Waste-Derived Fertilizers: Conversion Technologies, Circular Bioeconomy Perspectives and Agronomic Value
Abstract
1. Introduction
2. Biowaste Streams for Nutrient Recovery
2.1. Livestock Manure
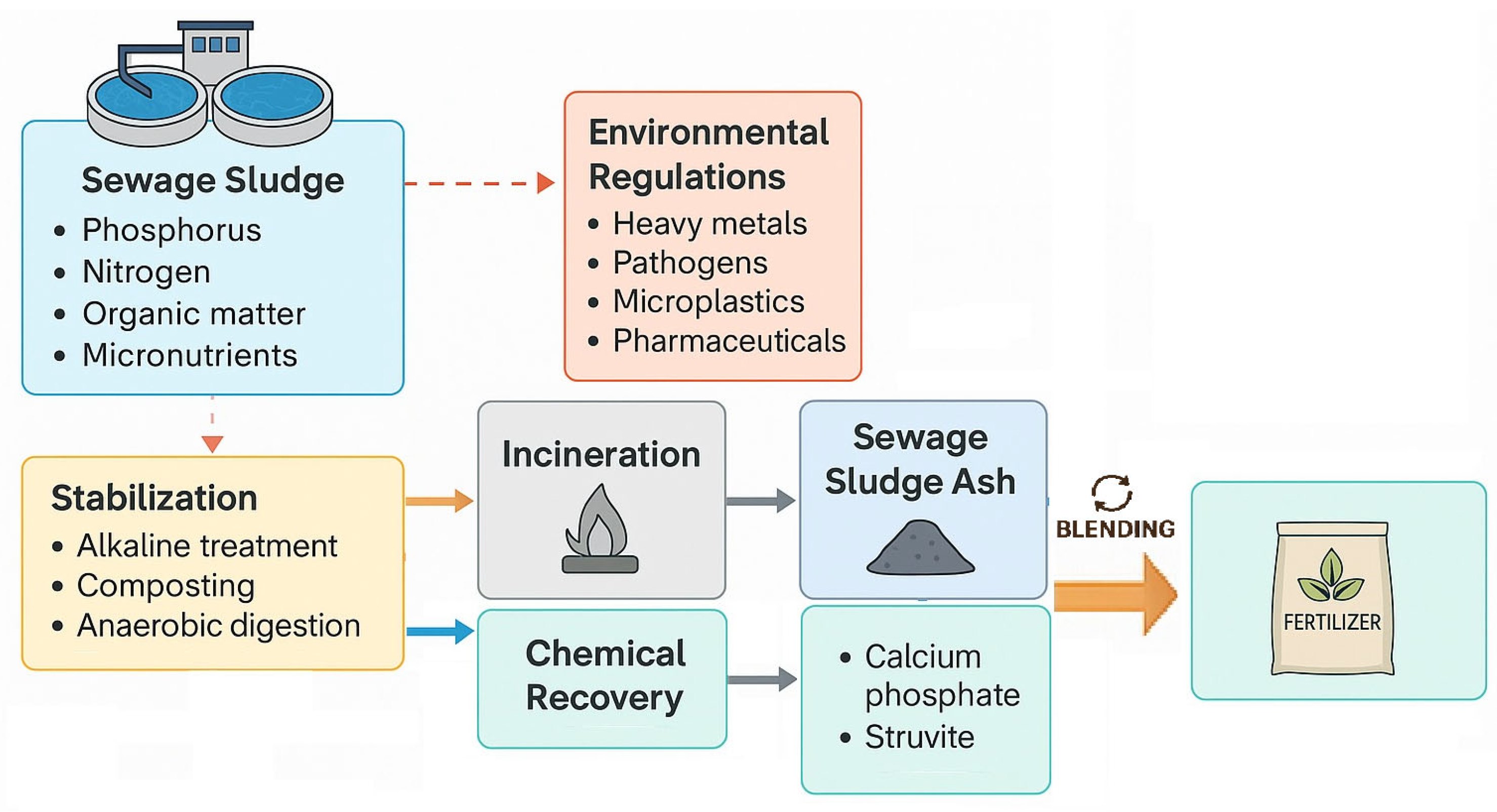
2.2. Sewage Sludge and Derivatives
2.3. Digestates
2.4. Food and Agro-Industrial By-Products
2.5. Biochar and Ashes
2.6. Municipal Wastewaters
3. Processing and Stabilization Strategies for Fertilizer-Compatible Bio-Based Inputs
3.1. Biological Processing Routes
3.2. Thermochemical Pathways
3.3. Chemical Processing and Nutrient Recovery Technologies
3.4. Tailoring Nutrient Profiles and Functionalization of Products
4. Agronomic Performance and Field Effectiveness of Waste-Derived Fertilizers
4.1. Compost and Digestate: Nutrient Release and Yield in Field Trials
4.2. Biochar Amendments: Yield Effects and Soil Health
4.3. Recovered Nutrient Fertilizers: Struvite, Ammonium Salts, and Ash-Based Products
4.4. Influence of Formulation and Application Strategies on Performance
4.5. Performance Variability Across Soils and Climates
4.6. Regulatory Considerations
5. Conclusions and Future Perspectives in Circular Fertilization
- -
- Optimization of treatment pathways to enhance agronomic performance, minimize environmental impacts, and ensure economic viability—especially for decentralized systems.
- -
- Development of multifunctional fertilizers incorporating biostimulants, microbial inoculants, or controlled-release mechanisms.
- -
- Harmonization of regulatory frameworks and certification procedures to facilitate market access and recognition of waste-derived products.
- -
- Long-term field trials under diverse soil–climate conditions to assess nutrient release, crop yield, and soil health outcomes.
- -
- Stakeholder engagement through training, field demonstrations, and transparent communication to build trust and encourage adoption.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CMCs | Component Material Categories |
| EC | European Commission |
| EU | European Union |
| FPR | Fertilising Products Regulation |
| HTC | Hydrothermal Carbonization |
| PFCs | Product Function Categories |
References
- FAO. Developments in International Fertilizer Markets. In Proceedings of the Committee on Commodity Problems, Seventy-Sixth Session, Rome, Italy, 11–13 September 2024; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/e379efe6-a955-4ffc-bb53-0c9a89f11f1a/content (accessed on 21 July 2025).
