Seedling Priming with Selenium Enhances the Biofortification Strategies in the Production of Broccoli Florets
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments and Experimental Conditions
2.2. Broccoli Harvest
2.3. Selenium and Zinc in Broccoli Floret
2.4. Statistical Analysis
3. Results
3.1. Agronomic Characteristics
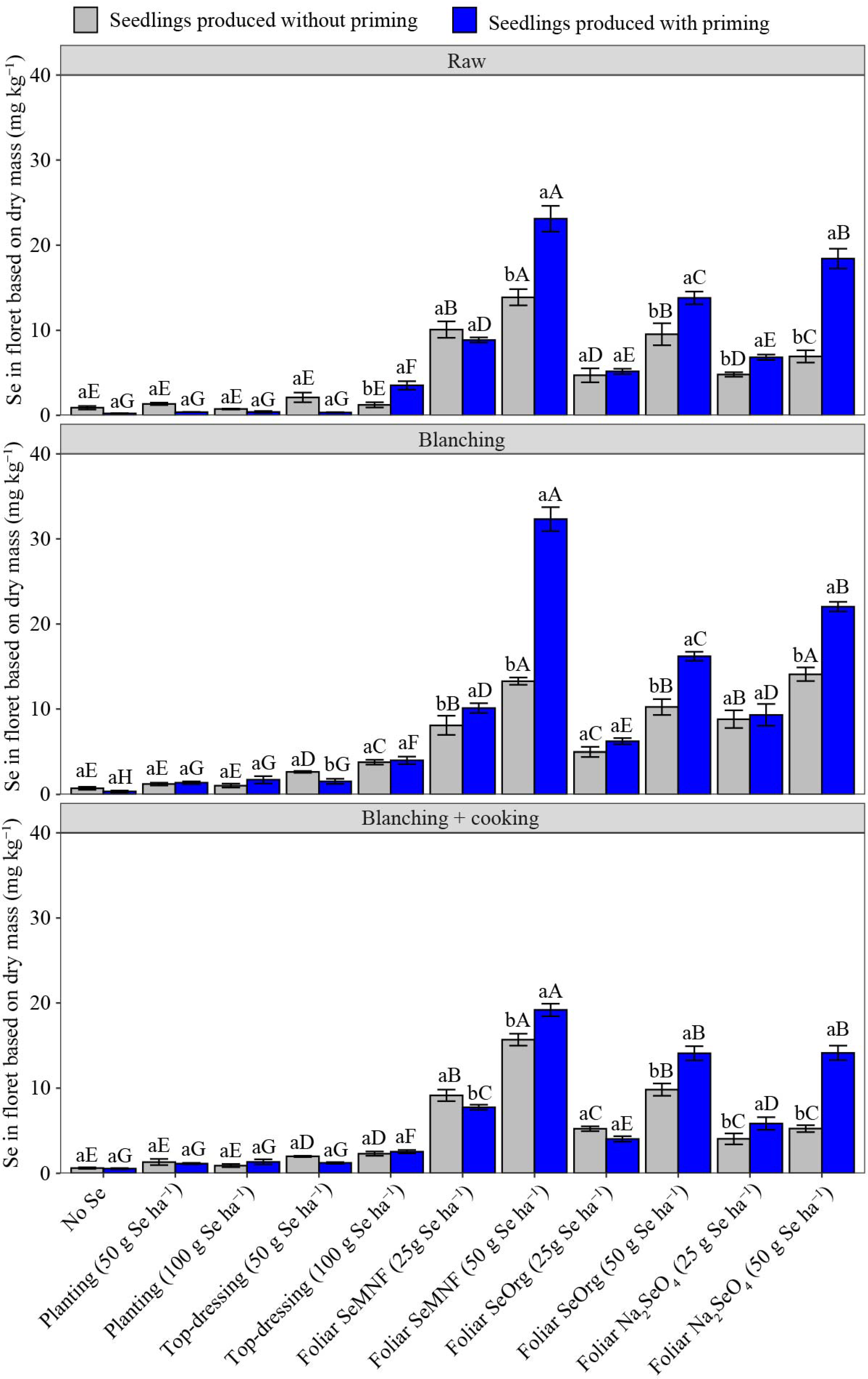
3.2. Selenium Concentration in Florets
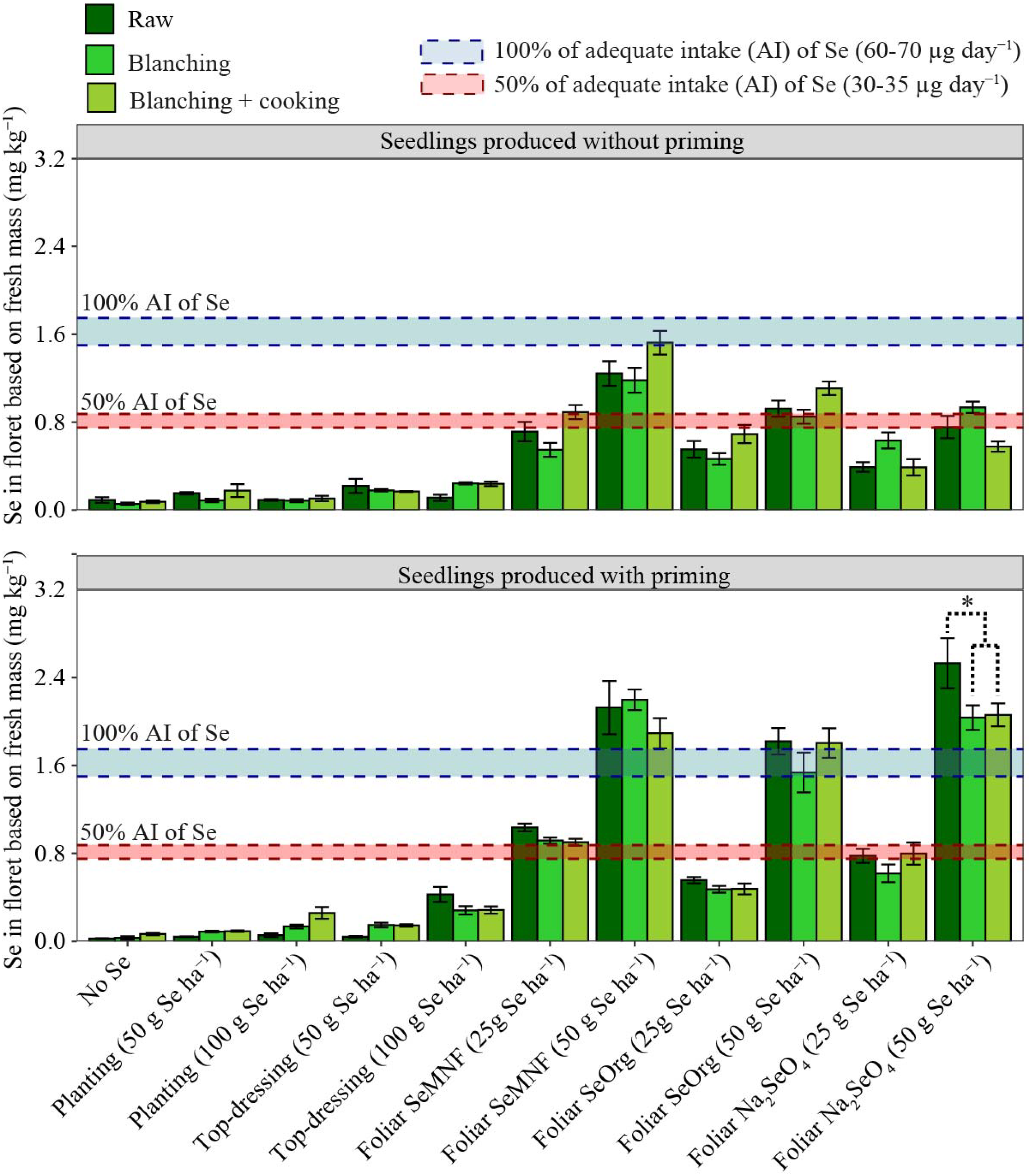
3.3. Effect of Processing on Se in Florets and Its Recommendation in the Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Treatment | Se Rate (g ha−1) | Priming | Raw (%) | Blanching (%) | Blanching + Cooking (%) |
|---|---|---|---|---|---|
| No Se | 0 | No | 90.4 ± 0.9 | 91.7 ± 0.8 | 87.6 ± 0.5 |
| Yes | 88.5 ± 0.6 | 93.0 ± 0.3 | 87.2 ± 0.6 | ||
| Planting | 50 | No | 87.6 ± 0.6 | 91.3 ± 0.7 | 88.3 ± 0.9 |
| Yes | 89.6 ± 1.0 | 93.2 ± 0.4 | 91.5 ± 0.2 | ||
| 100 | No | 90.7 ± 0.6 | 93.5 ± 0.5 | 89.5 ± 0.4 | |
| Yes | 92.8 ± 0.7 | 93.1 ± 0.4 | 90.0 ± 1.1 | ||
| Top-dressing | 50 | No | 90.8 ± 1.3 | 91.0 ± 1.0 | 90.2 ± 0.9 |
| Yes | 88.0 ± 1.0 | 90.6 ± 0.2 | 86.9 ± 1.0 | ||
| 100 | No | 89.8 ± 1.1 | 91.6 ± 0.6 | 88.6 ± 0.6 | |
| Yes | 91.9 ± 0.6 | 92.8 ± 0.1 | 90.5 ± 0.7 | ||
| Foliar SeMNF | 25 | No | 89.2 ± 1.0 | 93.3 ± 0.2 | 88.9 ± 0.6 |
| Yes | 88.0 ± 0.6 | 91.7 ± 0.6 | 88.2 ± 0.4 | ||
| 50 | No | 88.5 ± 0.2 | 93.5 ± 0.5 | 91.9 ± 0.6 | |
| Yes | 86.3 ± 1.3 | 91.6 ± 0.9 | 80.3 ± 0.8 | ||
| Foliar SeOrg | 25 | No | 87.7 ± 0.5 | 90.1 ± 0.4 | 88.2 ± 0.3 |
| Yes | 88.0 ± 0.4 | 92.9 ± 0.8 | 88.9 ± 0.6 | ||
| 50 | No | 88.3 ± 0.4 | 90.9 ± 0.3 | 88.4 ± 0.3 | |
| Yes | 90.9 ± 0.6 | 93.2 ± 0.3 | 90.1 ± 0.8 | ||
| Foliar Na2SeO4 | 25 | No | 89.1 ± 0.6 | 92.3 ± 0.6 | 88.2 ± 0.6 |
| Yes | 86.8 ± 0.4 | 90.6 ± 0.3 | 87.2 ± 0.7 | ||
| 50 | No | 88.7 ± 0.7 | 93.4 ± 0.2 | 86.2 ± 0.6 | |
| Yes | 86.4 ± 0.7 | 90.8 ± 0.3 | 85.4 ± 0.3 |
| Treatment | Se Rate (g ha−1) | Priming | Raw (mg kg−1) | Blanching (mg kg−1) | Blanching + Cooking (mg kg−1) |
|---|---|---|---|---|---|
| No Se | 0 | No | 48.1 ± 2.3 | 50.0 ± 7.0 | 38.6 ± 3.1 |
| Yes | 46.4 ± 3.3 | 73.5 ± 4.9 | 42.7 ± 1.9 | ||
| Planting | 50 | No | 49.8 ± 1.9 | 76.3 ± 9.5 | 51.0 ± 4.9 |
| Yes | 60.2 ± 1.7 | 91.0 ± 1.8 | 65.1 ± 1.1 | ||
| 100 | No | 60.9 ± 3.4 | 83.6 ± 7.9 | 60.0 ± 4.5 | |
| Yes | 64.1 ± 3.0 | 68.6 ± 5.2 | 42.5 ± 3.6 | ||
| Top-dressing | 50 | No | 49.9 ± 6.0 | 52.4 ± 8.5 | 38.2 ± 2.8 |
| Yes | 48.3 ± 3.1 | 61.6 ± 5.6 | 39.9 ± 2.1 | ||
| 100 | No | 49.1 ± 5.0 | 60.7 ± 5.0 | 36.2 ± 1.3 | |
| Yes | 65.4 ± 3.3 | 65.4 ± 4.2 | 47.4 ± 5.1 | ||
| Foliar SeMNF | 25 | No | 46.9 ± 5.4 | 70.6 ± 4.2 | 43.9 ± 1.8 |
| Yes | 32.2 ± 2.7 | 48.8 ± 4.0 | 34.5 ± 2.3 | ||
| 50 | No | 52.1 ± 2.7 | 77.1 ± 9.6 | 62.8 ± 7.1 | |
| Yes | 49.9 ± 2.2 | 76.7 ± 10.1 | 33.8 ± 2.9 | ||
| Foliar SeOrg | 25 | No | 51.0 ± 5.0 | 50.9 ± 4.3 | 48.2 ± 2.9 |
| Yes | 51.2 ± 3.7 | 79.5 ± 7.7 | 45.9 ± 3.3 | ||
| 50 | No | 35.4 ± 0.6 | 31.8 ± 2.7 | 29.4 ± 0.9 | |
| Yes | 41.6 ± 3.1 | 56.7 ± 1.4 | 34.5 ± 1.9 | ||
| Foliar Na2SeO4 | 25 | No | 42.2 ± 2.5 | 52.9 ± 2.4 | 33.1 ± 1.5 |
| Yes | 49.5 ± 3.0 | 53.8 ± 4.7 | 39.4 ± 2.3 | ||
| 50 | No | 45.8 ± 2.3 | 60.5 ± 3.8 | 31.8 ± 2.7 | |
