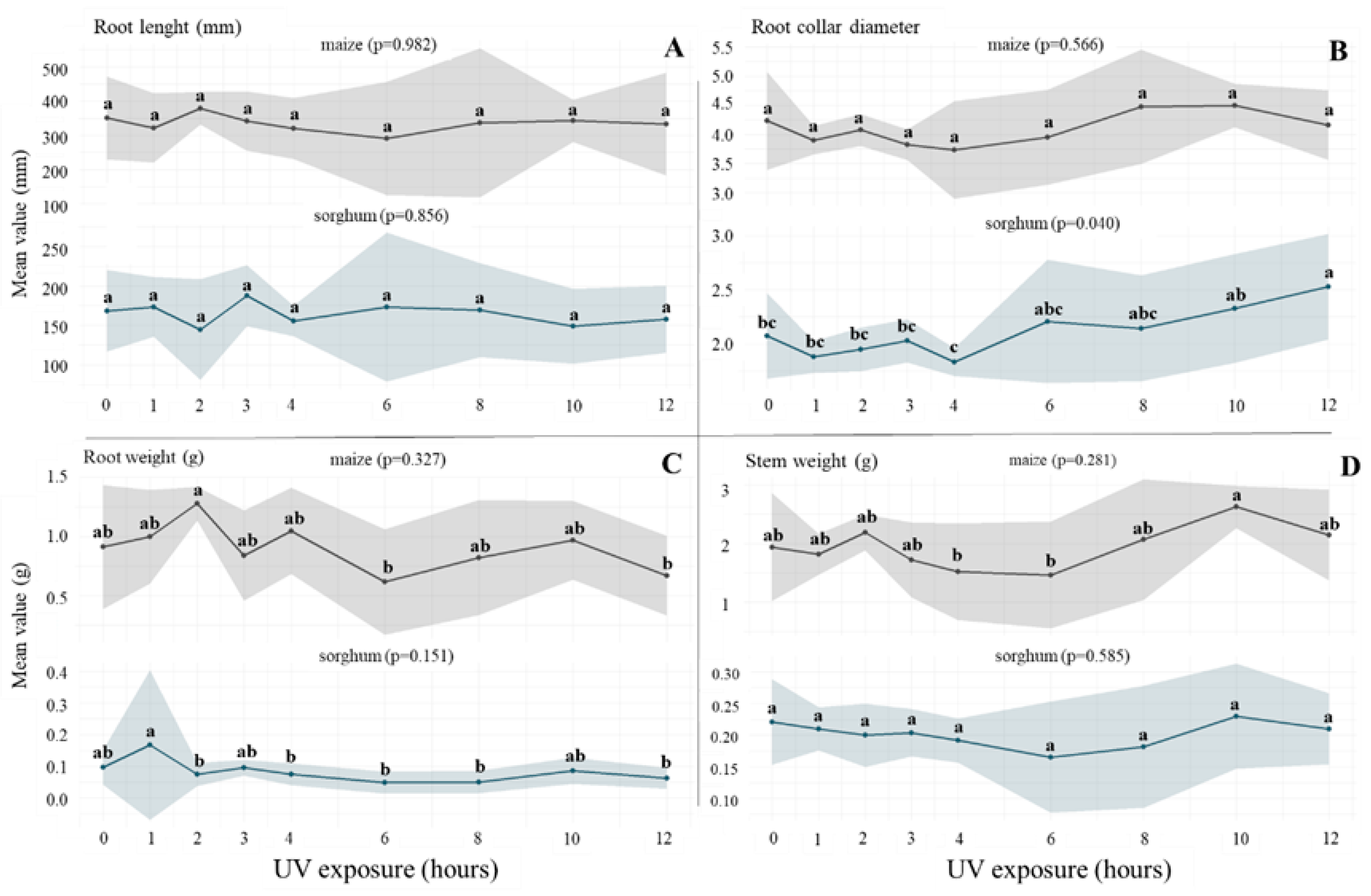
3.3. Comparison of the Root and Stem Characteristics of Maize and Sorghum as the Answer to UV-B Exposure
4. Discussion
4.1. Biophoton Emission as an Indicator of Stress
4.2. Chlorophyll Content of Leaves in Maize and Sorghum Under UV-B Exposure
4.3. Root and Stem Traits of Maize and Sorghum Under UV-B Exposure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tubiello, F.N.; Fabi, C.; Conchedda, G.; Casse, L.; Bottini, G. Comparison of WorldCereal with FAOSTAT Data: An Exploratory Analysis. In FAO Statistics Working Paper Series; FAO: Rome, Italy, 2025; pp. 25–46. [Google Scholar] [CrossRef]
- Bakari, H.; Djomdi Ruben, Z.F.; Roger, D.D.; Cedric, D.; Guillaume, P.; Pascal, D.; Philippe, M.; Gwendoline, C. Sorghum (Sorghum bicolor L. Moench) and Its Main Parts (By-Products) as Promising Sustainable Sources of Value-Added Ingredients. Waste Biomass Valorization 2023, 14, 1023–1044. [Google Scholar] [CrossRef]
- Popescu, A.; Condei, R. Some Considerations on the Prospects of Sorghum Crop. Sci. Pap. 2014, 14, 295–304. [Google Scholar]
- Gerik, T.; Bean, B.; Vanderlip, R. Sorghum Growth and Development. Agric. Commun. 2003, B-6137 7-03. [Google Scholar]
- Tao, Y.; Zhao, X.; Wang, X.; Hathorn, A.; Hunt, C.; Cruickshank, A.W.; van Oosterom, E.J.; Godwin, I.D.; Mace, E.S.; Jordan, D.R. Large-scale GWAS in Sorghum Reveals Common Genetic Control of Grain Size among Cereals. Plant Biotechnol. J. 2020, 18, 1093–1105. [Google Scholar] [CrossRef]
- Buhiniček, I.; Kaučić, D.; Kozić, Z.; Jukić, M.; Gunjača, J.; Šarčević, H.; Stepinac, D.; Šimić, D. Trends in Maize Grain Yields across Five Maturity Groups in a Long-Term Experiment with Changing Genotypes. Agriculture 2021, 11, 887. [Google Scholar] [CrossRef]
- Bais, A.F.; McKenzie, R.L.; Bernhard, G.; Aucamp, P.J.; Ilyas, M.; Madronich, S.; Tourpali, K. Ozone depletion and climate change: Impacts on UV radiation. Photochem. Photobiol. Sci. 2015, 14, 19–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Twine, T.E.; Girvetz, E. Climate Change and Maize Yield in Iowa. PLoS ONE. 2016, 11, 156083. [Google Scholar] [CrossRef]
- Cockell, C.S.; Horneck, G. The History of the UV Radiation Climate Ofthe Earth—Theoretical and Space-Based Observations. Photochem. Photobiol. Sci. 2001, 73, 447–451. [Google Scholar] [CrossRef]
- Horneck, G.; Klaus, D.M.; Mancinelli, R.L. Space microbiology. Microbiol. Mol. Biol. Rev. 2010, 74, 121–156. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, R.L.; Aucamp, P.J.; Bais, A.F.; Björn, L.O.; Ilyas, M.; Madronich, S. Ozone depletion and climate change: Impacts on UV radiation. Photochem. Photobiol. Sci. 2011, 10, 182–198. [Google Scholar] [CrossRef]
