Improving Root Nitrogen Uptake via Organic Fertilizer Substitution Enhances Yield and Efficiency in Dryland Maize
Abstract
1. Introduction
2. Materials and Methods
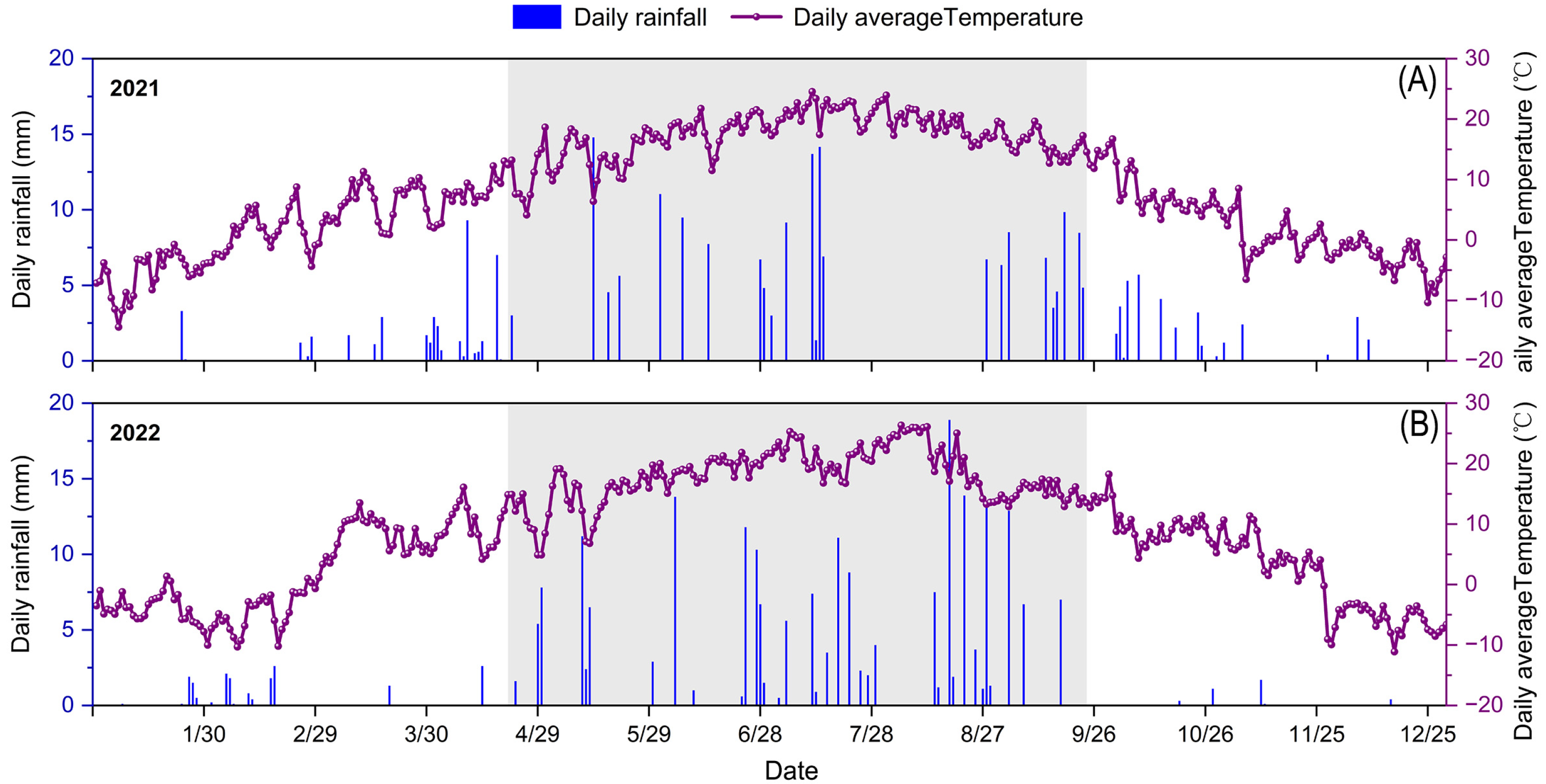
2.1. Experimental Site
2.2. Experimental Design and Field Management
2.3. Measurement Index and Methods
2.3.1. Root Dry Weight
2.3.2. Nitrogen Content in Roots
2.3.3. Roots Activity and Nitrogen Metabolism Enzyme Activities in Roots
Roots Sampling Method
Roots Activity
Nitrogen Metabolic Enzyme Activities in Roots
Nitrite reductase (NiR) activity = 192.2x − 12.34, R2 = 0.9973
Glutamine synthetase (GS) activity = 29.859x − 0.3773, R2 = 0.9952
Glutamate synthase (GOGAT) activity = 261.39x − 5.7096, R2 = 0.9974
2.3.4. Biomass, Yield, and Yield Components
2.3.5. Nitrogen Accumulation and Translocation Efficiency
2.3.6. Nitrogen Fertilizer Use Efficiency
2.4. Data Organization and Processing
3. Results
3.1. Effects of Organic Fertilizer Substitution for Basal Chemical Fertilizer on Physiological Characteristics of Maize Roots
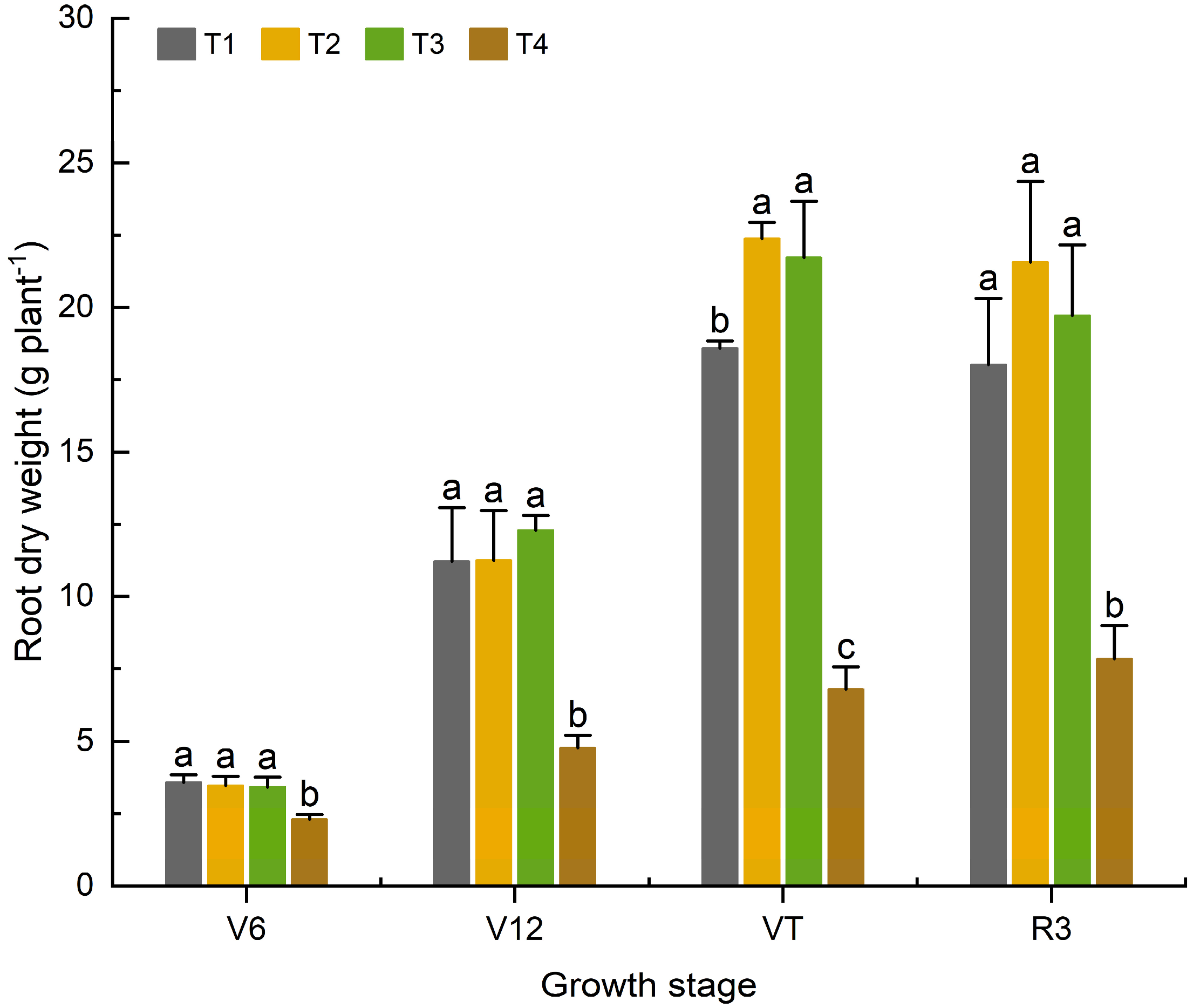
3.1.1. Effects of Organic Fertilizer Substitution for Basal Chemical Fertilizer on the Root Dry Weight of Maize
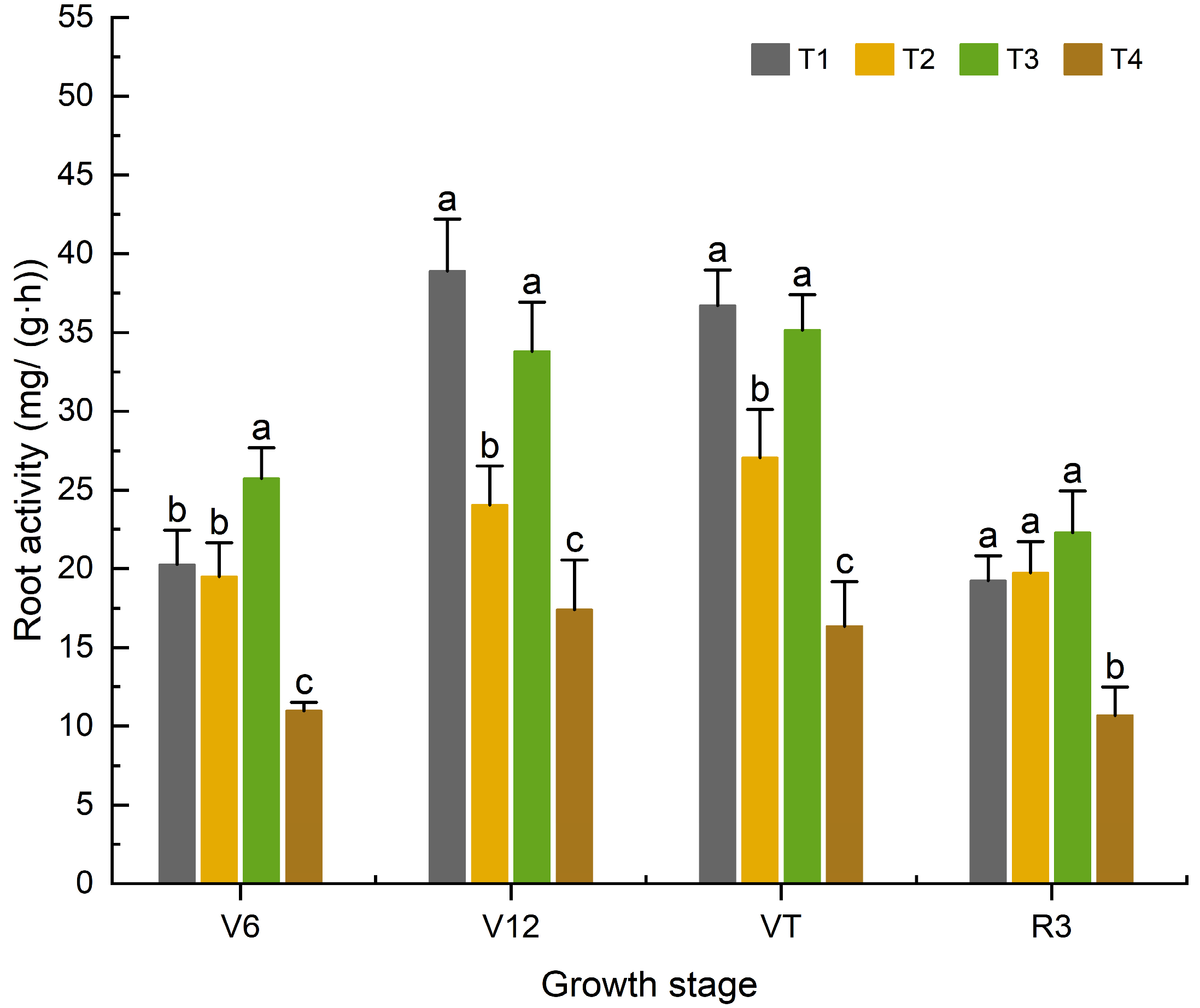
3.1.2. Effects of Organic Fertilizer Substitution for Basal Chemical Fertilizer on the Root Activity of Maize
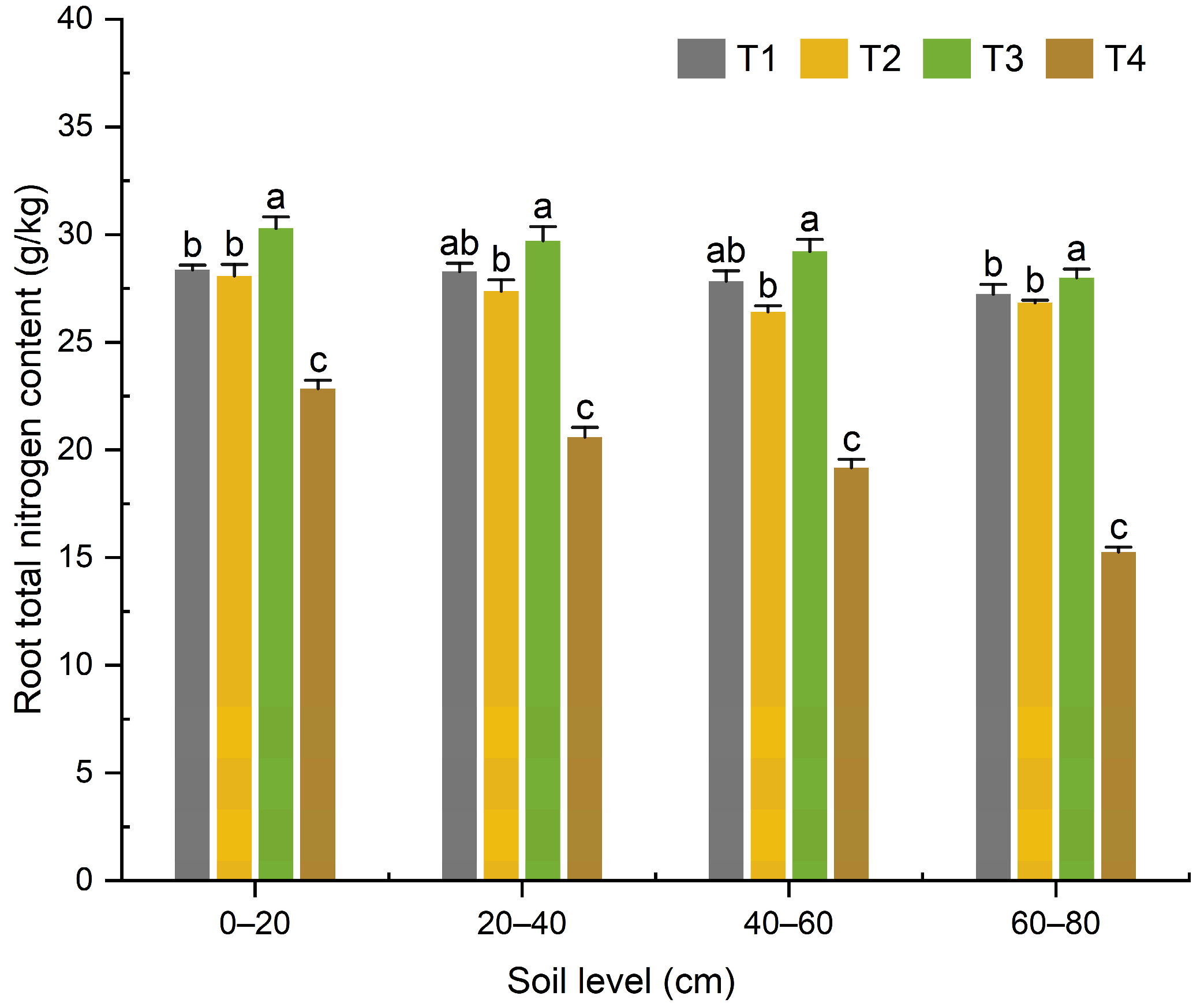
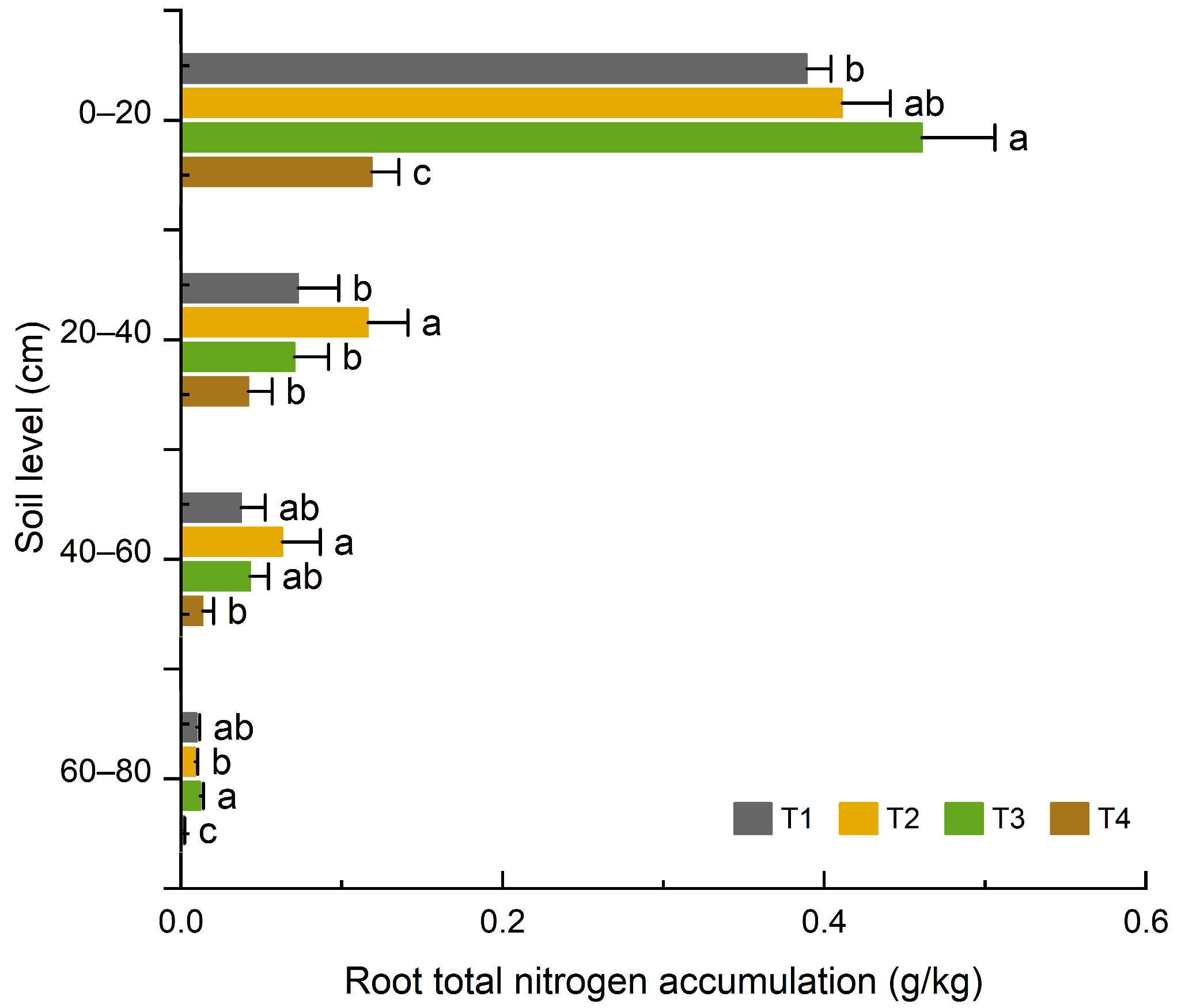
3.1.3. Effects of Organic Fertilizer Substitution for Basal Chemical Fertilizer on Nitrogen Content and Accumulation in Maize Roots
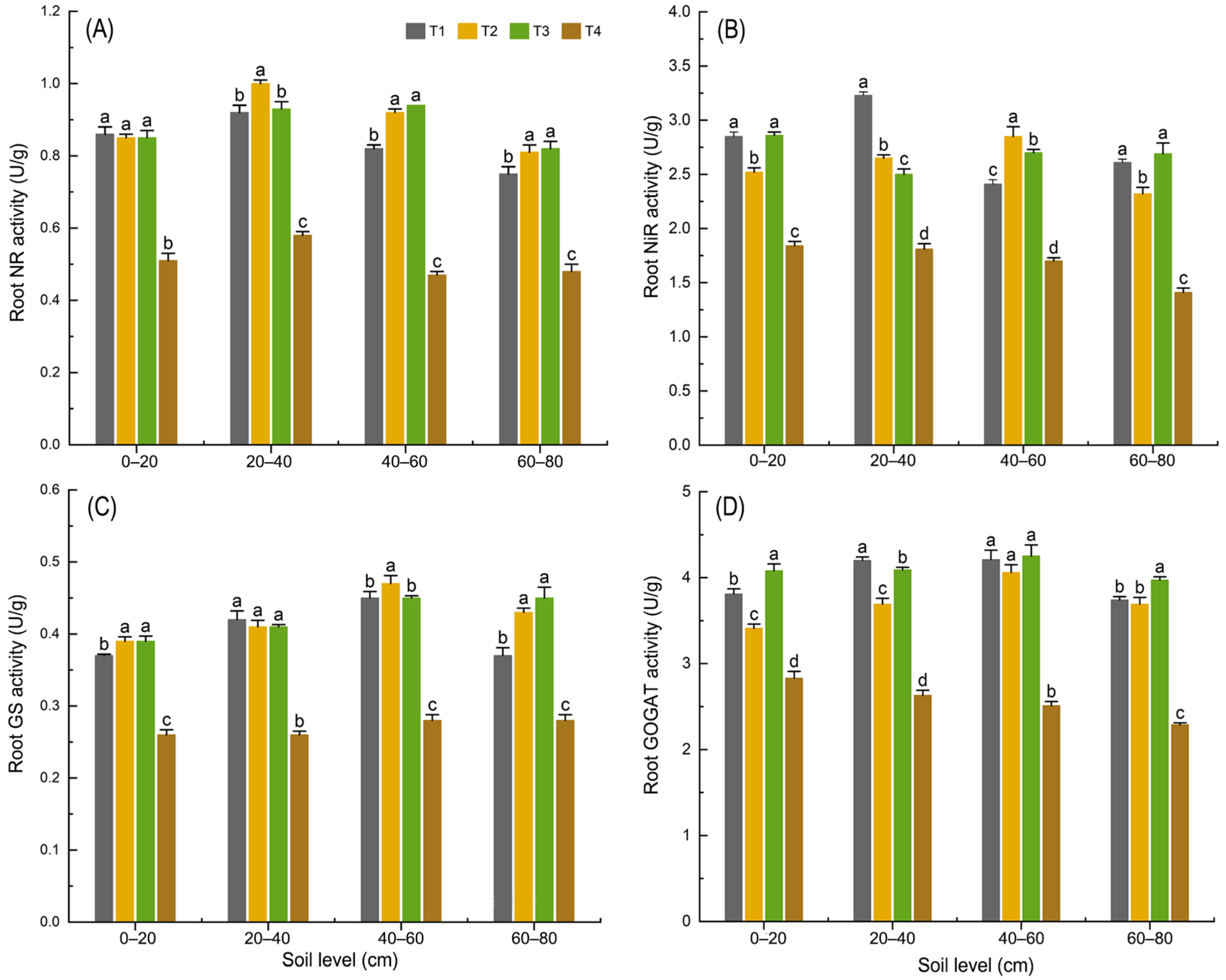
3.1.4. Effects of Organic Fertilizer Substitution for Basal Chemical Fertilizer on Nitrogen Metabolic Enzymes in Maize Roots
3.1.5. Effects of Organic Fertilizer Substituting Basal Chemical Fertilizer on Nitrogen Transport Efficiency in Maize Roots
3.2. Effects of Organic Fertilizer Substitution for Basal Chemical Fertilizer on Maize Yield and Nitrogen Fertilizer Use Efficiency
3.2.1. Effects of Organic Fertilizer Substitution for Basal Chemical Fertilizer on Maize Yield and Its Yield Components
3.2.2. Effects of Organic Fertilizer Substitution for Basal Chemical Fertilizer on Nitrogen Use Efficiency of Maize