| Yes | 39.9 ± 2.8 | 54.5 ± 2.4 | 28.3 ± 1.1 |
References
- Garousi, F. The Essentiality of Selenium for Humans, Animals, and Plants, and the Role of Selenium in Plant Metabolism and Physiology. Acta Univ. Sapientiae Aliment. 2017, 10, 75–90. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Bano, I.; Zare, H. A Comprehensive Review on Selenium and Its Effects on Human Health and Distribution in Middle Eastern Countries. Biol. Trace Elem. Res. 2022, 200, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhang, L.; Ma, F.; Xue, S.; Sun, T.; Xu, Z. Effects of Selenium on Chronic Kidney Disease: A Mendelian Randomization Study. Nutrients 2022, 14, 4458. [Google Scholar] [CrossRef]
- Lopes, G.; Ávila, F.W.; Guilherme, L.R.G. Selenium Behavior in the Soil Environment and Its Implication for Human Health. Ciênc. Agrotecnol. 2017, 41, 605–615. [Google Scholar] [CrossRef]
- Galić, L.; Vinković, T.; Ravnjak, B.; Lončarić, Z. Agronomic Biofortification of Significant Cereal Crops with Selenium—A Review. Agronomy 2021, 11, 1015. [Google Scholar] [CrossRef]
- Schiavon, M.; Nardi, S.; dalla Vecchia, F.; Ertani, A. Selenium Biofortification in the 21st Century: Status and Challenges for Healthy Human Nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef]
- Wangkahart, E.; Bruneel, B.; Chantiratikul, A.; de Jong, M.; Pakdeenarong, N.; Subramani, P.A. Optimum Dietary Sources and Levels of Selenium Improve Growth, Antioxidant Status, and Disease Resistance: Re-Evaluation in a Farmed Fish Species, Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2022, 121, 172–182. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Wise, L.A. Environmental Selenium and Human Health: An Update. Curr. Environ. Health Rep. 2018, 5, 464–485. [Google Scholar] [CrossRef]
- Finley, J.W.; Davis, C.D.; Feng, Y. Selenium from High Selenium Broccoli Protects Rats from Colon Cancer. J. Nutr. 2000, 130, 2384–2389. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Ligas, B.; Mikula, K.; Witek-Krowiak, A.; Moustakas, K.; Chojnacka, K. Biofortification of Edible Plants with Selenium and Iodine—A Systematic Literature Review. Sci. Total Environ. 2021, 754, 141983. [Google Scholar] [CrossRef]
- De Morais, E.G.; Silva, M.A.; Quispe, A.P.V.; Machado, G.G.L.; Prado, D.T.; Benevenute, P.A.N.; de Sousa Lima, J.; de Sousa, G.F.; de Barros Vilas Boas, E.V.; Guilherme, L.R.G. Foliar Sprays of Multi-Nutrient Fertilizer Containing Selenium Produce Functional Tomato Fruits with Higher Shelf Life. Plants 2024, 13, 2288. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; García-Latorre, C.; Velazquez, R.; Broadley, M.R. Effects of Selenate Application on Growth, Nutrient Bioaccumulation, and Bioactive Compounds in Broccoli (Brassica oleracea Var. italica L.). Horticulturae 2024, 10, 808. [Google Scholar] [CrossRef]
- Trod, B.S.; Buttarelli, M.S.; Stoffel, M.M.; Céccoli, G.; Olivella, L.; Barengo, P.B.; Llugany, M.; Guevara, M.G.; Muñoz, F.F.; Daurelio, L.D. Postharvest Commercial Quality Improvement of Broccoli (Brassica oleracea L.) after Foliar Biofortification with Selenium. Crop Sci. 2023, 63, 784–800. [Google Scholar] [CrossRef]
