Optimizing Growth, Physiology, and Saponin Production in Primula veris L. Through Tailored LED Light Spectra for Energy-Efficient Cultivation
Abstract
1. Introduction
2. Materials and Methods
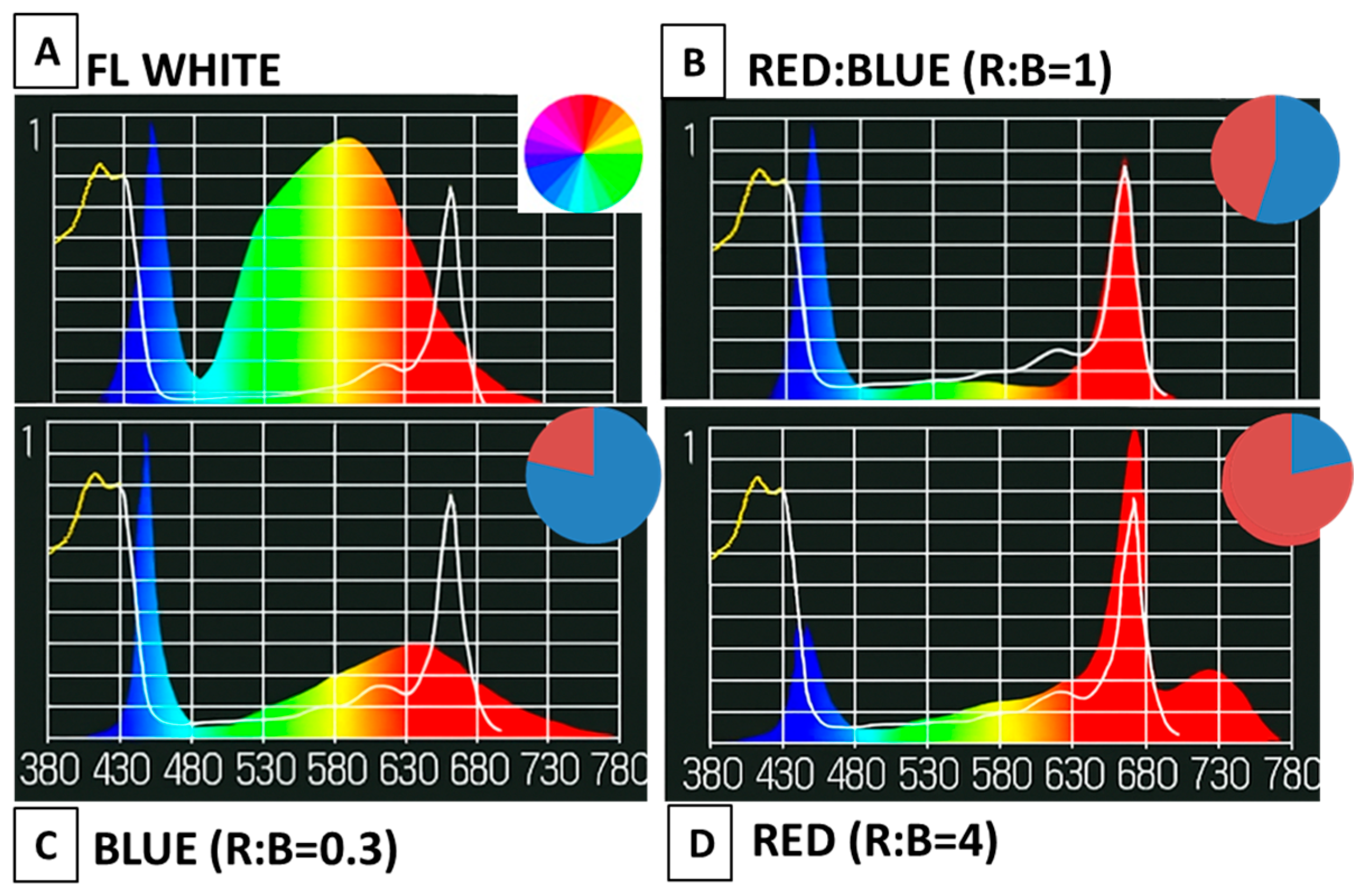
2.1. Plant Material, Growth Conditions, and Light Treatments
2.2. The Determination of the Relative Increase from Leaf Characteristics and Plant Biomass Production
2.3. The Determination of Leaf Photosynthetic Parameters
2.4. Determination of Total Chlorophylls and Carotenoids
2.5. Determination of Chlorophyll Fluorescence