3.3. Relationship Between Physiological Characteristics of Maize Roots and Yield
3.4. Partial Least Squares Structural Equation Modeling (PLS-SEM)
4. Discussion
4.1. Regulatory Effects on the Key Processes of Nitrogen Metabolism in the Roots of Dryland Maize Under the Optimal Proportion of Organic Fertilizer Substitution for Basal Chemical Fertilizer
4.2. Correlation Mechanism Between Root Nitrogen Metabolism Characteristics, Yield, and Nitrogen Use Efficiency of Dryland Maize Under the Substitution of Base Applied Chemical Fertilizers with Organic Fertilizers
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guillemot, J.R.; Zhang, X.; Warner, M.E. Population aging and decline will happen sooner than we think. Soc. Sci. 2024, 13, 190. [Google Scholar] [CrossRef]
- Roy, M.; Medhekar, A. Transforming smart farming for sustainability through agri-tech Innovations: Insights from the australian agricultural landscape. Farming Syst. 2025, 3, 100165. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Plaza, C.; Saiz, H.; Manzano, R.; Flagmeier, M.; Maestre, F.T. Aridity and reduced soil micronutrient availability in global drylands. Nat. Sustain. 2019, 2, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, Y.; Xu, K.; Pan, Y. Effects of Drought on vegetation productivity of farmland ecosystems in the drylands of northern china. Remote Sens. 2021, 13, 1179. [Google Scholar] [CrossRef]
- Wang, J.B.; Xie, J.H.; Li, L.L.; Adingo, S. Review on the fully mulched ridge–furrow system for sustainable maize production on the semi-arid loess plateau. J. Integr. Agric. 2023, 22, 1277–1290. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, X.W.; Diao, L.F.; Wang, Y.Q.; Wang, J.S.; An, S.Q.; Cheng, X.L.; Yang, W. Changes in soil organic carbon and nitrogen pool sizes, dynamics, and biochemical stability during 160 years natural vegetation restoration on the loess plateau, china. CATENA 2022, 211, 106014. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Xie, J.H.; Li, L.L.; Wang, L.L.; Luo, Z.Z.; Wang, J.B. Effects of long-term reduce/zero tillage and nitrogen fertilizer reducing on maize yield and soil carbon emission under fully plastic mulched ridge-furrow planting system. Sci. Agric. Sin. 2021, 54, 5054–5067. (In Chinese). [Google Scholar] [CrossRef]