- European Commission. Ensuring Availability and Affordability of Fertilisers. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, COM(2022) 590 Final, Brussels, Belgium, 9 November 2022. Available online: https://agriculture.ec.europa.eu/common-agricultural-policy/agri-food-supply-chain/ensuring-availability-and-affordability-fertilisers_en (accessed on 21 July 2025).
- EFG International. EU Natural Gas Prices to Gradually Normalise. 24 February 2025. Available online: https://www.efginternational.com/insights/2025/eu-natural-gas-prices-to-gradually-normalise.html (accessed on 21 July 2025).
- Hidalgo, D.; Corona, F.; Martín-Marroquín, J.M. Nutrient recycling: From waste to crop. Biomass Convers. Biorefin. 2021, 11, 207–217. [Google Scholar] [CrossRef]
- European Environment Agency. Air Pollution in Europe: 2024 Reporting Status Under the National Emission Reduction Commitments Directive (Briefing No. 07/2024). Emissions from Agriculture Account for 93% of Total Ammonia Emissions in the EU-27. Available online: https://www.eea.europa.eu/publications/national-emission-reduction-commitments-directive-2024 (accessed on 21 July 2025).
- International Fertilizer Association (IFA). Production Emissions. Mineral Fertilizer Production and Greenhouse Gas Emissions. Available online: https://www.fertilizer.org/key-priorities/fertilizers-climate-change/production-emissions/ (accessed on 21 July 2025).
- Chojnacka, K.; Mikula, K.; Skrzypczak, D.; Izydorczyk, G.; Gorazda, K.; Kulczycka, J.; Witek-Krowiak, A. Practical aspects of biowastes conversion to fertilizers. Biomass Convers Biorefin. 2024, 14, 1515–1533. [Google Scholar] [CrossRef]
- Bagga, D.; Chauhan, S.; Bhavanam, A.; Nikhil, G.N.; Meena, S.S.; Mohanty, A. Recent advancements in fermentation strategies for mass production and formulation of biofertilizers: Towards waste valorization. J. Soil Sci. Plant Nutr. 2024, 24, 5868–5897. [Google Scholar] [CrossRef]
- Hidalgo, D.; Corona, F. Livestock manure valorization to Biochemical’s and energy. In Manure Technology and Sustainable Development; Springer Nature: Singapore, 2023; pp. 211–239. [Google Scholar]
- Sharma, A.; Soni, R.; Soni, S.K. From waste to wealth: Exploring modern composting innovations and compost valorization. J. Mater. Cycles Waste Manag. 2024, 26, 20–48. [Google Scholar] [CrossRef]
- Orlandella, I.; Fiore, S. Life Cycle Assessment of the Production of Biofertilizers from Agricultural Waste. Sustainability 2025, 17, 421. [Google Scholar] [CrossRef]
- Rey-Martínez, N.; Torres-Sallan, G.; Morales, N.; Serra, E.; Bisschops, I.; van Eekert, M.H.; Sanchis, S. Combination of technologies for nutrient recovery from wastewater: A review. Clean. Waste Syst. 2024, 7, 100139. [Google Scholar] [CrossRef]
- Anuvia Plant Nutrients. ATP Nutrition. Anuvia Plant Nutrients and ATP Nutrition Launch SymTRX, the First Bio-Based Granular Fertilizer in Canada. Available online: https://atpag.com/news/anuvia-plant-nutrients-and-atp-nutrition-launch-symtrx-the-first-bio-based-granular-fertilizer-in-canada/ (accessed on 21 July 2025).
- Lystek. Leaders in Biosolids and Organics Management. Available online: https://lystek.com (accessed on 21 July 2025).
- Rosemarin, A.; Macura, B.; Carolus, J.; Barquet, K.; Ek, F.; Järnberg, L.; Lorick, D.; Johannesdottir, S.; Pedersen, S.M.; Koskiaho, J.; et al. Circular nutrient solutions for agriculture and wastewater—A review of technologies and practices. Curr. Opin. Environ. Sustain. 2020, 45, 78–91. [Google Scholar] [CrossRef]
- Burnham, J.C. Organic Containing Sludge to Fertilizer Alkaline Conversion Process. U.S. Patent US7662206B2, 15 September 2006. Available online: https://patents.google.com/patent/US7662206B2/en (accessed on 21 July 2025).
- Burnham, J.C.; Carr, J.P.; Dahms, G.L. Process for Treating Sludge and Manufacturing Bioorganically-Augmented High Nitrogen-Containing Inorganic Fertilizer. U.S. Patent US7947104B2, 19 February 2008. Available online: https://patents.google.com/patent/US7947104B2/en (accessed on 21 July 2025).
- Method and System for Treating Sludge Using Recycle. U.S. Patent US20050067348A1, 25 September 2003. Available online: https://patents.google.com/patent/US20050067348A1 (accessed on 21 July 2025).
- BCR Solid Solutions. Neutralizer. Available online: https://bcrinc.com/neutralizer/ (accessed on 21 July 2025).
- FEECO International, Inc. Available online: https://feeco.com/ (accessed on 21 July 2025).
- Fibrophos Fertilisers. PK Fertiliser Phosphate and Potash Fertiliser. Available online: https://www.fibrophos.co.uk/ (accessed on 21 July 2025).
- Géotexia. Available online: https://geotexia.wordpress.com/ (accessed on 21 July 2025).
- Italpollina. Available online: https://www.italpollina.com/ (accessed on 21 July 2025).
- Fertikal. Available online: https://www.fertikal.be/en/ (accessed on 21 July 2025).