2.6. Extraction of Triterpene Saponins and Phenolic Compounds and Ultra Performance Liquid Chromatography–Mass Spectrometry (UPLC-MS/MS, MRM) Analyses
2.7. Energy Usage, Water Usage, and Land Area Usage
2.8. Statistical Analysis
3. Results and Discussion
3.1. Relative Growth Rate with Focus on Leaf Characteristics Under Effect of Different LEDs
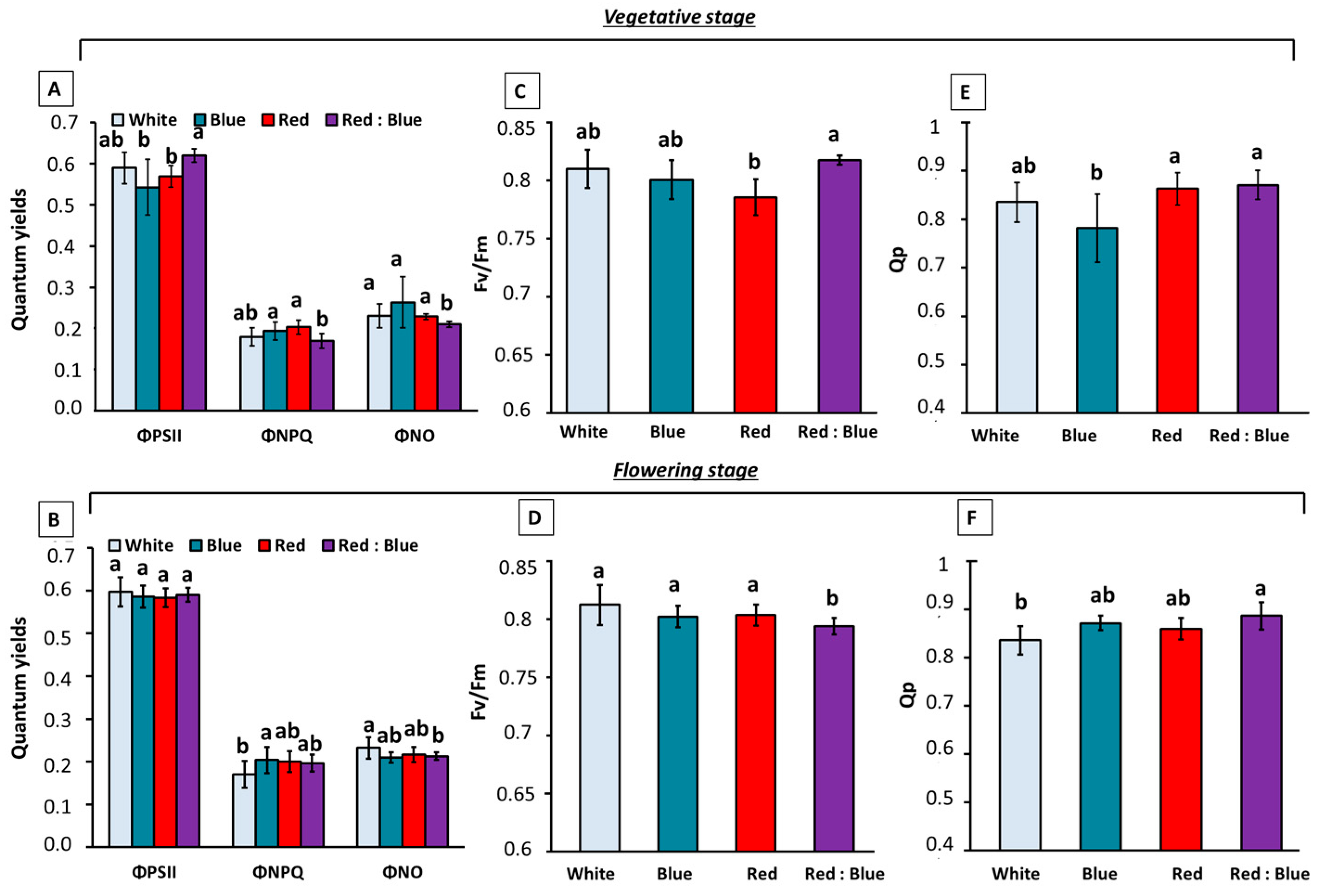
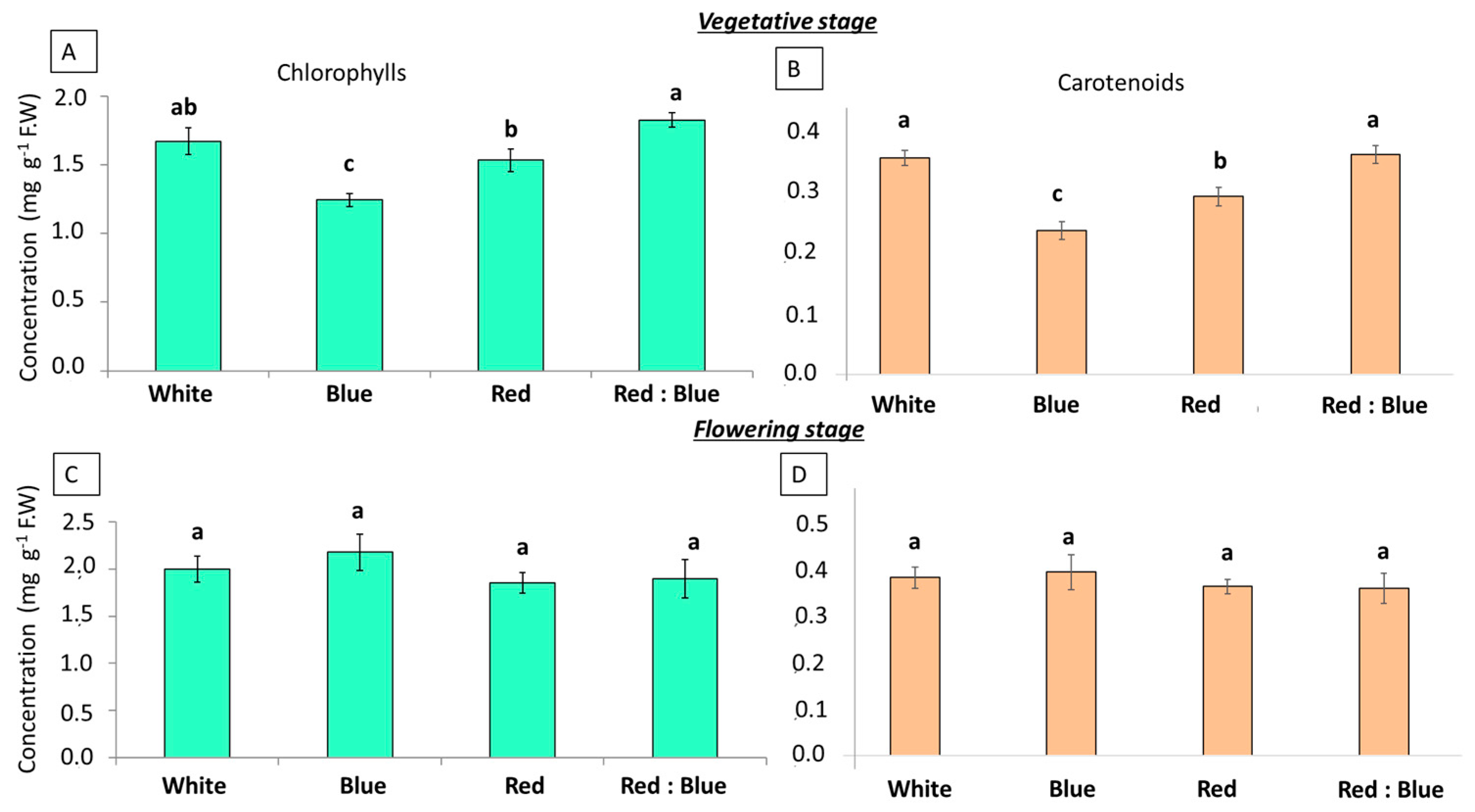
3.2. Photosynthetic Parameters Under the Effect of Different LEDs
3.3. Saponins and Phenolic Metabolites Under the Effect of Different LEDs
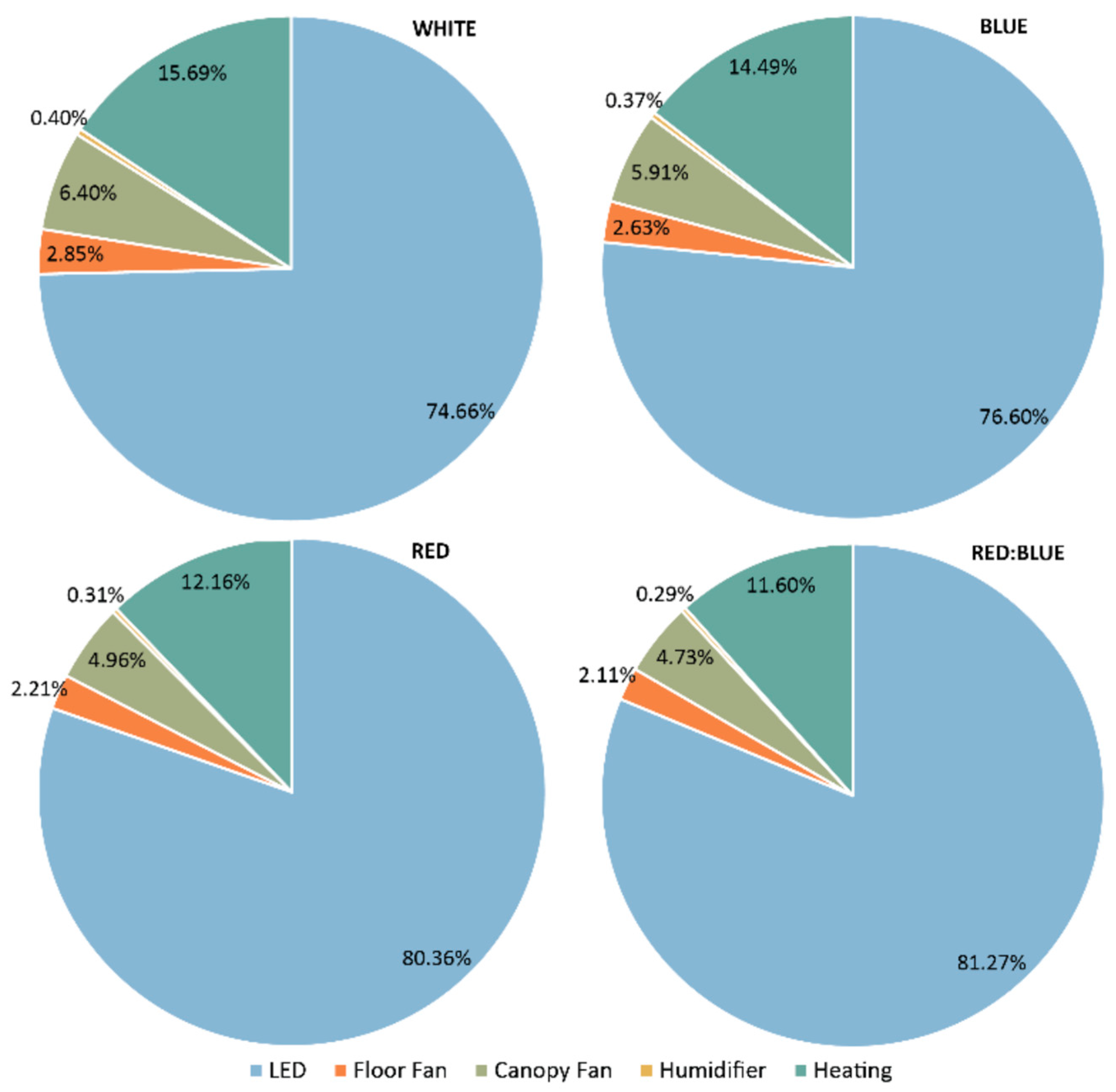
3.4. Energy Usage, Water Usage, and Land Area Usage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wichtl, M. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis; CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-0-8493-1961-7. [Google Scholar]
- Brys, R.; Jacquemyn, H. Biological Flora of the British Isles: Primula veris L. J. Ecol. 2009, 97, 581–600. [Google Scholar] [CrossRef]
- Hostettmann, K.; Marston, A. Chemistry and Pharmacology of Natural Products; Cambridge University Press: Cambridge, UK, 1995; Volume 548, pp. 326–327. [Google Scholar]
- Marchyshyn, S.; Slobodianiuk, L.; Budniak, L.; Shostak, L.; Gerush, O. Investigation on the Expectorant Effect of Extracts from Primula veris L. Open Access Maced. J. Med. Sci. 2022, 10, 1368–1372. [Google Scholar] [CrossRef]
- EMA/HMPC/104095/2012 Committee on Herbal Medicinal Products (HMPC) Community Herbal Monograph on Primula veris L. and/or Primula elatior (L.) Hill, Radix. European Medicines Agency. 2013. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-community-herbal-monograph-primula-veris-l-and-or-primula-elatior-l-hill-radix_en.pdf (accessed on 19 September 2012).