- Xie, J.H.; Wang, L.L.; Li, L.L.; Coulter, J.A.; Chai, Q.; Zhang, R.Z.; Luo, Z.Z.; Carberry, P.; Rao, K.P.C. Subsoiling increases grain yield, water use efficiency, and economic return of maize under a fully mulched ridge-furrow system in a semiarid environment in China. Soil Tillage Res. 2020, 199, 104584. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.L.; Liu, Y.X.; Shen, H.Z.; Huang, T.T.; Ma, X.Y. Optimization of a nitrogen fertilizer application scheme for spring maize in full-film double-ridge furrow in longzhong, china. Agric. Water Manag. 2023, 290, 108580. [Google Scholar] [CrossRef]
- Liao, Z.Q.; Zhang, C.; Yu, S.L.; Lai, Z.L.; Wang, H.D.; Zhang, F.C.; Wu, P.; Fan, J.L. Ridge-furrow planting with black film mulching increases rainfed summer maize production by improving resources utilization on the loess plateau of china. Agric. Water Manag. 2023, 289, 108558. [Google Scholar] [CrossRef]
- Tian, S.; Xu, Y.; Wang, Q.S.; Zhang, Y.J.; Yuan, X.L.; Ma, Q.; Feng, X.F.; Ma, H.C.; Liu, J.X.; Liu, C.Q.; et al. The effect of optimizing chemical fertilizers consumption structure to promote environmental protection, crop yield and reduce greenhouse gases emission in China. Sci. Total Environ. 2023, 857, 159349. [Google Scholar] [CrossRef] [PubMed]
- Boh, M.Y.; Clark, O.G. Nitrogen and phosphorus flows in Ontario’s food systems. Resour. Conserv. Recy. 2020, 154, 104639. [Google Scholar] [CrossRef]
- Budiastuti, M.T.S.; Purnomo, D.; Pujiasmanto, B.; Setyaningrum, D. Response of maize yield and nutrient uptake to indigenous organic fertilizer from corn cobs. Agriculture 2023, 13, 309. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Z.; Li, F.; Zhang, J. Effects of organic and inorganic fertilization on soil organic carbon and enzymatic activities. Agronomy 2022, 12, 3125. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, H.; Alandia, G.; Luo, L.; Cui, Z.; Niu, X.; Liu, R.; Zhang, X.; Zhang, Y.; Zhang, F. Long-term effect of manure and mineral fertilizer application rate on maize yield and accumulated nutrients use efficiencies in north China plain. Agronomy 2020, 10, 1329. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Jiang, P.P.; Wang, R.R.; Guo, J.H.; Xiao, H.S.; Wu, J.Z.; Shaaban, M.; Li, Y.J.; Huang, M. Organic fertilizer substituting 20% chemical N increases wheat productivity and soil fertility but reduces soil nitrate-N residue in drought-prone regions. Front. Plant Sci. 2024, 15, 1379485. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Xu, L. Effects of manure-based nitrogen substitution for chemical nitrogen fertilizers on economic benefits and water-use efficiency of maize. Agronomy 2023, 13, 3031. [Google Scholar] [CrossRef]
- Ren, J.H.; Liu, X.L.; Yang, W.P.; Yang, X.X.; Li, W.G.; Xia, Q.; Li, J.H.; Gao, Z.Q.; Yang, Z.P. Rhizosphere soil properties, microbial community, and enzyme activities: Short-term responses to partial substitution of chemical fertilizer with organic manure. J. Environ. Manag. 2021, 299, 113650. [Google Scholar] [CrossRef]
- Xie, J.H.; Chai, Q.; Li, L.L.; Zhang, R.Z.; Wang, L.L.; Liu, C. Effects of the substitution of inorganic nitrogen by organic nitrogen fertilizer on maize grain yield and water and nitrogen use efficiency under plastic film fully mulched ridge-furrow in semi-arid area. J. Appl. Ecol. 2019, 30, 1199–1206. (In Chinese). [Google Scholar] [CrossRef]
- Xie, L.H.; Li, L.L.; Xie, J.H.; Wang, J.B.; Mumtaz, M.Z.; Effah, Z.; Fudjoe, S.K.; Khaskheli, M.A.; Luo, Z.Z.; Li, L.Z. Optimal substitution of inorganic fertilizer with organic amendment sustains rainfed maize production and decreases soil N2O emissions by modifying denitrifying bacterial communities in northern china. Eur. J. Agron. 2024, 160, 127287. [Google Scholar] [CrossRef]
- Xie, L.H.; Li, L.L.; Xie, J.H.; Wang, J.B.; Zhou, Y.J.; Chen, Q.; Fudjoe, S.K. Effects of substitution of chemical fertilizer by organic fertilizer on maize growth and field carbon emission in dry farming area of Longzhong, Gansu Province. J. Plant Nutr. Fertil. 2022, 28, 1029–1038. (In Chinese). [Google Scholar] [CrossRef]