- Muñoz, F.F.; Stoffel, M.M.; Céccoli, G.; Trod, B.S.; Daurelio, L.D.; Bouzo, C.A.; Guevara, M.G. Improving the Foliar Biofortification of Broccoli with Selenium without Commercial Quality Losses. Crop Sci. 2021, 61, 4218–4228. [Google Scholar] [CrossRef]
- Li, H.; Xia, Y.; Liu, H.-Y.; Guo, H.; He, X.-Q.; Liu, Y.; Wu, D.-T.; Mai, Y.-H.; Li, H.-B.; Zou, L.; et al. Nutritional Values, Beneficial Effects, and Food Applications of Broccoli (Brassica oleracea Var italica Plenck). Trends Food Sci. Technol. 2022, 119, 288–308. [Google Scholar] [CrossRef]
- Thomas, M.; Badr, A.; Desjardins, Y.; Gosselin, A.; Angers, P. Characterization of Industrial Broccoli Discards (Brassica oleracea Var italica) for Their Glucosinolate, Polyphenol and Flavonoid Contents Using UPLC MS/MS and Spectrophotometric Methods. Food Chem. 2018, 245, 1204–1211. [Google Scholar] [CrossRef]
- Alanís-Garza, P.A.; Becerra-Moreno, A.; Mora-Nieves, J.L.; Mora-Mora, J.P.; Jacobo-Velázquez, D.A. Effect of Industrial Freezing on the Stability of Chemopreventive Compounds in Broccoli. Int. J. Food Sci. Nutr. 2015, 66, 282–288. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, W.; Fu, D.; Zhang, T.; Liang, Z.; Yan, L.; Liu, C.; Zheng, L. Microwave Bag Cooking Affects the Quality, Glucosinolates Content and Hydrolysate Production of Broccoli Florets. Food Res. Int. 2023, 164, 112401. [Google Scholar] [CrossRef]
- Severini, C.; Giuliani, R.; De Filippis, A.; Derossi, A.; De Pilli, T. Influence of Different Blanching Methods on Colour, Ascorbic Acid and Phenolics Content of Broccoli. J. Food Sci. Technol. 2016, 53, 501–510. [Google Scholar] [CrossRef]
- Lara, T.S.; de Lima Lessa, J.H.; de Souza, K.R.D.; Corguinha, A.P.B.; Martins, F.A.D.; Lopes, G.; Guilherme, L.R.G. Selenium Biofortification of Wheat Grain via Foliar Application and Its Effect on Plant Metabolism. J. Food Compos. Anal. 2019, 81, 10–18. [Google Scholar] [CrossRef]
- De Lima Lessa, J.H.; Araujo, A.M.; Ferreira, L.A.; da Silva Júnior, E.C.; de Oliveira, C.; Corguinha, A.P.B.; Martins, F.A.D.; de Carvalho, H.W.P.; Guilherme, L.R.G.; Lopes, G. Agronomic Biofortification of Rice (Oryza sativa L.) with Selenium and Its Effect on Element Distributions in Biofortified Grains. Plant Soil 2019, 444, 331–342. [Google Scholar] [CrossRef]
- Bachiega, P.; Salgado, J.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and Antiproliferative Activities in Different Maturation Stages of Broccoli (Brassica oleracea italica) Biofortified with Selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef]
- Sarwar, N.; Akhtar, M.; Kamran, M.A.; Imran, M.; Riaz, M.A.; Kamran, K.; Hussain, S. Selenium Biofortification in Food Crops: Key Mechanisms and Future Perspectives. J. Food Compos. Anal. 2020, 93, 103615. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium Biofortification and Interaction with Other Elements in Plants: A Review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef] [PubMed]
- Quispe, A.P.V.; de Morais, E.G.; Benevenute, P.A.N.; de Sousa Lima, J.; dos Santos, L.C.; Silva, M.A.; Chalfun-Júnior, A.; Marchiori, P.E.R.; Guilherme, L.R.G. Priming Effect with Selenium and Iodine on Broccoli Seedlings: Activation of Biochemical Mechanisms to Mitigate Cold Damages. Plant Physiol. Biochem. 2025, 223, 109876. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Tan, Z.; Kasiulienė, A.; Ai, P. Transformation Mechanism of Nutrient Elements in the Process of Biochar Preparation for Returning Biochar to Soil. Chin. J. Chem. Eng. 2017, 25, 477–486. [Google Scholar] [CrossRef]