- Zandalinas, S.I.; Sales, C.; Beltrán, J.; Gómez-Cadenas, A.; Arbona, V. Activation of Secondary Metabolism in Citrus Plants Is Associated to Sensitivity to Combined Drought and High Temperatures. Front. Plant Sci. 2017, 7, 1954. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Ganzera, M.; Stuppner, H. Analysis of Phenolic Glycosides and Saponins in Primula elatior and Primula veris (Primula Root) by Liquid Chromatography, Evaporative Light Scattering Detection and Mass Spectrometry. J. Chromatogr. A 2006, 1112, 218–223. [Google Scholar] [CrossRef]
- Apel, L.; Kammerer, D.R.; Stintzing, F.C.; Spring, O. Comparative Metabolite Profiling of Triterpenoid Saponins and Flavonoids in Flower Color Mutations of Primula veris L. Int. J. Mol. Sci. 2017, 18, 153. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Sarrou, E.; Angeli, A.; Martens, S.; Maloupa, E.; Grigoriadou, K. Developing an in Vitro Elicitation Strategy for Specialized Secondary Metabolites Production in Adventitious Root Cultures of Primula veris subsp. veris. Ind. Crops Prod. 2023, 197, 116618. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J.; Fankhauser, C. Light Signal Transduction in Higher Plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.W.; Bansal, V.; Prasad, K. (Eds.) Plant Secondary Metabolites, Volume Two: Stimulation, Extraction, and Utilization; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Appolloni, E.; Pennisi, G.; Zauli, I.; Carotti, L.; Paucek, I.; Quaini, S.; Orsini, F.; Gianquinto, G. Beyond Vegetables: Effects of Indoor LED Light on Specialized Metabolite Biosynthesis in Medicinal and Aromatic Plants, Edible Flowers, and Microgreens. J. Sci. Food Agric. 2022, 102, 472–487. [Google Scholar] [CrossRef]
- Cáceres-Cevallos, G.J.; Jordán, M.J. Effects of LED Light on Aromatic Medicinal Plants from Lavandula, Salvia, and Thymus Genera: A Systematic Review. Stresses 2024, 4, 627–640. [Google Scholar] [CrossRef]
- Tilbrook, K.; Arongaus, A.B.; Binkert, M.; Heijde, M.; Yin, R.; Ulm, R. The UVR8 UV-B Photoreceptor: Perception, Signaling and Response. Arab. Book 2013, 11, e0164. [Google Scholar] [CrossRef]
- Folta, K.M.; Carvalho, S.D. Photoreceptors and Control of Horticultural Plant Traits. HortScience 2015, 50, 1274–1280. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, X.; Gao, L.; Chen, Q.; Qu, M. Blue Light Is More Essential than Red Light for Maintaining the Activities of Photosystem II and I and Photosynthetic Electron Transport Capacity in Cucumber Leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef]
- Christiaens, A.; Gobin, B.; Van Labeke, M.C. Light Quality and Adventitious Rooting: A Mini-Review. Acta Hortic. 2016, 1134, 385–394. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Zhang, F.; Gu, Y.; Tian, W.; Tian, W.; Tong, Y.; Li, J. The Combination of Red and Blue Light Increases the Biomass and Steroidal Saponin Contents of Paris polyphylla var. Yunnanensis. Ind. Crops Prod. 2023, 194, 116311. [Google Scholar] [CrossRef]
- Watcharatanon, K.; Ingkaninan, K.; Putalun, W. Improved Triterpenoid Saponin Glycosides Accumulation in in Vitro Culture of Bacopa monnieri (L.) Wettst with Precursor Feeding and LED Light Exposure. Ind. Crops Prod. 2019, 134, 303–308. [Google Scholar] [CrossRef]
- Di, P.; Sun, Z.; Cheng, L.; Han, M.; Yang, L.; Yang, L. LED Light Irradiations Differentially Affect the Physiological Characteristics, Ginsenoside Content, and Expressions of Ginsenoside Biosynthetic Pathway Genes in Panax ginseng. Agriculture 2023, 13, 807. [Google Scholar] [CrossRef]
- Sobhani Najafabadi, A.; Khanahmadi, M.; Ebrahimi, M.; Moradi, K.; Behroozi, P.; Noormohammadi, N. Effect of Different Quality of Light on Growth and Production of Secondary Metabolites in Adventitious Root Cultivation of Hypericum perforatum. Plant Signal. Behav. 2019, 14, 1640561. [Google Scholar] [CrossRef]