- Liu, B.X.; Meng, H.F.; Li, L.L.; Xie, J.H.; Zhou, Y.J.; Tian, X.; Wang, C.Y.; Zhang, G.C. Effects of organic fertilizer substitution for basal chemical nitrogen on photosynthetic performance, yield, and nitrogen use efficiency in rainfed maize. J. Maize Sci. 2024, 32, 96–107. (In Chinese). [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.Y.; Sheng, D.C.; Zhang, S.; Gu, S.C.; Yan, Y.; Zhao, F.C.; Wang, P.; Huang, S.B. Optimizing root system architecture to improve root anchorage strength and nitrogen absorption capacity under high plant density in maize. Field Crop Res. 2023, 303, 109109. [Google Scholar] [CrossRef]
- Feng, W.H.; Sánchez-Rodríguez, A.R.; Bilyera, N.; Wang, J.Q.; Wang, X.Q.; Han, Y.H.; Ma, B.X.; Zhang, H.Y.; Li, F.Y.; Zhou, J.; et al. Mechanisms of biochar-based organic fertilizers enhancing maize yield on a Chinese Chernozem: Root traits, soil quality and soil microorganisms. Environ. Technol. Innov. 2024, 36, 103756. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, R.; Zhang, Y.; Li, W.; Zhang, Y.; Zhang, M.; Yang, X.; Yang, H. Improving maize carbon and nitrogen metabolic pathways and yield with nitrogen application rate and nitrogen forms. PeerJ 2024, 12, e16548. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Cai, H.G.; Ma, W.; Zhang, X.Z.; Ping, J.P.; Yan, X.G.; Liu, J.Z.; Yuan, J.C.; Wang, L.C.; Ren, J. Effect of subsoil tillage depth on nutrient accumulation root distribution grain yield in spring maize. Crop J. 2014, 2, 297–307. [Google Scholar] [CrossRef]
- Kirk, P.L. Kjeldahl method for total nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Barber, S.A. Effect of tillage practice on corn (Zea mays L.) root distribution and morphology. Agron. J. 1971, 63, 724–726. [Google Scholar] [CrossRef]
- Clemensson-Lindell, A. Triphenyltetrazolium chloride as an indicator of fine-root vitality and environmental stress in coniferous forest stands: Applications and limitations. Plant Soil 1994, 159, 297–300. [Google Scholar] [CrossRef]
- Campbell, W.; Remmler, J. Regulation of corn leaf nitrate reductase: I. immunochemical methods for analysis of the rnzyme’s protein component. Plant Physiol. 1986, 80, 435–441. [Google Scholar] [CrossRef]