- Mmbando, G.S.; Teranishi, M.; Hidema, J. Very High Sensitivity Of African Rice to Artificial Ultraviolet-B Radiation Caused by Genotypeand Quantity of Cyclobutane Pyrimidine Dimer Photolyase. Sci. Rep. 2020, 10, 3158. [Google Scholar] [CrossRef]
- Tevini, M. UV-B Effects on Plants. In Environmental Pollution and Plant Responses; Routledge: Oxford, UK, 2023; pp. 83–97. [Google Scholar]
- Middleton, E.M.; Teramura, A.H. The Role of Flavonol Glycosides and Carotenoids in Protecting Soybean from Ultraviolet-B Damage. Plant Physiol. 1993, 103, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, K.; Hidema, J.; Tamura, K.; Takagi, S.; Hara-Nishimura, I. Plant Nuclei Move to Escape Ultraviolet-Induced DNA Damage Andcell Death. Plant Physiol. 2016, 170, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Hermanowicz, P.; Banaś, A.K.; Sztatelman, O.; Gabryś, H.; Łabuz, J. UV-B Induces Chloroplast Movements in a Phototropin-Dependent Manner. Front. Plant Sci. 2019, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Rácz, A.; Hideg, É.; Czégény, G. Selective Responses of Class III Plant Peroxidase Isoforms to Environmentally Relevant UV-B Doses. J. Plant Physiol. 2018, 221, 101–106. [Google Scholar] [CrossRef]
- Petrov, V.D.; Breusegem, F. Hydrogen Peroxide—A Central Hub for Information Flow in Plant Cells. AoB Plants 2012, 2012, pls014. [Google Scholar] [CrossRef]
- Czégény, G.; Wu, M.; Dér, A.; Eriksson, L.A.; Strid, Å.; Hideg, É. Hydrogen Peroxide Contributes to the Ultraviolet-B(280–315 Nm) Induced Oxidative Stress of Plant Leaves through Multiple Pathways. FEBS Lett. 2014, 588, 2255–2261. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of Reactive Oxygen Species by Photosystem II as a Response to Light and Temperature Stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef]
- Li, X.; Ren, Q.; Zhao, W.; Liao, C.; Wang, Q.; Ding, T.; Hu, H.; Wang, M. Interaction between UV-B and Plant Anthocyanins. Funct. Plant Biol. 2023, 50, 599–611. [Google Scholar] [CrossRef]
- Li, Y.; Zu, Y.Q.; Chen, J.J.; Chen, H.Y. Intraspecific Responses in Crop Growth and Yield of 20 Soybean Cultivars to Enhanced Ultraviolet-B Radiation under Field Conditions. Field Crops Res. 2002, 78, 1–8. [Google Scholar] [CrossRef]
- Kataria, S.; Guruprasad, K.N. Solar UV-B and UV-A/B Exclusion Effects on Intraspecific Variations in Crop Growth and Yield of Wheat Varieties. Field Crops Res. 2012, 125, 8–13. [Google Scholar] [CrossRef]