- Cáceres-Cevallos, G.; Martínez-Conesa, C.; García-Aledo, I.; Quílez-Simón, M.; Romero-Espinar, P.; Jordán, M.J. Lavandula latifolia Medik. Grown with LED Emitting Diode Light Technology, an Initial Approach. Acta Hortic. 2023, 1358, 303–310. [Google Scholar] [CrossRef]
- Bantis, F.; Radoglou, K. Testing the Potential of LEDs to Enhance Growth and Quality Characteristics of Salvia fruticosa. Hortic. Sci. 2019, 46, 98–106. [Google Scholar] [CrossRef]
- Lee, H.R.; Kim, H.M.; Jeong, H.W.; Hwang, S.J. Growth and Bioactive Compounds of Salvia plebeia R. Br. Grown under Various Ratios of Red and Blue Light. Horticulturae 2020, 6, 35. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A.; Sabzalian, M.R. Thymol, Carvacrol, and Antioxidant Accumulation in Thymus Species in Response to Different Light Spectra Emitted by Light-Emitting Diodes. Food Chem. 2020, 307, 125521. [Google Scholar] [CrossRef]
- Costine, B.; Zhang, M.; Pearson, B.; Nadakuduti, S.S. Impact of Blue Light on Plant Growth, Flowering and Accumulation of Medicinal Flavones in Scutellaria baicalensis and S. lateriflora. Horticulturae 2022, 8, 1141. [Google Scholar] [CrossRef]
- d’Aquino, L.; Lanza, B.; Gambale, E.; Sighicelli, M.; Menegoni, P.; Modarelli, G.C.; Rimauro, J.; Chianese, E.; Nenna, G.; Fasolino, T.; et al. Growth and Metabolism of Basil Grown in a New-Concept Microcosm under Different Lighting Conditions. Sci. Hortic. 2022, 299, 111035. [Google Scholar] [CrossRef]
- Feldzensztajn, M.; Wierzba, P.; Mazikowski, A. Examination of Spectral Properties of Medicinal Plant Leaves Grown in Different Lighting Conditions Based on Mint Cultivation. Sensors 2021, 21, 4122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of Light on Secondary Metabolite Biosynthesis in Medicinal Plants. Front. Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef]
- Wintermans, J.F.G.M.; De Mots, A. Spectrophotometric Characteristics of Chlorophylls a and b and Their Phenophytins in Ethanol. Biochim. Et Biophys. Acta (BBA)—Biophys. Incl. Photosynth. 1965, 109, 448–453. [Google Scholar] [CrossRef]
- Moustakas, M.; Bayçu, G.; Sperdouli, I.; Eroğlu, H.; Eleftheriou, E.P. Arbuscular Mycorrhizal Symbiosis Enhances Photosynthesis in the Medicinal Herb Salvia fruticosa by Improving Photosystem II Photochemistry. Plants 2020, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Masuero, D.; Gasperotti, M.; Franceschi, P.; Caputi, L.; Viola, R.; Mattivi, F. A Versatile Targeted Metabolomics Method for the Rapid Quantification of Multiple Classes of Phenolics in Fruits and Beverages. J. Agric. Food Chem. 2012, 60, 8831–8840. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the Role of Red:Blue LED Lights on Resource Use Efficiency and Nutritional Properties of Indoor Grown Sweet Basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F.M. Optimal Light Intensity for Sustainable Water and Energy Use in Indoor Cultivation of Lettuce and Basil under Red and Blue LEDs. Scientia Horticulturae 2020, 272, 109508. [Google Scholar] [CrossRef]
- Park, H.; Yu, Y.J.; Choi, E.Y. Effects of Fluorescent Light and Light-Emitting Diodes on Leaf Morphology, Growth and Antioxidant Capacity of Salvia plebeia. J. Bio-Environ. Control. 2017, 26, 208–214. [Google Scholar] [CrossRef]
- Seyedi, F.S.; Nafchi, M.G.; Reezi, S. Effects of Light Spectra on Morphological Characteristics, Primary and Specialized Metabolites of Thymus vulgaris L. Heliyon 2024, 10, e23032. [Google Scholar] [CrossRef]
- Paik, I.; Huq, E. Plant Photoreceptors: Multi-Functional Sensory Proteins and Their Signaling Networks. Semin. Cell Dev. Biol. 2019, 92, 114–121. [Google Scholar] [CrossRef]
- Matysiak, B.; Kowalski, A. White, Blue and Red LED Lighting on Growth, Morphology and Accumulation of Flavonoid Compounds in Leafy Greens. Zemdirbyste-Agriculture 2019, 106, 281–286. [Google Scholar] [CrossRef]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of Seven Diverse Species to Blue and Green Light: Interactions with Photon Flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef]