- Bachiega, P.; de Almeida, E.; Salgado, J.M.; Arruda, M.A.Z.; Lehmann, E.L.; Morzelle, M.C.; de Carvalho, H.W.P. Benchtop and Handheld Energy-Dispersive X-Ray Fluorescence (EDXRF) as Alternative for Selenium Concentration Measurement in Biofortified Broccoli Seedling. Food Anal. Methods 2019, 12, 1520–1527. [Google Scholar] [CrossRef]
- Tian, M.; Yang, Y.; Ávila, F.W.; Fish, T.; Yuan, H.; Hui, M.; Pan, S.; Thannhauser, T.W.; Li, L. Effects of Selenium Supplementation on Glucosinolate Biosynthesis in Broccoli. J. Agric. Food Chem. 2018, 66, 8036–8044. [Google Scholar] [CrossRef]
- Wiesner-Reinhold, M.; Schreiner, M.; Baldermann, S.; Schwarz, D.; Hanschen, F.S.; Kipp, A.P.; Rowan, D.D.; Bentley-Hewitt, K.L.; McKenzie, M.J. Mechanisms of Selenium Enrichment and Measurement in Brassicaceous Vegetables, and Their Application to Human Health. Front. Plant Sci. 2017, 8, 1365. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Mazhar, M.W.; Maqbool, M.; Hussain, T.; Hussain, S.A.; Casini, R.; Abd-ElGawad, A.M.; Elansary, H.O. Seed Priming with the Selenium Nanoparticles Maintains the Redox Status in the Water Stressed Tomato Plants by Modulating the Antioxidant Defense Enzymes. Plants 2023, 12, 1556. [Google Scholar] [CrossRef]
- Waqas, M.; Korres, N.E.; Khan, M.D.; Nizami, A.-S.; Deeba, F.; Ali, I.; Hussain, H. Advances in the Concept and Methods of Seed Priming. In Priming and Pretreatment of Seeds and Seedlings; Springer: Singapore, 2019; pp. 11–41. [Google Scholar]
- Llorens, E.; González-Hernández, A.I.; Scalschi, L.; Fernández-Crespo, E.; Camañes, G.; Vicedo, B.; García-Agustín, P. Priming Mediated Stress and Cross-Stress Tolerance in Plants: Concepts and Opportunities. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–20. [Google Scholar]
- Hu, F.; Jiang, S.; Wang, Z.; Hu, K.; Xie, Y.; Zhou, L.; Zhu, J.; Xing, D.; Du, B. Seed Priming with Selenium: Effects on Germination, Seedling Growth, Biochemical Attributes, and Grain Yield in Rice Growing under Flooding Conditions. Plant Direct 2022, 6, e378. [Google Scholar] [CrossRef]
- Hussain, S.; Ahmed, S.; Akram, W.; Li, G.; Yasin, N.A. Selenium Seed Priming Enhanced the Growth of Salt-Stressed Brassica rapa L. through Improving Plant Nutrition and the Antioxidant System. Front. Plant Sci. 2023, 13, 1050359. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; de Sousa, G.F.; Van Opbergen, G.A.Z.; Van Opbergen, G.G.A.Z.; Corguinha, A.P.B.; Bueno, J.M.M.; Brunetto, G.; Leite, J.M.; dos Santos, A.A.; Lopes, G.; et al. Foliar Application of Selenium Associated with a Multi-Nutrient Fertilizer in Soybean: Yield, Grain Quality, and Critical Se Threshold. Plants 2023, 12, 2028. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 13143–13151. [Google Scholar]
- Santos, H.G.; Jacomine, P.K.T.; Dos Anjos, L.H.C.; De Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; De Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa: Brasília, DF, Brazil, 2018. [Google Scholar]
- IIUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; IIUSS Working Group WRB, Ed.; FAO: Rome, Italy, 2015. [Google Scholar]
- Soil Survey Staff (Ed.) Keys to Soil Taxonomy, 13th ed.; USDA Natural Resources Conservation Service: Washington, DC, USA, 2022. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. (Eds.) Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017; ISBN 978-85-7035-771-7. [Google Scholar]
- USEPA. Method 3051A (SW-846). In Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils; USEPA, Ed.; United States Environmental Protection Agency: Washington, DC, USA, 2007. [Google Scholar]
- Novais, R.F.; Neves, J.C.L.; Barros, N.F. Ensaio em Ambiente Controlado. In Métodos de Pesquisa em Fertilidade do Solo; Oliveira, A.J., Ed.; Embrapa-SEA: Brasília, Brazil, 1991; pp. 189–253. [Google Scholar]
- Rivera-Martin, A.; Broadley, M.R.; Poblaciones, M.J. Soil and Foliar Zinc Application to Biofortify Broccoli (Brassica oleracea Var italica L.): Effects on the Zinc Concentration and Bioavailability. Plant Soil Environ. 2020, 66, 113–118. [Google Scholar] [CrossRef]
- Rivera-Martin, A.; Broadley, M.R.; Poblaciones, M.J. Soil and Foliar Zinc Biofortification of Broccolini: Effects on Plant Growth and Mineral Accumulation. Crop Pasture Sci. 2020, 71, 484. [Google Scholar] [CrossRef]
- Guilherme, L.R.G.; Morais, E.G.; Quispé, A.P.V.; Nascimento, M.L. Pacote Tecnológico Para a Produção de Brócolis Biofortificado Com Selênio Por Meio Do Uso de Mudas Priming e Aplicação Foliar. Brazil Patent BR1020250158965, 2025. [Google Scholar]
- Shvetsova, S. Analysis of Changes in Moisture Levels in Broccoli during Long-Term Storage. BIO Web Conf. 2024, 93, 02012. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Hamner, B.; Frasco, M. Metrics: Evaluation Metrics for Machine Learning; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- De Souza Cardoso, A.A.; Namorato, F.A.; Guilherme, L.R.G.; de Souza Silva, M.L.; Liu, J.; Li, L. Glutathione Is Involved in Selenium Detoxification and Suppresses the Selenate-Induced SULTR1;1 Gene Expression in Plants. Environ. Exp. Bot. 2023, 213, 105424. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Nahar, K.; Fujita, M. Selenium Toxicity in Plants and Environment: Biogeochemistry and Remediation Possibilities. Plants 2020, 9, 1711. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Neysanian, M.; Iranbakhsh, A.; Ahmadvand, R.; Oraghi Ardebili, Z.; Ebadi, M. Comparative Efficacy of Selenate and Selenium Nanoparticles for Improving Growth, Productivity, Fruit Quality, and Postharvest Longevity through Modifying Nutrition, Metabolism, and Gene Expression in Tomato; Potential Benefits and Risk Assessment. PLoS ONE 2020, 15, e0244207. [Google Scholar] [CrossRef]
- Choukri, M.; Abouabdillah, A.; Bouabid, R.; Abd-Elkader, O.H.; Pacioglu, O.; Boufahja, F.; Bourioug, M. Zn Application through Seed Priming Improves Productivity and Grain Nutritional Quality of Silage Corn. Saudi J. Biol. Sci. 2022, 29, 103456. [Google Scholar] [CrossRef]
- Zhou, Y.; Nie, K.; Geng, L.; Wang, Y.; Li, L.; Cheng, H. Selenium’s Role in Plant Secondary Metabolism: Regulation and Mechanistic Insights. Agronomy 2024, 15, 54. [Google Scholar] [CrossRef]