- He, J.; Huang, L.K.; Chow, W.S.; Whitecross, M.I.; Anderson, J.M. Effects of Supplementary Ultraviolet-B Radiation on Rice and Pea Plants. Funct. Plant Biol. 1993, 20, 129–142. [Google Scholar] [CrossRef]
- Hofmann, R.W.; Campbell, B.D.; Bloor, S.J.; Swinny, E.E.; Markham, K.R.; Ryan, K.G.; Fountain, D.W. Responses to UV-B Radiation in Trifolium repens L.—Physiological Links to Plant Productivity and Water Availability. Plant Cell Environ. 2003, 26, 603–612. [Google Scholar] [CrossRef]
- Hunt, J.E.; McNeil, D.L. Nitrogen status affects UV-B sensitivity of cucumber. Funct. Plant Biol. 1998, 25, 79–86. [Google Scholar] [CrossRef]
- DeLong, J.M.; Steffen, K.L. Photosynthetic Function, Lipid Peroxidation, and α-Tocopherol Content in Spinach Leaves during Exposure to UV-B Radiation. Can. J. Plant Sci. 1997, 77, 453–459. [Google Scholar] [CrossRef]
- Shaukat, S.S.; Farooq, M.A.; Siddiqui, M.F.; Zaidi, S. Effect of Enhanced UV-B Radiation on Germination, Seedling Growth and Biochemical Responses of Vigna mungo (L.) Hepper. Pak. J. Bot. 2013, 45, 779–785. [Google Scholar]
- Carletti, P.; Masi, A.; Wonisch, A.; Grill, D.; Tausz, M.; Ferretti, M. Changes in Antioxidant and Pigment Pool Dimensions in UV-B Irradiated Maize Seedlings. Environ. Exp. Bot. 2003, 50, 149–157. [Google Scholar] [CrossRef]
- Gao, W.; Zheng, Y.; Slusser, J.R.; Heisler, G.M.; Grant, R.H.; Xu, J.; He, D. Effects of Suplementary Ultraviolet-B Irradiance on Maize Yield and Qualities: A Field Experiment. Photochem. Photobiol. 2004, 80, 127–131. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, R. Diurnal Changes of Gas Exchange, Chlorophyll Fluorescence, and Stomatal Aperture of Hybrid Poplar Clones Subjected to Midday Light Stress. Photosynthetica 2000, 37, 559–571. [Google Scholar] [CrossRef]
- Jovanić, B.R.; Radenković, B.; Despotović-Zrakić, M.; Bogdanović, Z.; Barać, D. Effect of UV-B Radiation on Chlorophyll Fluorescence, Photosynthetic Activity and Relative Chlorophyll Content of Five Different Corn Hybrids. J. Photochem. Photobiol. 2022, 10, 100115. [Google Scholar] [CrossRef]
- Kataria, S.; Guruprasad, K.N. Intraspecific Variations in Growth, Yield and Photosynthesis of Sorghum Varieties to Ambient UV (280–400 Nm) Radiation. Plant Sci. 2012, 196, 85–92. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, Y.; Ren, J.; Li, C. UV-Spectra Dependence of Seedling Injury and Photosynthetic Pigment Change in Cucumis Sativus and Glycine Max. Environ. Exp. Bot. 2006, 57, 160–167. [Google Scholar] [CrossRef]