- Oaks, A.; Wallace, W.; Stevens, D. Synthesis and turnover of nitrate reductase in corn roots. Plant Physiol. 1972, 50, 649–654. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamachi, K.; Yamaya, T.; Mae, T.; Ojima, K. A role for glutamine synthetase in the remobilization of leaf nitrogen during natural senescence in rice leaves. Plant Physiol. 1991, 96, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Sodek, L.; Silva, W. Glutamate synthase: A possible role in nitrogen metabolism of the developing maize endosperm. Plant Physiol. 1977, 60, 602–605. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Freschet, G.; McCormack, M.; Lambers, H.; Gu, J. Nutrient resorption of leaves and roots coordinates with root nutrient-acquisition strategies in a temperate forest. New Phytol. 2025, 246, 515–527. [Google Scholar] [CrossRef]
- Wang, J.Q.; Liu, Z.Y.; Shan, X.H.; Zhang, Y.L. Partial replacement of chemical fertilizer by manure affects maize root traits in north china plain. Soil Use Manag. 2023, 39, 1545–1556. [Google Scholar] [CrossRef]
- Rasool, R.; Kukal, S.S.; Hira, G.S. Root growth and soil water dynamics in relation to inorganic and organic fertilization in maize-wheat. Commun. Soil Sci. Plant Anal. 2010, 41, 2478–2490. [Google Scholar] [CrossRef]
- Costa-Broseta, Á.; Castillo, M.; León, J. Nitrite reductase 1 is a target of nitric oxide-mediated post-translational modifications and controls nitrogen flux and growth in arabidopsis. Int. J. Mol. Sci. 2020, 21, 7270. [Google Scholar] [CrossRef]
- Sullivan, D.M.; Moore, A.D.; Verhoeven, E.; Brewer, L.J. Baseline Soil Nitrogen Mineralization: Measurement and Interpretation. Oregon State University Extension Service. 2020. Available online: https://extension.oregonstate.edu/sites/default/files/documents/em9281.pdf (accessed on 15 August 2025).
- Liu, X.J.; Hu, B.; Chu, C.C. Nitrogen assimilation in plants: Current status and future prospects. J. Genet. Genom. 2021, 49, 394–404. [Google Scholar] [CrossRef]
- Hirel, B.; Tétu, T.; Lea, P.; Dubois, F. Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Zhai, L.C.; Wang, Z.B.; Zhai, Y.C.; Zhang, L.H.; Zheng, M.J.; Yao, H.P.; Lv, L.H.; Shen, H.P.; Zhang, J.T.; Yao, Y.R.; et al. Partial substitution of chemical fertilizer by organic fertilizer benefits grain yield, water use efficiency, and economic return of summer maize. Soil Tillage Res. 2022, 217, 105287. [Google Scholar] [CrossRef]