- Grooms, A.; Reusser, S.; Dose, A.; Britton, A.; Prasad, R. Operating experience with Ostara struvite harvesting process. In Proceedings of the WEFTEC 2015, Water Environment Federation, Chicago, IL, USA, 26–30 September 2015. [Google Scholar]
- Kokulan, V.; Schneider, K.; Macrae, M.L.; Wilson, H. Struvite application to field corn decreases the risk of environmental phosphorus loss while maintaining crop yield. Agric. Ecosyst. Environ. 2024, 366, 108936. [Google Scholar] [CrossRef]
- Aguilar-Pozo, V.B.; Chimenos, J.M.; Tribby, E.; Lopez, A.; Gómez, J.; Olaciregui-Arizmendi, K.; Astals, S. Phosphorus recovery using low-grade magnesium oxide as a magnesium source and an alkaline reagent: A pilot-scale study. J. Water Process Eng. 2025, 72, 107408. [Google Scholar] [CrossRef]
- Fang, L.; Wang, Q.; Li, J.; Poon, C.S.; Cheeseman, C.R.; Donatello, S.; Tsang, D.C.W. Feasibility of wet-extraction of phosphorus from incinerated sewage sludge ash (ISSA) for phosphate fertilizer production: A critical review. Crit. Rev. Environ. Sci. Technol. 2020, 51, 1–33. [Google Scholar] [CrossRef]
- Ohtake, H.; Tsuneda, S. Phosphorus Recovery and Recycling; Springer: Singapore, 2018. [Google Scholar]
- Aldaach, H.; Zaki, M.T.; Orner, K.D. Improving prediction of nutrient recovery via struvite precipitation from organic waste digestate. Environ. Eng. Sci. 2025, 42, 53–67. [Google Scholar] [CrossRef]
- Müller, B.; Wester-Larsen, L.; Jensen, L.S.; Salo, T.; Garrido, R.R.; Arkoun, M.; Bauerle, A. Agronomic performance of novel, nitrogen-rich biobased fertilizers across European field trial sites. Field Crops Res. 2024, 316, 109486. [Google Scholar] [CrossRef]
- Chojnacka, K. Valorization of biorefinery residues for sustainable fertilizer production: A comprehensive review. Biomass Convers. Biorefin. 2023, 13, 14359–14388. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1069/2009 Laying Down Health Rules as Regards Animal by-Products Not Intended for Human Consumption and Commission Regulation (EU) 2023/1605 on End-Points for Animal by-Products. Brussels, Belgium, 2009 & 2023. Available online: https://eur-lex.europa.eu/eli/reg/2009/1069/oj/eng (accessed on 9 September 2025).
- Garmendia-Lemus, S.; Moshkin, E.; Hung, Y.; Tack, J.; Buysse, J. European farmers’ perceptions and intentions to use bio-based fertilisers: Insights from the theory of planned behaviour and perceived utility. J. Clean. Prod. 2024, 434, 139755. [Google Scholar] [CrossRef]
- Tawo, O.E.; Mbamalu, M.I. Advancing waste valorization techniques for sustainable industrial operations and improved environmental safety. Int. J. Sci. Res. Arch. 2025, 14, 127–149. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Regulation (EU) 2019/1009 of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, L 170, 1–114. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj/eng (accessed on 24 July 2025).
- European Parliament; Council of the European Union. Regulation (EC) No 1069/2009 of 21 October 2009 Laying Down Health rules as Regards Animal by-Products and Derived Products not Intended for Human Consumption and Repealing Regulation (EC) No 1774/2002. Off. J. Eur. Union 2009, L 300, 1–33. Available online: https://eur-lex.europa.eu/eli/reg/2009/1069/oj (accessed on 24 July 2025).
- Maffia, A.; Marra, F.; Celano, G.; Oliva, M.; Mallamaci, C.; Hussain, M.I.; Muscolo, A. Exploring the potential and obstacles of agro-industrial waste-based fertilizers. Land 2024, 13, 1166. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Afshar, R.K. Livestock manure: From waste to resource in a circular economy. J. Agric. Food Res. 2024, 17, 101255. [Google Scholar] [CrossRef]
- Reyhanitabar, A.; Raji, M.; Khalkhal, K.; Hemati, A.; Sarikhani, M.R. The Effect of Biochar, Leonardite and Coal on Some Quality Characteristics of Compost Resulting from The Co-composting of Manure and Forest Organic Matter. Water Soil Sci. 2024, 34, 161–178. [Google Scholar] [CrossRef]
- Li, J.; Ma, W.; Wang, P.; Meng, X. Farmers’ Willingness to Transform Untreated Livestock Manure into Organic Fertilizer: Does Information Technology Adoption Make a Difference? Environ. Dev. Sustain. 2024, 26, 5025–5045. [Google Scholar] [CrossRef]
- Balkrishna, A.; Kaushik, P.; Singh, S.; Agrahari, P.; Kumar, B.; Kumar, P.; Arya, V.P. Potential Use of Sewage Sludge as Fertilizer in Organic Farming. Clean. Waste Syst. 2025, 10, 100245. [Google Scholar] [CrossRef]
- Pasalari, H.; Farzadkia, M.; Khosravani, F.; Ganachari, S.; Aminabhavi, T.M. Phosphorous recovery from sewage sludge via chemical and thermal technologies. Chem. Eng. J. 2024, 496, 153869. [Google Scholar] [CrossRef]
- European Sustainable Phosphorus Platform (ESPP). Catalogue of Nutrient Recovery Technologies. 2024. Available online: https://www.phosphorusplatform.eu/activities/p-recovery-technology-inventory (accessed on 24 July 2025).
- Galanakis, C.M.; Daskalakis, M.I.; Galanakis, I.M.; Nehrey, M.; Spanou, M.; Vetsou, A.; Agrafioti, E. Landscape of policies, standards, approaches, and projects for EU food security: An overview. Discov. Food 2025, 5, 1–26. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K. Anaerobic digestate management for carbon neutrality and fertilizer use: A review of current practices and future opportunities. Biomass Bioenergy 2024, 180, 106991. [Google Scholar] [CrossRef]
- Nowak, M.; Bojarski, W.; Czekała, W. Economic and energy efficiency analysis of the biogas plant digestate management methods. Energies 2024, 17, 3021. [Google Scholar] [CrossRef]
- International Energy Agency. Outlook for Biogas and Biomethane: Prospects for Organic Growth. 2025. Available online: https://www.iea.org/reports/outlook-for-biogas-and-biomethane (accessed on 24 July 2025).