- Dehariya, P.; Kataria, S.; Pandey, G.P.; Guruprasad, K.N. Photosynthesis and Yield in Cotton (Gossypium hirsutum L.) Var. Vikram after Exclusion of Ambient Solar UV-B/A. Acta Physiol. Plant. 2012, 34, 1133–1144. [Google Scholar] [CrossRef]
- Rrobson, T.M.; Klem, K.; Urban, O.; Jansen, M.A.K. Plant Morphological Responses to UV-B. Plant Cell Environ. 2015, 38, 856–866. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Y.; He, H. Influence of Ambient and Enhanced Ultraviolet-B Radiation on the Plant Growth and Physiological Properties in Contrasting Populations of Hippophae rhamnoides. J. Plant Res. 2008, 121, 377–385. [Google Scholar] [CrossRef]
- Klem, K.; Ac, A.; Holub, P.; Kovac, D.; Spunda, V.; Robson, T.M.; Urban, O. Interactive Effects of PAR and UV Radiation on the Physiology, Morphology and Leaf Optical Properties of Two Barley Varieties. Environ. Exp. Bot. 2012, 75, 52–64. [Google Scholar] [CrossRef]
- Robson, T.M.; Aphalo, P.J. Species-Specific Effect of UV-B Radiation on the Temporal Pattern of Leaf Growth. Physiol. Plant. 2012, 144, 146–160. [Google Scholar] [CrossRef]
- Liaqat, W.; Altaf, M.T.; Barutçular, C. Ultraviolet-B Radiation in Relation to Agriculture in the Context of Climate Change: A Review. Cereal Res. Commun. 2024, 52, 1–24. [Google Scholar] [CrossRef]
- Wong, H.M.; Hofmann, R.W.; Reichman, S.M. The Interactions of Iron Nutrition, Salinity and Ultraviolet-B Radiation on the Physiological Responses of Wheat (Triticum aestivum L.). Environ. Exp. Bot. 2023, 207, 105201. [Google Scholar] [CrossRef]
- Srivastava, G.; Kumar, S.; Dubey, G. Nickel and Ultraviolet-B Stresses Induce Differential Growth and Photosynthetic Responses in Pisum sativum L. Seedlings. Biol. Trace Elem. Res. 2012, 149, 86–96. [Google Scholar] [CrossRef]
- Schmitz-Hoerner, R.; Weissenböck, G. Contribution of Phenolic Compounds to the UV-B Screening Capacity of Developing Barley Primary Leaves in Relation to DNA Damage and Repair under Elevated UV-B Levels. Phytochemistry 2003, 64, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Ruhland, C.T.; Eatwell, M.F. The Effects of Ultraviolet Radiation on the Brown Midrib Mutants of Zea mays and Sorghum bicolor. Theor. Exp. Plant Physiol. 2017, 29, 87–94. [Google Scholar] [CrossRef]
- Zhang, L.; Allen, L.H.; Vaughan, M.M.; Hauser, B.A.; Boote, K.J. Solar Ultraviolet Radiation Exclusion Increases Soybean Internode Lengths and Plant Height. Agric. For. Meteorol. 2014, 184, 170–178. [Google Scholar] [CrossRef]