- Marchezan, C.; Ferreira, P.A.A.; Silva, L.S.; Bacca, A.; Krug, A.; Nicoloso, F.T.; Tarouco, C.P.; Tiecher, T.L.; Brunetto, G.; Ceretta, C.A. Nitrogen availability and physiological response of corn after 12 years with organic and mineral fertilization. J. Soil Sci. Plant Nutr. 2020, 20, 979–989. [Google Scholar] [CrossRef]
- Wang, X.; Liu, E.; Wang, L.; Lv, P. Changes in soil physical properties and maize productivity in response to nitrogen substitution by maize stover for mineral fertilizers. Agronomy 2025, 15, 587. [Google Scholar] [CrossRef]


| Code | Treatment | Base Fertilizer | Jointing Fertilizer | Large Belling Fertilizer | |||||
|---|---|---|---|---|---|---|---|---|---|
| Organic Fertilizer (kg/hm2) | Chemical Fertilizer (kg/hm2) | Chemical Fertilizer (kg/hm2) | |||||||
| N | P2O5 | K2O | N | P2O5 | K2O | N | |||
| T1 | 100% Chemical fertilizer | 0.0 | 0.0 | 0.0 | 100.0 | 150.0 | 10.6 | 60.0 | 40.0 |
| T2 | 50% Chemical fertilizer + 50% Organic fertilizer | 50.0 | 15.2 | 10.6 | 50.0 | 134.8 | 0.0 | 60.0 | 40.0 |
| T3 | 62.5% Chemical fertilizer + 37.5% Organic fertilizer | 37.5 | 11.4 | 8.0 | 62.5 | 138.6 | 2.6 | 60.0 | 40.0 |
| T4 | No fertilizer | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Treatment | NTA (kg/hm2) | NTE (%) | AANAA (kg/hm2) | NCP (%) |
|---|---|---|---|---|
| T1 | 10.3 ± 1.8 b | 37.3 ± 1.1 b | 7.0 ± 0.2 a | 16.9 ± 1.7 a |
| T2 | 14.7 ± 2.4 a | 38.4 ± 1.3 b | 7.5 ± 0.4 a | 17.1 ± 3.3 a |
| T3 | 12.7 ± 1.6 ab | 41.7 ± 1.3 a | 7.8 ± 0.7 a | 19.7 ± 2.8 a |
| Year | Treatment | Biomass Yield (kg/hm2) | Grain Yield (kg/hm2) | Grain Number per Ears | Grain Weight per Ears (g) | 1000-Kernel Weight (g) |
|---|---|---|---|---|---|---|
| 2021 | T1 | 23,021.50 ± 947.7 a | 8406.16 ± 213.5 a | 653 ± 19 a | 180.9 ± 5.2 a | 293.5 ± 12.05 a |
| T2 | 20,742.5 ± 1251.3 b | 7951.98 ± 205.2 a | 599 ± 25 a | 165.9 ± 0.9 b | 276.5 ± 4.45 b | |
| T3 | 22,132.5 ± 199.5 ab | 8118.07 ± 56 aa | 625 ± 32 a | 178.7 ± 7.6 ab | 282.5 ± 10.35 ab | |
| T4 | 6831.5 ± 909.9 c | 2457.82 ± 248.6 b | 260 ± 38 b | 50.5 ± 11.7 c | 217.5 ± 4.45 c | |
| 2022 | T1 | 20,125.3 ± 1058.6 a | 5711.5 ± 401.1 ab | 675 ± 16 a | 158.8 ± 2.8 b | 282.5 ± 3.51 a |
| T2 | 14,340.9 ± 1268.6 c | 5424.8 ± 490.4 b | 650 ± 6 b | 155.1 ± 1.6 b | 265.5 ± 1.51 b | |