- Tian, M.; Hui, M.; Thannhauser, T.W.; Pan, S.; Li, L. Selenium-Induced Toxicity Is Counteracted by Sulfur in Broccoli (Brassica oleracea L. Var. italica). Front. Plant Sci. 2017, 8, 1425. [Google Scholar] [CrossRef]
- Skrypnik, L.; Feduraev, P.; Golovin, A.; Maslennikov, P.; Styran, T.; Antipina, M.; Riabova, A.; Katserov, D. The Integral Boosting Effect of Selenium on the Secondary Metabolism of Higher Plants. Plants 2022, 11, 3432. [Google Scholar] [CrossRef]
- De Lima Lessa, J.H.; Raymundo, J.F.; Branco Corguinha, A.P.; Dias Martins, F.A.; Araujo, A.M.; Melo Santiago, F.E.; Pereira de Carvalho, H.W.; Guimarães Guilherme, L.R.; Lopes, G. Strategies for Applying Selenium for Biofortification of Rice in Tropical Soils and Their Effect on Element Accumulation and Distribution in Grains. J. Cereal Sci. 2020, 96, 103125. [Google Scholar] [CrossRef]
- Hassan, M.; Belal, H.; Abou-Sreea, A.; Rady, M. Exogenous Application of Selenium or Iodine Improves the Growth, Yield and Antioxidant Status of Capsicum annuum L. Labyrinth fayoum. J. Sci. Interdiscip. Stud. 2023, 1, 76–83. [Google Scholar] [CrossRef]
- USDA Food Availability (Per Capita) Data System. Available online: https://www.ers.usda.gov/data-products/food-availability-per-capita-data-system/ (accessed on 31 October 2024).
- Lu, X.; He, Z.; Lin, Z.; Zhu, Y.; Yuan, L.; Liu, Y.; Yin, X. Effects of Chinese Cooking Methods on the Content and Speciation of Selenium in Selenium Bio-Fortified Cereals and Soybeans. Nutrients 2018, 10, 317. [Google Scholar] [CrossRef]
- Farooq, M.R.; Zhang, Z.; Liu, X.; Chen, Y.; Wu, G.; Niu, S.; Song, J.; Chen, D.; Yin, X. Selenium Loss during Boiling Processes and Its Bioaccessibility in Different Crops: Estimated Daily Intake. Food Chem. 2024, 443, 138607. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Y.; Dong, G.; Wu, H. Effect of Boiling and Frying on the Selenium Content, Speciation, and in Vitro Bioaccessibility of Selenium-Biofortified Potato (Solanum tuberosum L.). Food Chem. 2021, 348, 129150. [Google Scholar] [CrossRef]
| Attributes | Values |
|---|---|
| pH in water | 4.8 |
| Soil organic matter (g kg−1) | 24.9 |
| Clay (g kg−1) | 670 |
| Silt (g kg−1) | 130 |
| Sand (g kg−1) | 200 |
| Total nitrogen (g kg−1) | 2.3 |
| Available potassium (mg kg−1) | 24.8 |
| Available phosphorus (mg kg−1) | 0.4 |
| Exchangeable calcium2+ (cmolc kg−1) | 0.4 |
| Exchangeable magnesium2+ (cmolc kg−1) | 0.2 |
| Available zinc (mg kg−1) | 0.2 |
| Available iron (mg kg−1) | 38.0 |
| Available manganese (mg kg−1) | 3.4 |
| Available copper (mg kg−1) | 1.2 |
| Available boron (mg kg−1) | 0.01 |
| Available sulfur (mg kg−1) | 2.9 |
| Total selenium (mg kg−1) | 0.38 |
| Acronym | Description |
|---|---|
| No Se | Control—standard field fertilization, adapted for pots [43] |
| Planting (50 g Se ha−1) # | Soil application of Se using Se-enriched planting fertilizer to achieve a dose of 50 g Se ha−1 |
| Planting (100 g Se ha−1) # | Soil application of Se using Se-enriched planting fertilizer to achieve a dose of 100 g Se ha−1 |
| Top-dressing (50 g Se ha−1) # | Soil application of Se using Se-enriched top-dressing fertilizer to achieve a dose of 50 g Se ha−1 |
