Development of Edible Flower Production and the Prospects of Modern Production Technology
Abstract
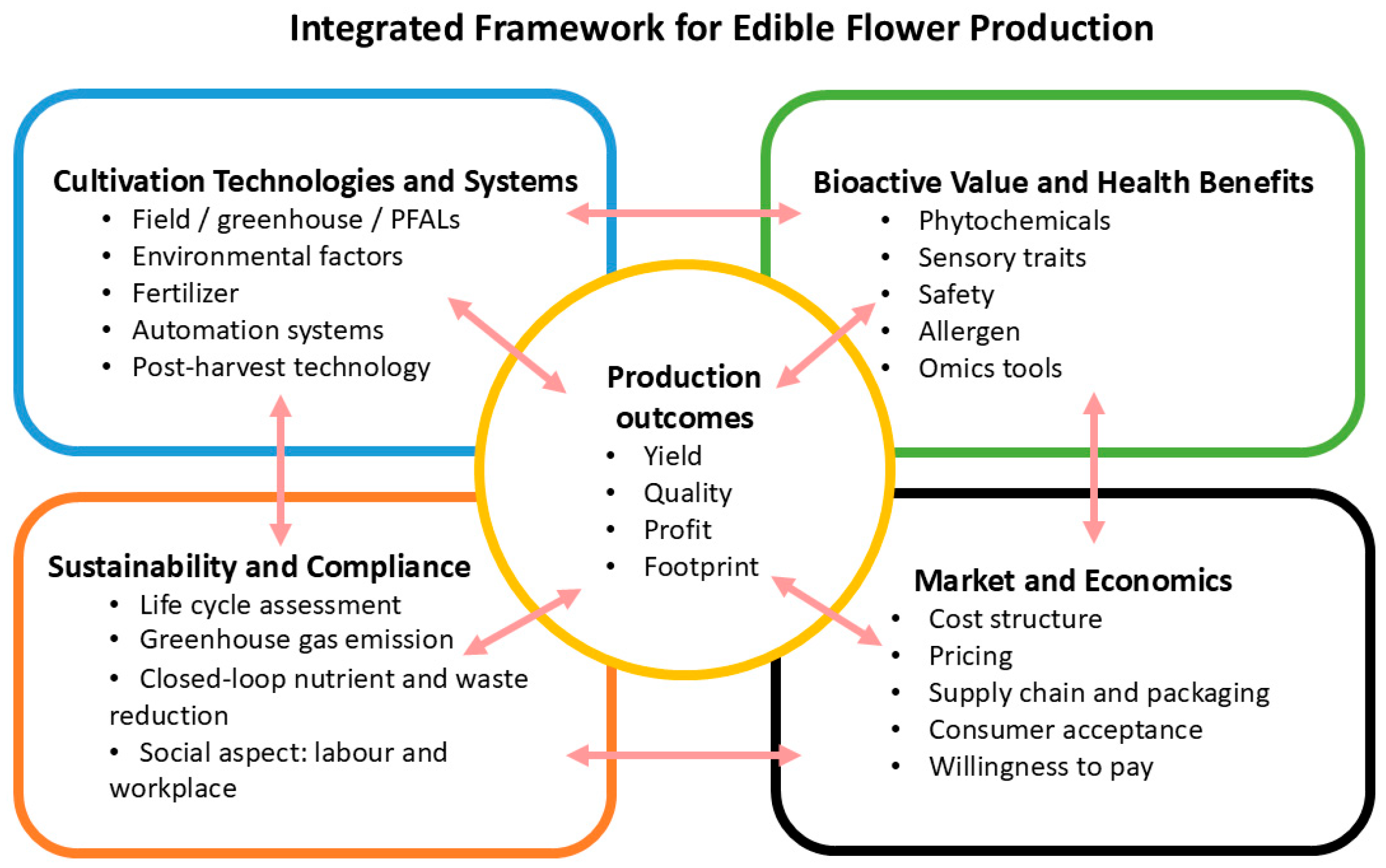
1. Introduction
2. Development of Edible Flower Production
2.1. Wild Collecting
2.2. Open-Field Farming
2.3. Greenhouse Cultivation
3. Plant Factories with Artificial Lighting (PFALs): A New Paradigm for Edible Flower Production
3.1. Overview of PFALs: Principles and Components
3.2. Advantages of PFALs for Edible Flowers
3.3. Current Status of Edible Flower Research in PFALs
4. Key Environmental Factors Influencing Plant Growth and Flowering
4.1. Light
4.2. Temperature
4.3. Nutrient Solution
| Environmental Factors | Plant Species | Effect on Flower Production | Citations | |
|---|---|---|---|---|
| Light | Shaded condition | Boronia heterophylla, Paeonia lactiflora | Reducing floral pigmentation | Lee et al., 2007 [48] Zhao et al., 2012 [49] |
| Photoperiod | Carthamus tinctorius | Flowering occurred earlier under longer photoperiods (up to 15 h day−1) | Torabi et al., 2020 [52] | |
| Night interruption lighting | Chrysanthemum | End-of-day low-intensity lighting extends the photoperiod and regulates flowering | Trivellini et al., 2023 [53] | |
| Night interruption lighting with white, blue, or far-red light | Chrysanthemum | Promote flowering | Park and Jeong, 2019 [54] Park and Jeong, 2019 [55] | |
| Far-red light | Campanula carpatica, tickseed | Lack of FR can inhibit flower initiation or development | Runkle and Heins, 2001 [59] | |
| Daily light integral | Tickseed, Echinacea × hybrida, Lavander, Lobelia × speciosa | Reduced flowering percentages and inflorescence/bud number under low DLI conditions compared to high DLI | Whitman et al., 2022 [61] | |
| Pelargonium × hortorum, Petunia | Earlier flowering under moderate to high DLI levels than under low DLI conditions | Chaudhary and Poudyal, 2025 [62] | ||
| Viola cornuta | Using supplemental LED light when growing Viola cornuta L. helps increase the number of flowers per plant, flower fresh weight and promotes the accumulation of secondary compounds | Koksal et al., 2015 [63] Locatelli et al., 2024 [64] | ||
| Temperature | hibiscus, miniature roses, sinningia, gerbera, kalanchoe, hydrangea, begonia, calceolaria, and pelargonium | Increasing the temperature from 18 °C to 24 °C reduced the time to flowering by 20% to 40% | Mortensen, 2014 [65] | |
| Nasturtium | The fastest flowering occurred at 25 °C (41 days), was slightly delayed at 30 °C (45 days), and was greatly delayed at 10 °C (91 days) | Munir et al., 2015 [66] | ||
| Chrysanthemum | At 25/20 °C, flowering was highest and most synchronized (2049 flowers, 281.8 g dry weight), while higher or lower temperatures reduced flower number and harvest uniformity | Nakano et al., 2020 [67] | ||
| Rose cv. ‘Belinda’s Dream Knock Outrose ‘RADrazz’ | Exposure to two weeks of high temperatures (36/28 °C day/night) during visible bud stage drastically reduced flower size and increased the likelihood of flower abscission | Greyvenstein et al., 2014 [68] | ||
| Pansy | All 12 pansy cultivars were grown at 30 °C exhibited a 20–77% reduction in flower bud number and a 14–44% reduction in flower diameter, with the overall color display dropping by 60–88% | Warner and Erwin, 2006 [69] | ||