- Pierre, B.S.; Casaroli, D.; Junior, J.A.; Evangelista, A.W.P.; Flores, R.A.; Teixeira, I.R. Water deficit, fertigation, and bio-inputs in sugarcane cultivation: A scientometric review and practical recommendations. Aust. J. Crop Sci. 2025, 19, 429–435. [Google Scholar]
- Yahya, A.; Khalid, N.A.; Salleh, M.M. Biocompost from Oil Producing Plants. In Biorefinery of Oil Producing Plants for Value-Added Products; Ahmad, A.L., Mubarak, N.M., Eds.; Wiley-VCH: Weinheim, Germany, 2022; Volume 2, pp. 605–629. [Google Scholar] [CrossRef]
- Rațu, R.N.; Veleșcu, I.D.; Stoica, F.; Usturoi, A.; Arsenoaia, V.N.; Crivei, I.C.; Brumă, I.S. Application of agri-food by-products in the food industry. Agriculture 2023, 13, 1559. [Google Scholar] [CrossRef]
- Samy, A.M.; Mahmoud, A.F.; Abdalla, O.A. Effect of application time on the efficacy of Trichoderma spp. to biologically control sunflower charcoal rot. Sultan Qaboos Univ. J. Sci. 2025, 30, 35–43. [Google Scholar]
- Gaur, P.; Kumari, K.; Sharma, P.; Gupta, V.; Gaur, V.K. Biological Processes for Food Waste Treatment. In Sustainable Technologies for Food Waste Management; CRC Press: Boca Raton, FL, USA, 2025; pp. 49–67. [Google Scholar]
- Lackner, M.; Besharati, M. Agricultural Waste: Challenges and Solutions, a Review. Waste 2025, 3, 18. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, M.; Lu, J.; Zhang, Y.; Lu, R.; Li, Y.; Yu, B. Optimizing fabrication of coated fertilizers integrated with biochar for enhanced slow-release properties: Mechanisms and cost-effectiveness analysis. Ind. Crop. Prod. 2024, 222, 120077. [Google Scholar] [CrossRef]
- Kan, S.; Yilmaz, F.G.; Yagcioglu, K.D.; Kadioglu, Y.K.; Gezgin, S.; Gunes, A.; Taskin, M.B. Valorization of poultry litter incineration ash as a sustainable and balanced fertilizer source. J. Soil Sci. Plant Nutr. 2024, 24, 7570–7580. [Google Scholar] [CrossRef]
- Maj, I.; Niesporek, K.; Płaza, P.; Maier, J.; Łój, P. Biomass Ash: A Review of Chemical Compositions and Management Trends. Sustainability 2025, 17, 4925. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, N.V.; Biriș, S.Ș.; Gheorghiță, N.E.; Ionescu, M.; Milea, O.E.; Dincă, M. Management of waste and by–products from meat industry. Acta Tech. Corviniensis–Bull. Eng. 2024, 17, 49–58. [Google Scholar]
- European Commission. Final Report—Heavy Metals and Organic Compounds from Wastes Used as Organic Fertilisers, ENV.A.2./ETU/2001/0024. European Commission: Brussels, Belgium, 2004; Available online: https://ec.europa.eu/environment/pdf/waste/compost/hm_finalreport.pdf (accessed on 24 July 2025).
- Canziani, R.; Boniardi, G.; Turolla, A. Phosphorus recovery—Recent Developments and Case Studies. In Sustainable and Circular Management of Resources and Waste Towards a Green Deal; Elsevier: Amsterdam, The Netherlands, 2023; pp. 269–281. [Google Scholar] [CrossRef]
- Martín-Marroquín, J.M.; Hidalgo, D.; Corona, F.; Sánchez-Gatón, M.A. Innovative nutrient recovery from digestate through integrated crystallization and stripping technologies. In Proceedings of the 11th International Conference on Sustainable Solid Waste Management (RHODES2024), Rhodes, Greece, 19–22 June 2024. [Google Scholar]
- Henriksen, C.B. Towards a more plant-based bioeconomy for Europe. Nat. Food 2025, 6, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ruan, S.; Zhang, R.; Wu, W. Odor nuisance, environmental impact and health risk of priority-controlled VOCs generated from three decentralized aerobic biological modes in treating rural perishable waste. Environ. Sci. Pollut. Res. 2025, 32, 11040–11051. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.; Bułkowska, K. Sustainable Management and Advanced Nutrient Recovery from Biogas Energy Sector Effluents. Energies 2024, 17, 3705. [Google Scholar] [CrossRef]
- Mortadha, H.; Kerrouchi, H.B.; Al-Othman, A.; Tawalbeh, M.A. Comprehensive Review of Biomass Pellets and Their Role in Sustainable Energy: Production, Properties, Environment, Economics, and Logistics. Waste Biomass Valor. 2025, 1–33. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K. Challenges and opportunities in biofertilizer commercialization. SVOA Microbiol. 2024, 5, 1–14. [Google Scholar] [CrossRef]
- Waqas, M.; Hashim, S.; Humphries, U.W.; Ahmad, S.; Noor, R.; Shoaib, M.; Lin, H.A. Composting processes for agricultural waste management: A comprehensive review. Processes 2023, 11, 731. [Google Scholar] [CrossRef]
- Yin, J.; Xie, M.; Yu, X.; Feng, H.; Wang, M.; Zhang, Y.; Chen, T. A review of the definition, influencing factors, and mechanisms of rapid composting of organic waste. Environ. Pollut. 2024, 342, 123125. [Google Scholar] [CrossRef]
- Kelbesa, W.A. Effect of compost in improving soil properties and its consequent effect on crop production–A review. J. Nat. Sci. Res. 2021, 12, 15–25. [Google Scholar] [CrossRef]
- Altıkat, A.; Alma, M.H.; Altıkat, A.; Bilgili, M.E.; Altıkat, S. A comprehensive study of biochar yield and quality concerning pyrolysis conditions: A multifaceted approach. Sustainability 2024, 16, 937. [Google Scholar] [CrossRef]
- Villada, E.; Velasquez, M.; Gómez, A.M.; Correa, J.D.; Saldarriaga, J.F.; López, J.E.; Tamayo, A. Combining anaerobic digestion slurry and different biochars to develop a biochar-based slow-release NPK fertilizer. Sci. Total Environ. 2024, 927, 171982. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.P.; Silva, C.A. Biochar–Nitrogen Composites: Synthesis, Properties, and Use as Fertilizer for Maize. Appl. Chem. 2024, 4, 157–173. [Google Scholar] [CrossRef]
- Salehi, B. Iron-Based Nanocomposites for Enhancing the Bioconversion of Agricultural Wastes. Ph.D. Thesis, North Carolina Agricultural and Technical State University, Greensboro, NC, USA, 2024. Available online: https://www.proquest.com/openview/77b34e8690f35838db13b8fc3ee4bf81/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 25 July 2025).