| Top-dressing (100 g Se ha−1) # | Soil application of Se using Se-enriched top-dressing fertilizer to achieve a dose of 100 g Se ha−1 |
| Foliar SeMNF (25 g Se ha−1) †* | Foliar application of Se using foliar Se multi-nutrient fertilizer to achieve a dose of 25 g Se ha−1 |
| Foliar SeMNF (50 g Se ha−1) †* | Foliar application of Se using foliar Se multi-nutrient fertilizer to achieve a dose of 50 g Se ha−1 |
| Foliar SeOrg (25 g Se ha−1) * | Foliar application of Se using foliar Se + organic compounds fertilizer to achieve a dose of 25 g Se ha−1 |
| Foliar SeOrg (50 g Se ha−1) * | Foliar application of Se using foliar Se + organic compounds fertilizer to achieve a dose of 50 g Se ha−1 |
| Foliar Na2SeO4 (25 g Se ha−1) * | Foliar application of Se using foliar sodium selenate to achieve a dose of 25 g Se ha−1 |
| Foliar Na2SeO4 (50g Se ha−1) * | Foliar application of Se using foliar sodium selenate to achieve a dose of 50 g Se ha−1 |
| Treatment | Se Rate (g ha−1) | Priming | DF (Days) | DH (Days) | TP (g plant−1) | HD (cm plant−1) | FW (g plant−1) | SW (g plant−1) | F:S Ratio (g g−1) |
|---|---|---|---|---|---|---|---|---|---|
| No Se | 0 | No | 69 ± 1 aA | 95 ± 1 aA | 166 ± 16 aA | 169 ± 6 aA | 130 ± 14 aA | 36 ± 3 aA | 3.7 ± 0.3 aA |
| Yes | 65 ± 3 bB | 90 ± 3 bC | 165 ± 8 aB | 175 ± 7 aA | 127 ± 8 aB | 39 ± 2 aA | 3.3 ± 0.3 bA | ||
| Planting | 50 | No | 61 ± 2 bB | 87 ± 2 aB | 177 ± 17 aA | 163 ± 8 aA | 137 ± 15 aA | 40 ± 3 aA | 3.4 ± 0.3 bA |
| Yes | 65 ± 4 aB | 87 ± 4 aC | 201 ± 24 aA | 178 ± 9 aA | 158 ± 19 aA | 43 ± 6 aA | 3.7 ± 0.3 aA | ||
| 100 | No | 66 ± 1 aB | 92 ± 1 aA | 167 ± 17 aA | 159 ± 8 aB | 120 ± 14 aA | 47 ± 3 aA | 2.5 ± 0.2 bB | |
| Yes | 68 ± 2 aA | 95 ± 3 aB | 131 ± 9 aB | 140 ± 4 aB | 98 ± 9 aB | 32 ± 2 bB | 3.1 ± 0.3 aB | ||
| Top-dressing | 50 | No | 64 ± 1 aB | 85 ± 2 bB | 177 ± 17 aA | 168 ± 13 aA | 132 ± 13 aA | 44 ± 5 aA | 3.0 ± 0.1 bB |
| Yes | 65 ± 2 aB | 90 ± 3 aC | 186 ± 21 aA | 168 ± 6 aA | 140 ± 16 aA | 46 ± 5 aA | 3.1 ± 0.2 aB | ||
| 100 | No | 66 ± 3 aB | 91 ± 3 aA | 185 ± 13 aA | 172 ± 4 aA | 142 ± 10 aA | 43 ± 3 aA | 3.3 ± 0.1 bA | |
| Yes | 67 ± 4 aA | 91 ± 4 aC | 187 ± 12 aA | 178 ± 7 aA | 146 ± 10 aA | 42 ± 3 aA | 3.5 ± 0.3 aA | ||
| Foliar SeMNF | 25 | No | 69 ± 4 aA | 93 ± 4 aA | 166 ± 15 aA | 170 ± 14 aA | 127 ± 14 aA | 39 ± 2 aA | 3.2 ± 0.4 bA |
| Yes | 62 ± 2 bB | 88 ± 3 aC | 186 ± 9 aA | 169 ± 7 aA | 147 ± 7 aA | 39 ± 2 aA | 3.8 ± 0.2 aA | ||
| 50 | No | 69 ± 1 aA | 94 ± 2 aA | 184 ± 15 aA | 168 ± 9 aA | 138 ± 14 aA | 46 ± 3 aA | 3.1 ± 0.4 bB | |
| Yes | 66 ± 3 aB | 89 ± 3 bC | 204 ± 20 aA | 171 ± 8 aA | 157 ± 16 aA | 47 ± 5 aA | 3.4 ± 0.2 aB | ||
| Foliar SeOrg | 25 | No | 71 ± 2 aA | 96 ± 2 aA | 140 ± 15 aA | 134 ± 15 aB | 103 ± 12 aA | 37 ± 3 aA | 2.8 ± 0.1 bB |
| Yes | 68 ± 4 aA | 94 ± 5 aB | 157 ± 20 aB | 147 ± 13 aB | 118 ± 16 aB | 39 ± 4 aA | 3.0 ± 0.1 aB | ||
| 50 | No | 66 ± 0 aB | 92 ± 1 aA | 193 ± 4 aA | 171 ± 5 aA | 149 ± 2 aA | 44 ± 3 aA | 3.4 ± 0.2 bA | |
| Yes | 65 ± 2 aB | 88 ± 2 aC | 152 ± 14 bB | 162 ± 10 aA | 119 ± 12 aB | 32 ± 3 bB | 3.7 ± 0.2 aA | ||
| Foliar Na2SeO4 | 25 | No | 65 ± 2 aB | 89 ± 2 aB | 193 ± 10 aA | 169 ± 5 aA | 147 ± 10 aA | 46 ± 2 aA | 3.2 ± 0.2 bB |