- Coleman, R.S.; Day, T.A. Response of Cotton and Sorghum to Several Levels of Subambient Solar UV-B Radiation: A Test of the Saturation Hypothesis. Physiol. Plant. 2004, 122, 362–372. [Google Scholar] [CrossRef]
- Chen, W.; Jia, B.; Chen, J.; Feng, Y.; Li, Y.; Chen, M.; Yin, Z. Effects of Different Planting Densities on Photosynthesis in Maize Determined via Prompt Fluorescence, Delayed Fluorescence and P700 Signals. Plants 2021, 10, 276. [Google Scholar] [CrossRef]
- Pónya, Z.s.; Jócsák, I.; Keszthelyi, S. Detection of Ultra-Weak Photon Emission in Sunflower (Helianthus annuus L.) Infested by Two Spotted-Spider Mite, Tetranychus Urticae Koch-Research Note. Phytoparasitica 2022, 50, 43–50. [Google Scholar] [CrossRef]
- Jócsák, I.; Csima, F.; Somfalvi-Tóth, K. Alterations of Photosynthetic and Oxidative Processes Influenced by the Presence of Different Zinc and Cadmium Concentrations in Maize Seedlings: Transition from Essential to Toxic Functions. Plants 2024, 13, 1150. [Google Scholar] [CrossRef]
- Sánchez-Moreiras, A.M.; Graña, E.; Reigosa, M.J.; Araniti, F. Imaging of Chlorophyll a Fluorescence in Natural Compound-Induced Stress Detection. Front. Plant Sci. 2020, 11, 583590. [Google Scholar] [CrossRef]
- Lukács, H.; Jócsák, I.; Somfalvi-Tóth, K.; Keszthelyi, S. Physiological Responses Manifested by Some Conventional Stress Parameters and Biophoton Emission in Winter Wheat as a Consequence of Cereal Leaf Beetle Infestation. Front. Plant Sci. 2022, 13, 839855. [Google Scholar] [CrossRef]
- Jocsak, I.; Malgwi, I.; Rabnecz, G.; Szegő, A.; Varga-Visi, E.; Végvári, G.; Ponya, Z. Effect of Cadmium Stress on Certain Physiological Parameters Antioxidative Enzyme Activities Biophoton Emission of Leaves in Barley (Hordeum vulgare L.) Seedlings. PLoS ONE 2020, 15, 240470. [Google Scholar] [CrossRef]
- Wu, G.; Bornman, J.F.; Bennett, S.J.; Clarke, M.W.; Fang, Z.; Johnson, S.K. Individual Polyphenolic Profiles and Antioxidant Activity in Sorghum Grains Are Influenced by Very Low and High Solar UV Radiation and Genotype. J. Cereal Sci. 2017, 77, 17–23. [Google Scholar] [CrossRef]
- Podolec, R.; Demarsy, E.; Ulm, R. Perception and Signaling of Ultraviolet-B Radiation in Plants. Annu. Rev. Plant Biol. 2021, 72, 793–822. [Google Scholar] [CrossRef] [PubMed]
- Haripriya Anand, M.; Byju, G. Chlorophyll Meter and Leaf Colour Chart to Estimate Chlorophyll Content, Leaf Colour, and Yield of Cassava. Photosynthetica 2008, 46, 511–516. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 18 July 2025).
- Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R package version 4.3.2. 2023.