| Chamomile | Chamomile grown at 25/15 °C (day/night) produces larger inflorescences, greater biomass, higher essential oil yield, and elevated chamazulene content | Saleh, 1970 [74] | ||
| Nutrient solution | Begonia | Flower production was maximized at a fertilizer EC of 1.6 dS m−1, with both lower and higher EC levels reducing inflorescence number | James and van Iersel, [79] | |
| Calendula | 1.5 dS m−1 EC plus 0.5 µM brassinosteroid yielded the most flowers, while 4.5 dS m−1 reduced flowering but boosted alpha-cadinol, delta-/sigma-cadinene, and flavonoids | Mokhtari and Afshari, 2016 [80] | ||
| Pansy | Flower number peaked at 5 dS m−1 EC across all color variants, but declined at 6.5 dS m−1 | Kentelky et al., 2022 [82] | ||
| Begonia | Supplying 50–100 mg L−1 P increased inflorescences compared to no P, as P-deficient plants were smaller and produced fewer flowers | James and van Iersel, 2001 [87] | ||
| Calendula | An extra-low P supply (5 mg L−1) with intermittent watering maximized buds and capitula, while higher P increased leaf biomass but reduced flowering | Stewart and Lovett-Doust, 2003 [88] | ||
| Rose | Increasing KH2PO4 to 3.0 g L−1 enhanced flower diameter, but 4.0–5.0 g L−1 reduced it | Ma et al., 2021 [90] | ||
5. Challenges, Research Gaps, and Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dourson, D.; Dourson, J. Wild Yet Tasty: A Guide to Edible Plants of Eastern Kentucky; University Press of Kentucky: Lexington, KY, USA, 2019. [Google Scholar] [CrossRef]
- Rajbanshi, N.; Thapa, L.B. Traditional knowledge and practices on utilizing medicinal plants by endangered Kisan ethnic group of eastern Nepal. Ethnobot. Res. Appl. 2019, 18, 23. [Google Scholar] [CrossRef]
- Harmayani, E.; Anal, A.K.; Wichienchot, S.; Bhat, R.; Gardjito, M.; Santoso, U.; Siripongvutikorn, S.; Puripaatanavong, J.; Payyappallimana, U. Healthy food traditions of Asia: Exploratory case studies from Indonesia, Thailand, Malaysia, and Nepal. J. Ethn. Foods 2019, 6, 1. [Google Scholar] [CrossRef]
- Panda, S.K.; Mohanta, Y.K.; Padhi, L.; Luyten, W. Antimicrobial activity of select edible plants from Odisha, India against food-borne pathogens. LWT 2019, 113, 108246. [Google Scholar] [CrossRef]
- Tembo-Phiri, C. Edible Fynbos Plants: A Soil Types and Irrigation Regime Investigation on Tetragonia decumbens and Mesembryanthemum crystallinum. Doctoral Dissertation, Stellenbosch University, Stellenbosch, South Africa, 2019. [Google Scholar]
- Dujmović, M.; Radman, S.; Opačić, N.; Uher, S.F.; Mikuličin, V.; Voća, S.; Žlabur, J.Š. Edible flower species as a promising source of specialized metabolites. Plants 2022, 11, 2529. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Liangli, Y. Total Phenolic Contents and Antioxidant Properties of Commonly Consumed Vegetables Grown in Colorado. LWT-Food Sci. Technol. 2006, 39, 1155–1162. [Google Scholar] [CrossRef]
- Ninama, V.; Hardik, S.; Chintan, K.; Ashok, I.; Rahu, D.; Susheel, S.; Alka, S. Assessment of Phytochemicals, Nutritional Compositions and Metabolite Profiling Using GCMS– from Annual Edible Flowers. Sci. Hortic. 2023, 323, 112551. [Google Scholar] [CrossRef]