- Mendonca Cidreira, A.C.; Wei, L.; Aldekhail, A.; Islam Rubel, R. Controlled-release nitrogen fertilizers: A review on bio-based and smart coating materials. J. Appl. Polym. Sci. 2025, 142, e56390. [Google Scholar] [CrossRef]
- NUTRIMAN Website. Farmer Platform 2023. Available online: https://nutriman.net/farmer-platform/product-categories (accessed on 25 July 2025).
- Häfner, F.; Hartung, J.; Möller, K. Digestate composition affecting N fertiliser value and C mineralisation. Waste Biomass Valor. 2022, 13, 3445–3462. [Google Scholar] [CrossRef]
- Guilayn, F.; Jimenez, J.; Monlau, F.; Vaneeckhaute, C. Valorisation of anaerobic digestate: Towards value-added products. In Renewable Energy Technologies for Energy Efficient Sustainable Development; Springer International Publishing: Cham, Switzerland, 2022; pp. 227–262. [Google Scholar] [CrossRef]
- Hidalgo, D.; Urueña, A.; Díez, D.; Martín-Marroquín, J.M. Hydrothermal Carbonization of Industrial Sludge: Recent Advances, Challenges, and Perspectives. In Recent Trends in Management and Utilization of Industrial Sludge; Springer: Cham, Switzerland, 2024; pp. 95–123. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, L.; Trakal, L.; Wang, S.; Shaheen, S.M.; Rinklebe, J.; Chen, Q. Pyrolytic and hydrothermal carbonization affect the transformation of phosphorus fractions in the biochar and hydrochar derived from organic materials: A meta-analysis study. Sci. Total. Environ. 2024, 906, 167418. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Liu, T.; Yang, L.; Liu, H.; Xu, D. Evaluation on phosphorus extraction potential in hydrochar obtained from hydrothermal liquefaction of sewage sludge. Biomass Bioenergy 2024, 182, 107121. [Google Scholar] [CrossRef]
- Ștefan, V.; Găgeanu, I.; Ciupercă, R.; Zaica, A.; Milea, O.E.; Cismaru, M. Phosphorus Extraction Technologies. Acta Tech. Corviniensis–Bull. Eng. 2024, 17, 137–142. [Google Scholar] [CrossRef]
- Mironiuk, M.; Izydorczyk, G.; Witek-Krowiak, A.; Chojnacka, K.; Górecki, H. Valorization of poultry slaughterhouse waste into fertilizers with designed properties. Environ. Sci. Pollut. Res. 2024, 31, 17822–17834. [Google Scholar] [CrossRef]
- Hidalgo, D.; Urueña, A.; Martín-Marroquín, J.M.; Díez, D. Integrated approach for biomass conversion using thermochemical routes with anaerobic digestion and syngas fermentation. Sustainability 2025, 17, 3615. [Google Scholar] [CrossRef]
- Durán-Valle, C.J.; López-Coca, I.M. Biochemical and Thermochemical Conversion Technologies for Agriculture Waste Transformation. In Transforming Agriculture Residues for Sustainable Development; Waste as a Resource; Arora, J., Joshi, A., Ray, R.C., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Cherif, H.; Labbaoui, A.; Risse, H.; Boughanmi, H.; Elfil, H. Magnesium recovery from brackish water desalination brine and valorization in fertilizer production. J. Environ. Chem. Eng. 2024, 12, 113799. [Google Scholar] [CrossRef]
- Tao, W.; Badsha, M.A.; Arachchilage, P.W.; Mostafa, A. Simultaneous ammonia recovery and treatment of sludge digestate using the vacuum stripping and absorption process: Scale-up design and pilot study. Chem. Eng. J. 2024, 480, 148336. [Google Scholar] [CrossRef]
- Im, S.; Lee, H.; Kim, T.; Jeon, H.; Jang, A. Application of non-acid stripping solution in hydrophobic membrane process for high-purity ammonia recovery from high-strength ammonium wastewater. Sep. Purif. Technol. 2025, 357, 129998. [Google Scholar] [CrossRef]
- Abdolrezayi, A.; Puricelli, S.; Dolci, G.; Turolla, A.; Canziani, R.; Rigamonti, L. Phosphorus recovery from sewage sludge ash: Life cycle inventory and critical review of LCA case studies. J. Environ. Manag. 2025, 389, 125620. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Ji, J.; Hong, T.; Dong, Y.; Zhang, Q.; Luo, L.; Tang, Y. Exploring the effect of low-temperature thermal-alkaline pretreatment on struvite recovery from metals-phosphorus rich sludge. J. Environ. Chem. Eng. 2025, 13, 115803. [Google Scholar] [CrossRef]
- Parde, D.; Ghosh, R.; Rajpurohit, P.; Bhaduri, S.; Behera, M. Nutrient retrieval techniques in wastewater treatment. In Biological and Hybrid Wastewater Treatment Technology: Recent Developments in India; Springer Nature: Cham, Switzerland, 2024; pp. 159–195. [Google Scholar] [CrossRef]
- Medici, M.; Calvia, M.; Greggio, N.; Buscaroli, A.; Marazza, D.; Canavari, M. How do farmers value organic fertilisers? An exploratory study on conventional and innovative products. Farm. Syst. 2025, 3, 100156. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Trzaska, K.; Mironiuk, M.; Mikula, K.; Izydorczyk, G.; Polomska, X.; Chojnacka, K. Recent innovations in fertilization with treated digestate from food waste to recover nutrients for arid agricultural fields. Environ. Sci. Pollut. Res. 2024, 31, 41563–41585. [Google Scholar] [CrossRef]
- Aka, R.J.N.; Agyekum-Oduro, E.; Zhu, J.; Wu, S. Integrating electrolytic struvite precipitation with ammonia scrubbing toward complete recovery of nitrogen and phosphorus from anaerobic digestate of poultry litter. Sep. Purif. Technol. 2025, 370, 133287. [Google Scholar] [CrossRef]
- Rosa, D.; Petruccelli, V.; Iacobbi, M.C.; Brasili, E.; Badiali, C.; Pasqua, G.; Di Palma, L. Functionalized biochar from waste as a slow-release nutrient source: Application on tomato plants. Heliyon 2024, 10, e29455. [Google Scholar] [CrossRef]
- Gong, M.; Chu, H.; Xu, Q. Influences of reaction parameters and complexation pretreatments on the distribution of phosphorus during hydrothermal carbonization of dewatered sewage sludge. J. Water Process Eng. 2024, 60, 105209. [Google Scholar] [CrossRef]