- Santos, I.; Almeida, J.; Salema, R. The Influence of UV-B Radiation on the Superoxide Dismutase of Maize, Potato, Sorghum, and Wheat Leaves. Can. J. Bot. 2016, 77, 70–76. [Google Scholar] [CrossRef]
- Correia, C.M.; Coutinho, J.F.; Björn, L.O.; Torres-Pereira, J.M. Ultraviolet-B Radiation and Nitrogen Effects on Growth and Yield of Maize under Mediterranean Field Conditions. Eur. J. Agron. 2000, 12, 117–125. [Google Scholar] [CrossRef]
- Goltsev, V.; Zaharieva, I.; Chernev, P.; Strasser, R. Delayed Chlorophyll Fluorescence as a Monitor for Physiological State of Photosynthetic Apparatus. Biotechnol. Biotechnol. Equip. 2009, 23, 452–457. [Google Scholar] [CrossRef]
- Hideg, É.; Juhász, M.; Bornman, J.F. The Distribution and Possible Origin of Blue—Green Fluorescence in Control and Stressed Barley Leaves. Photochem. Photobiol. Sci. 2002, 1, 934–941. [Google Scholar] [CrossRef]
- Shine, M.B.; Guruprasad, K.N. Oxyradicals and PSII Activity in Maize Leaves in the Absence of UV Components of Solar Spectrum. J. Biosci. 2012, 37, 703–712. [Google Scholar] [CrossRef]
- Papadopoulos, Y.A.; Gordon, R.J.; Mcrae, K.; Bush, R.; Belanger, G.; Butler, G.E.; Sherry, A.E.; Morrison, F.M. Current and Elevated Levels of UV-B Radiation Have Few Impacts on Yields of Perennial Forage Crops. Glob. Change Biol. 1999, 5, 847–856. [Google Scholar] [CrossRef]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Sailaja, K. Field Crop Responses to Ultraviolet-B Radiation: A Review. Agric. For. Meteorol. 2003, 120, 191–218. [Google Scholar] [CrossRef]
- Casati, P.; Walbot, V. Rapid Transcriptome Responses of Maize (Zea mays) to UV-B in Irradiated and Shielded Tissues. Genome Biol. 2004, 5, R16. [Google Scholar] [CrossRef] [PubMed]
- Pitz, A.; Somfalvi-Tóth, K. A Kukorica És a Cirok Vizsgálata Juvenilis Fejlődési Szakaszban Különböző Abiotikus Stresszorok (Víz, UV-B Sugárzás) Hatása Mellett. In Proceedings of the 2. Alkalmazott Növénytudományi Konferencia: Roncsolásmentes Képalkotó Technológiák a Növénytermesztésben: Legújabb Eredmények és Alkalmazási Lehetőségek, Balatonkenese, Hungary, 28 September 2023; Keszthelyi, S., Jócsák, I., Eds.; Magyar Agrár- és Élettudományi Egyetem, Növénytermesztési-tudományok Intézet: Kaposvár, Magyarország, 2023; p. 35. [Google Scholar]
- Pitz, A.; Somfalvi-Tóth, K. Abraktakarmányok (Kukorica, Szemes Cirok) Stresszreakciója Magas UV-B Besugárzás Hatására. In Proceedings of the XIV, Magyar Növénybiológiai Kongresszus: Összefoglaló Kötet, Balatonkenese, Hungary, 28–30 August 2024; Magyar Növénybiológiai Társaság, Ed.; Szegedi Biológiai Kutatóközpont: Szeged, Magyarország, 2024; p. 92. [Google Scholar]
- Selye, H. The Stress of Life, Rev ed; McGraw Hill: New York, NY, USA, 1978. [Google Scholar]
- Höhner, R.; Aboukila, A.; Kunz, H.H.; Venema, K. Proton Gradients and Proton-Dependent Transport Processes in the Chloroplast. Front. Plant Sci. 2016, 7, 218. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding Oxidative Stress and Antioxidant Functions to Enhance Photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef]
- Wilson, S.; Johnson, M.P.; Ruban, A. V Proton Motive Force in Plant Photosynthesis Dominated by ΔpH in Both Low and High Light. Plant Physiol. 2021, 187, 263–275. [Google Scholar] [CrossRef]
- Lima-Melo, Y.; Alencar, V.T.; Lobo, A.K.; Sousa, R.H.; Tikkanen, M.; Aro, E.M.; Gollan, P.J. Photoinhibition of Photosystem I Provides Oxidative Protection during Imbalanced Photosynthetic Electron Transport in Arabidopsis Thaliana. Front. Plant Sci. 2019, 10, 916. [Google Scholar] [CrossRef]