- Hegde, A.S.; Smriti, G.; Poonam, K.; Robin, J.; Vidyashankar, S. Wild Edible Flowers of Western Himalayas: Nutritional Characterization, UHPLC-QTOF-IMS-Based Phytochemical Profiling, Antioxidant Properties, and In Vitro Bioaccessibility of Polyphenols. ACS Omega 2023, 8, 40212. [Google Scholar] [CrossRef]
- Kozicka, M.; Ewelina, H. Identification and Quantification of Bioactive Compounds in Organic and Conventional Edible Pansy Flowers (Viola × Wittrockiana) and Their Antioxidant Activity. Plants 2023, 12, 1264. [Google Scholar] [CrossRef]
- Gonçalves, A.F.K.; Rossana, B.F.; Aline, A.B.; Mariana, P.; Ruy, C.R.B.; Margareth, L.A. Anti-oxidant Capacity, Total Phenolic Contents and HPLC Determination of Rutin in Viola Tricolor (L) Flowers. Free Radic. Antioxid. 2012, 4, 32–37. [Google Scholar] [CrossRef]
- Kalisz, A.; Zofia, W.; Monika, B.; Sylwester, S.; Jarmila, N.; Bożena, S.; Bożena, P. Petals of Different Ornamental Rose Cultivars as a Rich Source of Bioactive Compounds for Functional Foods. Sci. Hortic. 2023, 321, 112240. [Google Scholar] [CrossRef]
- Fernandes, L.; Elsa, R.; José, A.P.; Jorge, A.S.; Susana, C. Borage, Camellia, Centaurea and Pansies: Nutritional, Fatty Acids, Free Sugars, Vitamin E, Carotenoids and Organic Acids Characterization. Food Res. Int. 2020, 132, 109070. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Zagórska-Dziok, M.; Klimek-Szczykutowicz, M.; Szopa, A. Phenolic Profile and Comparison of the Antioxidant, Anti-Ageing, Anti-Inflammatory, and Protective Activities of Borago officinalis Extracts on Skin Cells. Molecules 2023, 28, 868. [Google Scholar] [CrossRef]
- Różyło, R.; Monika, S.C.; Urszula, G.D.; Dariusz, D. Spectroscopic, Mineral, and Antioxidant Characteristics of Blue Colored Powders Prepared from Cornflower Aqueous Extracts. Food Chem. 2021, 346, 128889. [Google Scholar] [CrossRef]
- Kaisoon, O.; Konczak, I.; Siriamornpun, S. Potential health enhancing properties of edible flowers from Thailand. Food Res. Int. 2011, 46, 563–571. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Pereira, E.L.; Saraiva, J.A.; Ramalhosa, E. Physicochemical, antioxidant and microbial properties of crystallized pansies (Viola × wittrockiana) during storage. Food Sci. Technol. Int. 2019, 25, 472–479. [Google Scholar] [CrossRef]
- Matyjaszczyk, E.; Śmiechowska, M. Edible flowers. Benefits and risks pertaining to their consumption. Trends Food Sci. Technol. 2019, 91, 670–674. [Google Scholar] [CrossRef]
- Burger, A.; Lichtscheidl, I. Strontium in the environment: Review about reactions of plants towards stable and radioactive strontium isotopes. Sci. Total Environ. 2018, 653, 1458–1512. [Google Scholar] [CrossRef]
- Koike, A.; Barreira, J.C.M.; Barros, L.; Santos-Buelga, C.; Villavicencio, A.L.C.H.; Ferreira, I.C.F.R. Edible flowers of Viola tricolor L. as a new functional food: Antioxidant activity, individual phenolics and effects of gamma and electron-beam irradiation. Food Chem. 2015, 179, 6–14. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007. Available online: https://eur-lex.europa.eu/eli/reg/2018/848/oj/eng (accessed on 15 August 2025).