- Trzcińska-Wencel, J.; Golińska, P.; Gade, A.; Ingle, P.U.; Shende, S.S.; Rai, M. Microbial Biosynthesis of Biostimulant Nanomaterials and Nanofertilizers. In Plant Biostimulation with Nanomaterials; Springer Nature: Singapore, 2025; pp. 301–356. [Google Scholar] [CrossRef]
- Lyons, S.E.; Arnall, D.B.; Ashford-Kornburger, D.; Brouder, S.M.; Christian, E.; Dobermann, A.; Haefele, S.M.; Haegele, J.; Helmers, M.J.; Wagner-Riddle, C. Field trial guidelines for evaluating enhanced efficiency fertilizers. Soil Sci. Soc. Am. J. 2025, 89, e20787. [Google Scholar] [CrossRef]
- Reimer, M.; Möller, K.; Magid, J.; Siebers, N.; Bai, Z.; Müller-Stöver, D. Urban waste fertilizer: Effects on yield, nutrient dynamics, and potentially toxic element accumulation. Nutr. Cycl. Agroecosyst. 2025, 130, 459–480. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Brienza, C.; Regelink, I.; Egene, C.E.; Reuland, G.; Luo, H.; Satvar, M.; Zilio, M.; Meers, E. Product Composition and Performance in Lab-and Field Trials for Biobased Fertilisers and Soil Improvers Recovered from Digestate: A Product from the H2020 Project SYSTEMIC; Wageningen Environmental Research: Wageningen, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Jaufmann, E.; Schmid, H.; Hülsbergen, K.J. Soil carbon accrual and yield response to biochar and compost in a four-year organic field study. Nutr. Cycl. Agroecosyst. 2025, 1–23. [Google Scholar] [CrossRef]
- Chen, H.; Levavasseur, F.; Houot, S. Substitution of mineral N fertilizers with organic wastes in two long-term field experiments: Dynamics and drivers of crop yields. Soil Use Manag. 2024, 40, e13079. [Google Scholar] [CrossRef]
- Trzaska, K.; Skrzypczak, D.; Izydorczyk, G.; Gil, F.; Chojnacka, K. Sustainable Nutrient Management with Digestate–Based Fertilization: A Two–Year Field Study on Maize Yield and Soil Fertility. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5355881 (accessed on 9 September 2025).
- Casini, D.; Barsali, T.; Rizzo, A.M.; Chiaramonti, D. Production and characterization of co-composted biochar and digestate from biomass anaerobic digestion. Biomass Convers. Biorefin. 2021, 11, 2271–2279. [Google Scholar] [CrossRef]
- Tadayon, M.S.; Mousavi, S.M.; Hosseini, S.M. Salicylic acid and biochar-biofertilizer improve soil fertility, drought tolerance, and fig yield in a semi-arid region. J. Soil Sci. Plant Nutr. 2025, 1–15. [Google Scholar] [CrossRef]
- Engedal, T.; Messmer, B.; Magid, J.; Jensen, L.S.; Hansen, V. Bio-based fertilizers typically deliver on either high nutrient release or soil health parameters. Geoderma 2025, 460, 117424. [Google Scholar] [CrossRef]
- Zin, M.M.T.; Sarker, M.; Lim, T.T. Biochar-seeded struvite production from combination of on-farm and industrial wastewater: A review. Resour. Conserv. Recycl. Adv. 2025, 27, 200273. [Google Scholar] [CrossRef]
- Hertzberger, A.J.; Cusick, R.D.; Margenot, A.J. A review and meta-analysis of the agricultural potential of struvite as a phosphorus fertilizer. Soil Sci. Soc. Am. J. 2020, 84, 653–671. [Google Scholar] [CrossRef]
- Leon, P.; Nakayama, Y.; Margenot, A.J. Field-scale evaluation of struvite phosphorus and nitrogen leaching relative to monoammonium phosphate. J. Environ. Qual. 2024, 53, 23–34. [Google Scholar] [CrossRef]
- Ghosh, S.; Lobanov, S.; Lo, V.K. An overview of technologies to recover phosphorus as struvite from wastewater: Advantages and shortcomings. Environ. Sci. Pollut. Res. 2019, 26, 19063–19077. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; Chen, Z.; Osman, A.I.; Rooney, D.W.; Elgarahy, A.M.; Al-Kutti, W.A. Strategies for ammonia recovery from wastewater: A review. Environ. Chem. Lett. 2024, 22, 2699–2751. [Google Scholar] [CrossRef]
- Deinert, L.; Ashekuzzaman, S.M.; Forrestal, P.; Schmalenberger, A. One-time application of struvites, ashes and superphosphate had no major impact on the microbial phosphorus mobilization capabilities over 15-months in a grassland field trial. Appl. Soil Ecol. 2025, 212, 106198. [Google Scholar] [CrossRef]
- Cruz, N.; Avellan, A.; Ruivo, L.; Silva, F.C.; Römkens, P.F.A.M.; Tarelho, L.A.C.; Rodrigues, S.M. Biomass ash-based soil improvers: Impact of formulation and stabilization conditions on materials’ properties. J. Clean. Prod. 2023, 391, 136049. [Google Scholar] [CrossRef]
- Usman, M.; Anastopoulos, I.; Hamid, Y.; Wakeel, A. Recent trends in the use of fly ash for the adsorption of pollutants in contaminated wastewater and soils: Effects on soil quality and plant growth. Environ. Sci. Pollut. Res. 2023, 30, 124427–124446. [Google Scholar] [CrossRef]
- Chojnacka, K.; Baltrusaitis, J. Organo-mineral fertilizers for sustainable agriculture. Sustain. Sci. Technol. 2025, 2, 022001. [Google Scholar] [CrossRef]
- Sica, P.; Sitzmann, T.J.; Müller-Stöver, D.; Magid, J. Strategic placement of mineral and biobased fertilizers for optimizing phosphorus use efficiency: A comprehensive review. Soil Use Manag. 2025, 41, e70039. [Google Scholar] [CrossRef]
- Boarino, A.; Carrara, N.; Padoan, E.; Celi, L.; Klok, H.A. Biodegradable polymers for plant nutrient delivery and recovery. Macromol. Biosci. 2025, 25, 2500042. [Google Scholar] [CrossRef]
- Verdi, L.; Kuikman, P.J.; Orlandini, S.; Mancini, M.; Napoli, M.; Dalla Marta, A. Does the use of digestate to replace mineral fertilizers have less emissions of N2O and NH3? Agric. For. Meteorol. 2019, 269–270, 112–118. [Google Scholar] [CrossRef]