| Yes | 65 ± 4 aB | 90 ± 4 aC | 177 ± 10 aA | 159 ± 9 aA | 134 ± 7 aA | 43 ± 4 aA | 3.2 ± 0.2 aB | ||
| 50 | No | 64 ± 1 bB | 87 ± 2 bB | 186 ± 12 aA | 167 ± 6 aA | 141 ± 11 aA | 46 ± 2 aA | 3.1 ± 0.2 bB | |
| Yes | 71 ± 1 aA | 101 ± 2 aA | 127 ± 12 bB | 156 ± 7 aA | 97 ± 10 bB | 30 ± 2 bB | 3.2 ± 0.3 aB |
| Treatment | Se Rate (g ha−1) | Priming | Raw (N) | Blanching (N) | Blanching + Cooking (N) |
|---|---|---|---|---|---|
| No Se | 0 | No | 75.5 ± 4.6 | 55.1 ± 4.1 | 19.1 ± 2.0 |
| Yes | 62.2 ± 2.7 | 64.0 ± 4.6 | 15.2 ± 2.0 | ||
| Planting | 50 | No | 76.4 ± 3.3 | 67.7 ± 3.9 | 15.6 ± 1.9 |
| Yes | 72.3 ± 5.3 | 59.7 ± 5.2 | 14.3 ± 1.6 | ||
| 100 | No | 71.2 ± 5.3 | 54.6 ± 4.6 | 16.6 ± 1.9 | |
| Yes | 62.9 ± 5.5 | 57.5 ± 3.4 | 16.4 ± 1.4 | ||
| Top-dressing | 50 | No | 79.0 ± 7.7 | 51.0 ± 4.3 | 19.0 ± 1.1 |
| Yes | 83.1 ± 5.3 | 69.6 ± 4.6 | 16.7 ± 1.5 | ||
| 100 | No | 71.7 ± 3.0 | 59.7 ± 3.4 | 22.0 ± 0.8 | |
| Yes | 69.4 ± 8.0 | 55.1 ± 2.5 | 16.7 ± 0.9 | ||
| Foliar SeMNF | 25 | No | 71.7 ± 8.8 | 56.0 ± 2.6 | 14.6 ± 0.9 |
| Yes | 72.5 ± 6.7 | 54.1 ± 6.0 | 20.2 ± 1.3 | ||
| 50 | No | 71.8 ± 5.4 | 59.2 ± 4.2 | 18.2 ± 1.5 | |
| Yes | 88.4 ± 5.9 | 58.1 ± 7.6 | 12.4 ± 0.9 | ||
| Foliar SeOrg | 25 | No | 76.5 ± 2.3 | 61.9 ± 3.7 | 14.2 ± 0.8 |
| Yes | 60.1 ± 5.7 | 53.3 ± 7.4 | 16.7 ± 2.4 | ||
| 50 | No | 74.6 ± 3.8 | 56.9 ± 7.5 | 16.6 ± 1.4 | |
| Yes | 73.9 ± 4.2 | 52.2 ± 3.1 | 15.0 ± 1.7 | ||
| Foliar Na2SeO4 | 25 | No | 78.7 ± 3.8 | 59.2 ± 3.7 | 14.9 ± 1.3 |
| Yes | 71.5 ± 6.2 | 57.1 ± 3.2 | 16.8 ± 1.0 | ||
| 50 | No | 77.6 ± 7.8 | 72.1 ± 2.2 | 13.5 ± 0.8 | |
| Yes | 72.4 ± 5.6 | 57.5 ± 4.5 | 13.7 ± 1.1 | ||
| Treatments mean | 73.3 ± 1.4 A | 58.7 ± 1.2 B | 16.3 ± 0.50 C | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quispe, A.P.V.; de Morais, E.G.; Prado, D.T.; Machado, G.G.L.; Benevenute, P.A.N.; Cezar, J.V.d.C.; Vilas Boas, E.V.d.B.; Lopes, G.; Guilherme, L.R.G. Seedling Priming with Selenium Enhances the Biofortification Strategies in the Production of Broccoli Florets. Agronomy 2025, 15, 2207. https://doi.org/10.3390/agronomy15092207
Quispe APV, de Morais EG, Prado DT, Machado GGL, Benevenute PAN, Cezar JVdC, Vilas Boas EVdB, Lopes G, Guilherme LRG. Seedling Priming with Selenium Enhances the Biofortification Strategies in the Production of Broccoli Florets. Agronomy. 2025; 15(9):2207. https://doi.org/10.3390/agronomy15092207
Chicago/Turabian StyleQuispe, Anyela Pierina Vega, Everton Geraldo de Morais, Debora Teixeira Prado, Gilson Gustavo Lucinda Machado, Pedro Antônio Namorato Benevenute, João Victor da Costa Cezar, Eduardo Valério de Barros Vilas Boas, Guilherme Lopes, and Luiz Roberto Guimarães Guilherme. 2025. "Seedling Priming with Selenium Enhances the Biofortification Strategies in the Production of Broccoli Florets" Agronomy 15, no. 9: 2207. https://doi.org/10.3390/agronomy15092207
APA StyleQuispe, A. P. V., de Morais, E. G., Prado, D. T., Machado, G. G. L., Benevenute, P. A. N., Cezar, J. V. d. C., Vilas Boas, E. V. d. B., Lopes, G., & Guilherme, L. R. G. (2025). Seedling Priming with Selenium Enhances the Biofortification Strategies in the Production of Broccoli Florets. Agronomy, 15(9), 2207. https://doi.org/10.3390/agronomy15092207