- Li, Y.; Li, J.; Gao, L.; Tian, Y. Irrigation has more influence than fertilization on leaching water quality and the potential environmental risk in excessively fertilized vegetable soils. PLoS ONE 2018, 13, e0204570. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-F.; Chen, Y.-J.; Chen, P.-A.; Kuo, C.-C.; Chen, K.-H.; Chen, C.-H.; Su, T.-C.; Chen, I.-Z.; Chang, Y.-S. Impact of Temperature on Growth, Photosynthetic Efficiency, Yield, and Functional Components of Bud-Leaves and Flowers in Edible Chrysanthemum (Chrysanthemum morifolium Ramat). Horticulturae 2025, 11, 448. [Google Scholar] [CrossRef]
- Munir, M. Application of photo-thermal models to quantify flowering time and development response of Chrysanthemum (Chrysanthemum × morifolium Ramat.). J. Appl. Hortic. 2023, 25, 230–237. [Google Scholar] [CrossRef]
- Vargo, M.; James, E.F. Modeling the Effect of Temperature on the Flower Development Rate of Hybrid Impatiens. Hort. Technol. 2022, 32, 16–20. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, B.; Xu, J.; Liang, W.; Huang, W.; Li, H. Effects of heat stress on changes in physiology and anatomy in two cultivars of Rhododendron. S. Afr. J. Bot. 2017, 112, 338–345. [Google Scholar] [CrossRef]
- Proietti, S.; Scariot, V.; De Pascale, S.; Paradiso, R. Flowering Mechanisms and Environmental stimuli for flower transition: Bases for Production Scheduling in Greenhouse Floriculture. Plants 2022, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Mugnozza, G.S.; De Lucia Zeller, B. Environmental improvements of greenhouse flower cultivation by means of LCA methodology. Acta Hortic. 2008, 801, 301–308. [Google Scholar] [CrossRef]
- Rahman, S.; Sarmah, R.; Bora, S.; Das, S.; Barua, S.; Sarmah, R. Growth and flowering of chrysanthemum in hydroponics. Pharma Innov. J. 2022, 11, 2786–2791. [Google Scholar]
- Sarmah, R.; Bora, S.; Sarmah, R. Quality blooming of marigold in hydroponics. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1792–1799. [Google Scholar] [CrossRef]
- Melo, E.F.R.; Santos, O.S.D. Growth and production of nasturtium flowers in three hydroponic solutions. Hortic. Bras. 2011, 29, 584–589. [Google Scholar] [CrossRef][Green Version]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Luna-Maldonado, A.I.; Vidales-Contreras, J.A.; Rodríguez-Fuentes, H. Editorial: Advances and Trends in Development of Plant Factories. Front. Plant Sci. 2016, 7, 1848. [Google Scholar] [CrossRef] [PubMed]
- Graamans, L.; Baeza, E.; Van Den Dobbelsteen, A.; Tsafaras, I.; Stanghellini, C. Plant factories versus greenhouses: Comparison of resource use efficiency. Agric. Syst. 2017, 160, 31–43. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Plant Factory as a Resource-Efficient closed plant production system. In Plant Factory: An Indoor Vertical Farming System for Efficient Crop Production; Kozai, T., Niu, G., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 69–90. [Google Scholar] [CrossRef]
- Vatistas, C.; Avgoustaki, D.D.; Bartzanas, T. A Systematic Literature Review on Controlled-Environment Agriculture: How Vertical Farms and Greenhouses Can Influence the Sustainability and Footprint of Urban Microclimate with Local Food Production. Atmosphere 2022, 13, 1258. [Google Scholar] [CrossRef]