- Al Murad, M.; Razi, K.; Jeong, B.R.; Samy, P.M.A.; Muneer, S. Light Emitting Diodes (LEDs) as Agricultural Lighting: Impact and Its Potential on Improving Physiology, Flowering, and Secondary Metabolites of Crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- Inoue, S.; Kinoshita, T. Blue Light Regulation of Stomatal Opening and the Plasma Membrane H+-ATPase. Plant Physiol. 2017, 174, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Chapter Two—Light-Regulated Plant Growth and Development. In Current Topics in Developmental Biology; Timmermans, M.C.P., Ed.; Plant Development; Academic Press: Cambridge, MA, USA, 2010; Volume 91, pp. 29–66. [Google Scholar]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The Role of Photosynthesis Related Pigments in Light Harvesting, Photoprotection and Enhancement of Photosynthetic Yield in Planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-C.; Kathare, P.K.; Paik, I.; Huq, E. Phytochrome Signaling Networks. Annu. Rev. Plant Biol. 2021, 72, 217–244. [Google Scholar] [CrossRef] [PubMed]
- Li, L. The Influence of Elicitor on the Accumulation of Several Metabolites in the Seedling Salvia przewalskii Maxim. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2014. [Google Scholar]
- Kapoor, S.; Raghuvanshi, R.; Bhardwaj, P.; Sood, H.; Saxena, S.; Chaurasia, O.P. Influence of Light Quality on Growth, Secondary Metabolites Production and Antioxidant Activity in Callus Culture of Rhodiola imbricata Edgew. J. Photochem. Photobiol. B Biol. 2018, 183, 258–265. [Google Scholar] [CrossRef]
- Stepanova, A.; Solov’yova, A.; Salamaikina, S. Influence of Spectral Light Composition on Flavones Formation in Callus Culture of Scutellaria baicalensis Georgi. Pharmacogn. Mag. 2020, 16, 156–160. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Prokopiuk, B.; Komsta, Ł.; Pawłowska, B.; Ekiert, H. The Influence of Light Quality on the Production of Bioactive Metabolites—Verbascoside, Isoverbascoside and Phenolic Acids and the Content of Photosynthetic Pigments in Biomass of Verbena officinalis L. Cultured in Vitro. J. Photochem. Photobiol. B Biol. 2020, 203, 111768. [Google Scholar] [CrossRef]
- Ueda, T.; Murata, M.; Yokawa, K. Single Wavelengths of LED Light Supplement Promote the Biosynthesis of Major Cyclic Monoterpenes in Japanese Mint. Plants 2021, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Shohael, A.M.; Ali, M.B.; Yu, K.W.; Hahn, E.J.; Islam, R.; Paek, K.Y. Effect of Light on Oxidative Stress, Secondary Metabolites and Induction of Antioxidant Enzymes in Eleutherococcus senticosus Somatic Embryos in Bioreactor. Process Biochem. 2006, 41, 1179–1185. [Google Scholar] [CrossRef]
- Cirak, C.; Radusiene, J.; Camas, N.; Caliskan, O.; Odabas, M.S. Changes in the Contents of Main Secondary Metabolites in Two Turkish Hypericum Species during Plant Development. Pharm. Biol. 2013, 51, 391–399. [Google Scholar] [CrossRef]
- Sarrou, E.; Martens, S.; Chatzopoulou, P. Metabolite Profiling and Antioxidative Activity of Sage (Salvia fruticosa Mill.) under the Influence of Genotype and Harvesting Period. Ind. Crops Prod. 2016, 94, 240–250. [Google Scholar] [CrossRef]
- Sarrou, E.; Tsivelika, N.; Chatzopoulou, P.; Tsakalidis, G.; Menexes, G.; Mavromatis, A. Conventional Breeding of Greek Oregano (Origanum vulgare ssp. hirtum) and Development of Improved Cultivars for Yield Potential and Essential Oil Quality. Euphytica 2017, 213, 104. [Google Scholar] [CrossRef]
- Önder, S.; Tonguç, M.; Erbaş, S.; Önder, D.; Mutlucan, M. Investigation of Phenological, Primary and Secondary Metabolites Changes during Flower Developmental of Rosa damascena. Plant Physiol. Biochem. 2022, 192, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; Pennisi, G.; Zulfiqar, F.; Gianquinto, G. Sustainable Use of Resources in Plant Factories with Artificial Lighting (PFALs). Eur. J. Hortic. Sci. 2020, 85, 297–309. [Google Scholar] [CrossRef]