| T3 | 17,770.3 ± 116.1 b | 6388.9 ± 279.8 a | 691 ± 7 a | 167.2 ± 3.5 a | 276.5 ± 3.21 a | |
| T4 | 8082.3 ± 298.4 d | 2723.7 ± 353.3 c | 212 ± 8 c | 40 ± 0.8 c | 198.5 ± 8.51 c | |
| Average | T1 | 21,573.4 ± 1801.2 A | 7058.9 ± 1503.6 A | 663.6 ± 24.5 A | 170.1 ± 12.7 AB | 288 ± 9.97 A |
| T2 | 17,541.7 ± 3606.8 B | 6684.8 ± 1420 A | 624.7 ± 32.5 A | 160.2 ± 5.8 B | 271 ± 6.7 B | |
| T3 | 19,951.4 ± 2459.7 AB | 7253.5 ± 964.2 A | 658.2 ± 42 A | 172.8 ± 7.9 A | 279.5 ± 7.6 AB | |
| T4 | 7456.7 ± 952.4 C | 2590.8 ± 309.6 B | 236.2 ± 36.7 B | 45.6 ± 6.9 C | 20.8 ± 12 C |
| Year | Treatment | PFP (kg/kg) | AE (kg/kg) | FCR (%) |
|---|---|---|---|---|
| 2021 | T1 | 42.0 ± 1.07 a | 29.7 ± 1.15 a | 70.7 ± 0.75 a |
| T2 | 39.7 ± 0.97 b | 27.1 ± 1.58 b | 69.1 ± 0.75 ab | |
| T3 | 40.6 ± 0.28 b | 28.3 ± 1.52 ab | 69.7 ± 0.21 b | |
| 2022 | T1 | 28.6 ± 1.2 ab | 14.9 ± 1 b | 52.2 ± 1.9 b |
| T2 | 27.1 ± 1.4 b | 13.5 ± 1.1 b | 49.5 ± 2.6 b | |
| T3 | 31.9 ± 0.8 a | 18.3 ± 1.4 a | 57.3 ± 1.1 a | |
| Average | T1 | 35.3 ± 7.5 A | 22.3 ± 8.3 A | 61.5 ± 10.3 A |
| T2 | 33.4 ± 7.1 A | 20.3 ± 8 A | 59.3 ± 10.9 A | |
| T3 | 36.3 ± 4.8 A | 23.3 ± 5.6 A | 63.5 ± 6.9 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, H.; Tian, X.; Liu, B.; Li, L.; Xie, J.; Zhu, Z. Improving Root Nitrogen Uptake via Organic Fertilizer Substitution Enhances Yield and Efficiency in Dryland Maize. Agronomy 2025, 15, 2216. https://doi.org/10.3390/agronomy15092216
Meng H, Tian X, Liu B, Li L, Xie J, Zhu Z. Improving Root Nitrogen Uptake via Organic Fertilizer Substitution Enhances Yield and Efficiency in Dryland Maize. Agronomy. 2025; 15(9):2216. https://doi.org/10.3390/agronomy15092216
Chicago/Turabian StyleMeng, Haofeng, Xin Tian, Bingxin Liu, Lingling Li, Junhong Xie, and Zhen Zhu. 2025. "Improving Root Nitrogen Uptake via Organic Fertilizer Substitution Enhances Yield and Efficiency in Dryland Maize" Agronomy 15, no. 9: 2216. https://doi.org/10.3390/agronomy15092216
APA StyleMeng, H., Tian, X., Liu, B., Li, L., Xie, J., & Zhu, Z. (2025). Improving Root Nitrogen Uptake via Organic Fertilizer Substitution Enhances Yield and Efficiency in Dryland Maize. Agronomy, 15(9), 2216. https://doi.org/10.3390/agronomy15092216