- Horta, C. Bioavailability of phosphorus from composts and struvite in acid soils. Rev. Bras. Eng. Agríc. Ambient. 2017, 21, 459–464. [Google Scholar] [CrossRef]
- Mancho, C.; Diez-Pascual, S.; Alonso, J.; Gil-Díaz, M.; Lobo, M.C. Assessment of Recovered Struvite as a Safe and Sustainable Phosphorous Fertilizer. Environments 2023, 10, 22. [Google Scholar] [CrossRef]
- Miao, C.; Zeller, V. Nutrient circularity from waste to fertilizer: A perspective from LCA studies. Sci. Total. Environ. 2025, 965, 178623. [Google Scholar] [CrossRef]
- Brousseau, V.D.; Goldstein, B.P.; Leroux, D.; Giguère, T.; MacPherson, S.; Lefsrud, M. Estimating the global warming potential of animal waste-based organic liquid fertilizer for urban hydroponic farms. J. Clean. Prod. 2024, 472, 143434. [Google Scholar] [CrossRef]
- Meng, X.; Knudsen, M.T.; Petersen, S.O.; Møller, H.B.; Hashemi, F. Climate impact of alternative organic fertilizers using life cycle assessment. Environ. Res. Lett. 2024, 19, 124050. [Google Scholar] [CrossRef]

| Biowaste Origin | Processing Method or Additive Agents | Main Nutrients (%) | Secondary Components | Physical Form | Reference |
|---|---|---|---|---|---|
| Sewage sludge | Treated with oxidants (e.g., ClO2, ferrates), ammonia or phosphoric acid | N: 10, P2O5: 23, K2O: 12 | Fe: 1, S: 20 | Granular | [13,14] |
| Sewage sludge | Alkali hydrolysis using NaOH or KOH | N: 4.5, P2O5: 7, K2O: 2.5 | S, Ca, Fe, Mg | Liquid | [15] |
| Sewage sludge | Acid-ammonia treatment (H2SO4, H3PO4, NH3) | N: 13–20, P2O5: 1–13 | S: 14–24, Fe: 0.5–3 | Granular/pellets | [16] |
| Sludge–ash blend | Stabilized with fly ash and lime kiln dust | N: 0.5, P2O5: 0.3, K2O: 0.1 | Ca: 10, Mg: 4 | Powdered/soil-like | [17] |
| Sludge-derived solid | Treated with Fe salts, acids, and ammonia | N: 16, P2O5: 4.6 | S: 16, Fe: 1 | Granular | [18] |
| Biosolids | Mixed with conventional mineral fertilizers | N: 4–15, P2O5: 1–10, K2O: 4–15 | Ca: 2, Fe: 1 | Granular | [19] |
| Poultry manure ash | Blended with KCl, TSP, and chalk | P2O5: 5–14, K2O: 12–20 | Ca: 15 | Granular | [20] |
| Poultry litter | Composted or dried | P2O5: 6, K2O: 3 | Ca: 12 | Pellets | [21] |
| Agro-industrial residues | Pressed cakes and vegetable meals | P2O5: 2.8–9, K2O: 2.5–15 | S: 2–15 | Granular | [22] |
| Livestock manure and digestate | Composting and drying | P2O5: 4, K2O: 3 | S: 3 | Granular | [23] |
| Meat and bone meal | Combined with dolomite, lime or acids | P2O5: 21–23, K2O: 3–4 | SO3: 4.5 | Granular | [24] |
| Municipal wastewaters | Struvite precipitation using magnesium compounds | N: 5, P2O5: 28 | Mg: 10 | Granular/pellets | [25,26,27] |
| Sewage sludge ash | Phosphate recovery via acid–alkali extraction and precipitation | P2O5: 21–22 | Ca: 15–18 | Powdered/granular | [28,29] |
| Processing Strategy | Key Objectives | Typical Inputs | Products | Challenges | Reference |
|---|---|---|---|---|---|
| Composting | Stabilization, hygienization, organic matter recovery | Biodegradable organic waste (food, garden, manure) | Compost with 1–2% N total, C/N ~12–15 | Odor, slow nutrient release, space requirement | [67,68,69] |
| Anaerobic Digestion | Biogas production, nutrient recovery in solid/liquid fractions | Manure, sewage sludge, food waste | Liquid: ~3–5 g/L N-NH4+; Solid: high in P and organics | Salinity, pathogen content, low dry matter | [11,39] |
| Pyrolysis | Carbon sequestration, creation of sorbent biochar | Dry biomass, digestate solids | Biochar with modest nutrient content | Low nutrient content, energy cost | [70,71] |
| Hydrothermal Carbonization | Production of reactive hydrochar and nutrient-rich liquid | Wet biomass (e.g., sludge, food waste, digestate) | Hydrochar enriched in reactive N and P fractions | Process water management, feedstock variability | [53,70,72] |
| Struvite Precipitation | Recovery of P as slow-release fertilizer | Digestate, centrate, wastewater | Struvite with 28% P2O5 and 5% N | Mg source cost, pH control, impurity management | [30,43,47] |
| Ammonia Stripping | Recovery of N as ammonium salts | Digestate, slurry | Ammonium sulfate or nitrate salts (6–9% N) | Energy input, acid handling, scaling | [61] |
| Ash-Based Recovery | Recovery of P/K from incinerated residues | Sewage sludge ash, meat/bone meal ash | Phosphate salts or phosphoric acid (20–30% P2O5) | Heavy metals, low solubility, regulatory status | [20,28,29] |
| Nutrient Blending & Functionalization | Adjusting NPK ratios, adding micronutrients or coatings | Digestate solids, compost, biochar | Tailored fertilizers with defined NPK ratios | Contaminants, stability, compatibility of additives | [73,74] |
| Fertilizer Type | Nutrient Release Characteristics | Relative Yield Performance vs. Mineral Fertilizers | Main Strengths/Weaknesses |
|---|---|---|---|
| Compost | Slow N release (MFE ~19% in year 1); high P and micronutrients; gradual multi-season availability | Lower short-term yields than mineral NPK; ~5–10% above unfertilized; builds long-term fertility | Strengths: Improves soil structure, water holding, microbial activity; safe in terms of pollutant uptake Weaknesses: Low immediate N supply; potential nutrient surpluses (P, S) |
| Liquid digestate | High ammonium content; immediate N availability; requires rapid incorporation to avoid losses | Up to 95–97% of mineral N yields when applied at equivalent N rates; partial substitution reduces yields | Strengths: Effective mineral N substitute; can blend with mineral fertilizers for optimal results Weaknesses: High water content; risk of volatilization |