- Michelet, L.; Zaffagnini, M.; Morisse, S.; Sparla, F.; Pérez-Pérez, M.E.; Francia, F.; Lemaire, S.D. Redox Regulation of the Calvin–Benson Cycle: Something Old, Something New. Front. Plant Sci. 2013, 4, 470. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Oelmüller, R. Reactive Oxygen Species Generation and Signaling in Plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef]
- Zhang, H.; He, H.; Song, W.; Zheng, L. Pre-Harvest UVB Irradiation Enhances the Phenolic and Flavonoid Content, and Antioxidant Activity of Green- and Red-Leaf Lettuce Cultivars. Horticulturae 2023, 9, 695. [Google Scholar] [CrossRef]
- Yao, Y.; Xuan, Z.; He, Y.; Lutts, S.; Korpelainen, H.; Li, C. Principal Component Analysis of Intraspecific Responses of Tartary Buckwheat to UV-B Radiation under Field Conditions. Environ. Exp. Bot. 2007, 61, 237–245. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, G.; Wang, L.; Zhou, Q.; Huang, X. Effects of Elevated Ultraviolet-B Radiation on Root Growth and Chemical Signaling Molecules in Plants. Ecotoxicol. Environ. Saf. 2019, 171, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Bheemanahalli, R.; Jumaa, S.H.; Kakar, N.; Reddy, V.R.; Gao, W.; Reddy, K.R. Impact of Ultraviolet-B Radiation on Early-Season Morpho-Physiological Traits of Indica and Japonica Rice Genotypes. Front. Plant Sci. 2024, 15, 1369397. [Google Scholar] [CrossRef]
- Yang, J.; Li, M.R.; Qin, L.; Feng, S.H.; Zhan, F.D.; Li, Y.; He, Y.M. The Effects of Enhanced UV-B Radiation on Root Morphology and Secretion Content of Rice (Oryza sativa Linn.). IOP Conf. Ser. Earth Environ. Sci. 2020, 446, 032073. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Silicon Enhances Plant Vegetative Growth and Soil Water Retention of Soybean (Glycine max) Plants under Water-Limiting Conditions. Plants 2022, 11, 1687. [Google Scholar] [CrossRef]
- Canbay, S.; Polat, E. UV-B ışın uygulamalarının domates, hıyar ve patlıcan fidelerinde fide gelişimi ve kalitesi üzerine etkileri. Mediterr. Agric. Sci. 2019, 32, 79–84. [Google Scholar] [CrossRef]
- Meng, F.; Xiang, D.; Zhu, J.; Li, Y.; Mao, C. Molecular Mechanisms of Root Development in Rice. Rice 2019, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Cybulski, W.J.I.; Peterjohn, W.T. Effects of Ambient UV-B Radiation on the above-Ground Biomass of Seven Temperate-Zone Plant Species. Plant Ecol. 1999, 145, 175–181. [Google Scholar] [CrossRef]
- Wijewardana, C.; Henry, W.B.; Gao, W.; Reddy, K.R. Interactive Effects on CO2, Drought, and Ultraviolet-B Radiation on Maize Growth and Development. J. Photochem. Photobiol. 2016, 160, 198–209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitz, A.; Jócsák, I.; Varga, C.; Somfalvi-Tóth, K. Comparative UV-B Stress Responses in Maize and Sorghum Based on Biophoton Emission Measurements and Morphophysiological Traits. Agronomy 2025, 15, 2224. https://doi.org/10.3390/agronomy15092224
Pitz A, Jócsák I, Varga C, Somfalvi-Tóth K. Comparative UV-B Stress Responses in Maize and Sorghum Based on Biophoton Emission Measurements and Morphophysiological Traits. Agronomy. 2025; 15(9):2224. https://doi.org/10.3390/agronomy15092224
Chicago/Turabian StylePitz, András, Ildikó Jócsák, Csaba Varga, and Katalin Somfalvi-Tóth. 2025. "Comparative UV-B Stress Responses in Maize and Sorghum Based on Biophoton Emission Measurements and Morphophysiological Traits" Agronomy 15, no. 9: 2224. https://doi.org/10.3390/agronomy15092224
APA StylePitz, A., Jócsák, I., Varga, C., & Somfalvi-Tóth, K. (2025). Comparative UV-B Stress Responses in Maize and Sorghum Based on Biophoton Emission Measurements and Morphophysiological Traits. Agronomy, 15(9), 2224. https://doi.org/10.3390/agronomy15092224