- Bantis, F.; Chatzigeorgiou, I.; Sismanis, M.; Ntinas, G.K.; Koukounaras, A. Vegetable Production in PFALs: Control of Micro-Environmental Factors, Principal Components and Automated Systems. Agriculture 2024, 14, 642. [Google Scholar] [CrossRef]
- Kong, Y.; Nemali, A.; Mitchell, C.; Nemali, K. Spectral Quality of Light Can Affect Energy Consumption and Energy-Use Efficiency of Electrical Lighting in Indoor Lettuce Farming. HortScience 2019, 54, 865–872. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource Use Efficiency of Indoor Lettuce (Lactuca sativa L.) Cultivation as Affected by Red:Blue Ratio Provided by LED Lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef] [PubMed]
- Pistillo, A.; Pennisi, G.; Crepaldi, A.; Giorgioni, M.E.; Minelli, A.; Orsini, F.; Gianquinto, G. Influence of Red:Blue Ratio in LED Lighting for Indoor Cultivation of Edible Marigold Flowers. Acta Hortic. 2022, 1337, 249–254. [Google Scholar] [CrossRef]



| Vegetative Stage | ||||
|---|---|---|---|---|
| Light Treatments | Total Plant Fresh Weight (g) | Root Fresh Weight (g) | Aerial Part Fresh Weight (g) | Single Leaf Fresh Weight (g) |
| White | 10.66 a ± 1.86 | 4.9 b ± 0.28 | 5.71 a ± 1.01 | 0.51 a ± 0.08 |
| Blue | 11.81 a ± 2.58 | 6.08 a ± 0.41 | 5.67 a ± 1.07 | 0.57 a ± 0.14 |
| Red | 11.24 a ± 1.51 | 5.88 ab ± 0.18 | 5.33 a ± 0.73 | 0.39 a ± 0.05 |
| Red:Blue | 11.62 a ± 0.93 | 6.16 a ± 0.21 | 5.37 a ± 0.56 | 0.49 a ± 0.04 |
| Flowering Stage | ||||
| White | 18.59 b ± 0.83 | 8.47 b ± 0.35 | 9.89 a ± 2.34 | 0.89 a ± 0.15 |
| Blue | 17.14 b ± 0.55 | 8.79 b ± 0.95 | 8.24 a ± 1.42 | 0.82 a ± 0.14 |
| Red | 25.77 a ± 0.82 | 14.13 a ± 0.47 | 12.53 a ± 1.57 | 1.07 a ± 0.12 |
| Red:Blue | 26.34 a ± 1.33 | 14.82 a ± 0.68 | 11.30 a ± 1.16 | 0.98 a ± 0.07 |
| Whole Plant Fresh Weight (g m−2) | Water Use Efficiency (g FW L−1 H2O) | Energy Use Efficiency (g FW kWh−1) | 1 Land Use Efficiency—3 Cultivation/year (kg m−2 a−1) | ||
|---|---|---|---|---|---|
| 1 Layer | 4 Layers | ||||
| White | 1062.57 ± 47.43 b | 4.92 ± 0.22 b | 4.32 ± 0.19 ab | 3.19 ± 0.14 b | 12.75 ± 0.57 b |
| Blue | 979.52 ± 31.25 b | 4.53 ± 0.14 b | 3.68 ± 0.14 b | 2.94 ± 0.09 b | 11.75 ± 0.38 b |
| Red | 1472.38 ± 46.65 a | 6.82 ± 0.22 a | 4.64 ± 0.15 a | 4.42 ± 0.14 a | 17.67 ± 0.56 a |
| Red:Blue | 1505.24 ± 76.14 a | 6.97 ± 0.35 a | 4.53 ± 0.23 a | 4.52 ± 0.23 a | 18.06 ± 0.91 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsivelika, N.; Koumparelou, D.; Chatzigeorgiou, I.; Sperdouli, I.; Abraham, E.; Panajiotidis, S.; Angeli, A.; Ntinas, G.K.; Martens, S.; Sarrou, E. Optimizing Growth, Physiology, and Saponin Production in Primula veris L. Through Tailored LED Light Spectra for Energy-Efficient Cultivation. Agronomy 2025, 15, 2184. https://doi.org/10.3390/agronomy15092184
Tsivelika N, Koumparelou D, Chatzigeorgiou I, Sperdouli I, Abraham E, Panajiotidis S, Angeli A, Ntinas GK, Martens S, Sarrou E. Optimizing Growth, Physiology, and Saponin Production in Primula veris L. Through Tailored LED Light Spectra for Energy-Efficient Cultivation. Agronomy. 2025; 15(9):2184. https://doi.org/10.3390/agronomy15092184
Chicago/Turabian StyleTsivelika, Nektaria, Danai Koumparelou, Ioanna Chatzigeorgiou, Ilektra Sperdouli, Eleni Abraham, Sampson Panajiotidis, Andrea Angeli, Georgios K. Ntinas, Stefan Martens, and Eirini Sarrou. 2025. "Optimizing Growth, Physiology, and Saponin Production in Primula veris L. Through Tailored LED Light Spectra for Energy-Efficient Cultivation" Agronomy 15, no. 9: 2184. https://doi.org/10.3390/agronomy15092184
APA StyleTsivelika, N., Koumparelou, D., Chatzigeorgiou, I., Sperdouli, I., Abraham, E., Panajiotidis, S., Angeli, A., Ntinas, G. K., Martens, S., & Sarrou, E. (2025). Optimizing Growth, Physiology, and Saponin Production in Primula veris L. Through Tailored LED Light Spectra for Energy-Efficient Cultivation. Agronomy, 15(9), 2184. https://doi.org/10.3390/agronomy15092184