| Solid digestate | Slower N release; higher P content; similar to compost in nutrient dynamics | Lower immediate yields than mineral NPK; improves soil organic matter | Strengths: Boosts soil carbon; P-rich Weaknesses: Limited short-term N |
| Biochar | Low nutrient content unless enriched; retains nutrients and improves soil properties | Little to no immediate yield benefit in temperate soils; benefits in degraded/acidic/drought-prone soils | Strengths: Long-term soil C sequestration, improved water retention, reduced leaching Weaknesses: Cost; may immobilize N initially |
| Struvite | Slow-release P (5–12% P); faster dissolution in acidic soils; some N (~5%) | Comparable to mineral P in acidic soils; slower early growth in neutral/alkaline soils | Strengths: High P use efficiency; reduces P losses; low impurities Weaknesses: Less effective in high-pH soils; higher cost |
| Recovered ammonium salts | 100% mineral N (NH4+ or NH4NO3); immediate availability; similar to synthetic fertilizers | 70–100% equivalence to mineral N in first year | Strengths: Direct substitute for synthetic N; recycles waste N Weaknesses: Dilute solutions; handling/storage issues |
| Ash-based products | High P and/or K; liming value; solubility varies by source | Variable; poultry litter ash effective; sewage sludge ash slow-release P | Strengths: Recycles P, K; raises pH Weaknesses: Potential heavy metal buildup; nutrient imbalances |
| Fertilizer Type/Context | Key Regulatory Challenge | Practical Implication |
|---|---|---|
| General/Cross-cutting | Fertilising Products Regulation (EU) 2019/1009 (FPR) sets CE-marking criteria for safety, contaminants, nutrient content, and labeling; complementary regulations like EC 1069/2009 impose hygiene and processing rules for animal-origin materials. | Producers must navigate multi-layered compliance; certification can be costly and time-consuming, especially for SMEs; national implementation differences add complexity. |
| Compost | Accepted under CMC of FPR when from source-separated biowaste; if containing animal by-products, must comply with EC 1069/2009 hygiene treatments. | Additional processing required for ABP-containing compost; differences in national rules affect cross-border marketability. |
| Digestate | Recognized under FPR CMCs if from source-separated biodegradable waste; inclusion of manure/sewage sludge triggers EC 1069/2009 pathogen and hygiene standards. | Dual compliance requirements (FPR + ABP); some Member States impose extra restrictions or permit limitations. |
| Manure-derived fertilizers | Regulated under EC 1069/2009 as Category 2/3 ABPs; must undergo specific hygienization (time–temperature) before use; FPR imposes additional safety and nutrient standards. | Hygienization increases costs; national derogations may create uneven market access. |
| Biochar | Eligible under FPR only if produced from listed biomass materials; must comply with contaminant thresholds and not contain excluded waste types. | Feedstock restrictions limit eligible sources; quality control required to meet contaminant and safety standards. |
| Struvite | Listed as a permissible CMC in FPR; must meet contaminant limits, nutrient content specifications, and labeling requirements. | Slow inclusion in markets due to farmer awareness and placement strategies; stricter requirements in certain Member States. |
| Recovered ammonium salts | Permitted under FPR if purity, contaminant, and safety criteria are met; handling and storage must comply with chemical safety legislation. | Transport and storage logistics affected by dilute nature; odor and handling concerns may influence acceptance. |
| Sewage sludge ash | Not currently listed in Annex II of FPR; national bans or phase-outs in some Member States; must comply with contaminant thresholds for specific uses. | Market access restricted to non-fertilizer uses (e.g., soil reclamation); regulatory inclusion under discussion. |
| Other ashes (e.g., poultry litter, wood ash) | Some ashes permitted under FPR if meeting safety and contaminant criteria; others excluded unless included in future Annex II updates. | Inconsistent acceptance across EU; potential heavy metal contamination can limit fertilizer use. |
| Emerging materials | Excluded from current CMC list; Draft CMC 11 proposed to include residues from agro-industrial, fermentation, and smelting sectors. | Until adopted, such materials remain subject to national rules, causing fragmented market access. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, D.; Martín-Marroquín, J.M.; Corona, F.; Verdugo, F. Waste-Derived Fertilizers: Conversion Technologies, Circular Bioeconomy Perspectives and Agronomic Value. Agronomy 2025, 15, 2167. https://doi.org/10.3390/agronomy15092167
Hidalgo D, Martín-Marroquín JM, Corona F, Verdugo F. Waste-Derived Fertilizers: Conversion Technologies, Circular Bioeconomy Perspectives and Agronomic Value. Agronomy. 2025; 15(9):2167. https://doi.org/10.3390/agronomy15092167
Chicago/Turabian StyleHidalgo, Dolores, Jesús M. Martín-Marroquín, Francisco Corona, and Francisco Verdugo. 2025. "Waste-Derived Fertilizers: Conversion Technologies, Circular Bioeconomy Perspectives and Agronomic Value" Agronomy 15, no. 9: 2167. https://doi.org/10.3390/agronomy15092167
APA StyleHidalgo, D., Martín-Marroquín, J. M., Corona, F., & Verdugo, F. (2025). Waste-Derived Fertilizers: Conversion Technologies, Circular Bioeconomy Perspectives and Agronomic Value. Agronomy, 15(9), 2167. https://doi.org/10.3390/agronomy15092167