- Zhuang, Y.; Lu, N.; Shimamura, S.; Maruyama, A.; Kikuchi, M.; Takagaki, M. Economies of scale in constructing plant factories with artificial lighting and the economic viability of crop production. Front. Plant Sci. 2022, 13, 992194. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Jindamol, H.; Thongtip, A.; Korinsak, S.; Romyanon, K.; Toojinda, T.; Darwell, C.T.; Wanichananan, P.; Panya, A.; Kaewsri, W.; et al. Physiological responses and variation in secondary metabolite content among Thai holy basil cultivars (Ocimum tenuiflorum L.) grown under controlled environmental conditions in a plant factory. Front. Plant Sci. 2022, 13, 1008917. [Google Scholar] [CrossRef]
- Munyanont, M.; Lu, N.; Rachma, D.F.; Ruangsangaram, T.; Takagaki, M. Lighting Patterns Regulate Flowering and Improve the Energy Use Efficiency of Calendula Cultivated in Plant Factories with Artificial Lighting. Agriculture 2024, 14, 2208. [Google Scholar] [CrossRef]
- Munyanont, M.; Lu, N.; Joilek, T.; Rachma, D.; Takagaki, M. Growth and flowering characteristics of Calendula under different light spectra in a controlled environment. Acta Hortic. 2024, 1404, 1371–1378. [Google Scholar] [CrossRef]
- Xu, W.; Lu, N.; Kikuchi, M.; Takagaki, M. Effects of Node Position and Electric Conductivity of Nutrient Solution on Adventitious Rooting of Nasturtium (Tropaeolum majus L.) Cuttings. Agronomy 2021, 11, 363. [Google Scholar] [CrossRef]
- Xu, W.; Lu, N.; Kikuchi, M.; Takagaki, M. Continuous Lighting and High Daily Light Integral Enhance Yield and Quality of Mass-Produced Nasturtium (Tropaeolum majus L.) in Plant Factories. Plants 2021, 10, 1203. [Google Scholar] [CrossRef]
- Kwak, N.Y.; Sung, B.H.; Kumaratenna, K.P.S.; Cho, Y.Y. Selection of optimal marigold (Tagetes patula) cultivar as an edible flower in a plant factory. Acta Hortic. 2024, 1404, 1379–1384. [Google Scholar] [CrossRef]
- Kwak, N.; Sung, B.H.; Kumaratenna, K.; Cho, Y. The optimum photoperiod on floral differentiation of French marigold grown in a closed-type plant factory. J. Bio-Environ. Control 2024, 33, 71–78. [Google Scholar] [CrossRef]
- Fujiwara, K. Radiometric, photometric and photonmetric quantities and their units. In Plant Factory: An Indoor Vertical Farming System for Efficient Crop Production; Toyoki, K., Ed.; Springer: Tokyo, Japan, 2016; pp. 367–376. [Google Scholar] [CrossRef]
- Sandoval-Herazo, L.C.; Alvarado-Lassman, A.; Nani, G.; Nakase-Rodríguez, C. Influence of light intensity on growth and flowering ornamental plants in constructed wetlands. Renew. Energy Biomass Sustain. 2022, 2, 27–36. [Google Scholar] [CrossRef]
- Pires, M.V.; Almeida, A.F.; Figueiredo, A.L.; Gomes, F.P.; Souza, M.M. Photosynthetic characteristics of ornamental passion flowers grown under different light intensities. Photosynthetica 2011, 49, 593–602. [Google Scholar] [CrossRef]
- Lee, K.M.; Jeong, T.Y.; Song, J.Y.; Jeong, B.R. Flower color change of Boronia heterophylla as affected by light intensity and preservative chemicals. Korean J. Hortic. Sci. Technol. 2007, 25, 458–462. [Google Scholar]
- Zhao, D.; Hao, Z.; Tao, J. Effects of shade on plant growth and flower quality in the herbaceous peony (Paeonia lactiflora Pall.). Plant Physiol. Biochem. 2012, 61, 187–196. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Jeong, B.R. Growth and Photosynthetic Responses to Increased LED Light Intensity in Korean Ginseng (Panax ginseng C.A. Meyer) Sprouts. Agronomy 2023, 13, 2375. [Google Scholar] [CrossRef]
- Jackson, S.D. Plant responses to photoperiod. New Phytol. 2008, 181, 517–531. [Google Scholar] [CrossRef]
- Torabi, B.; Mahin, A.; Asghar, R.; Arman, A. Modeling Flowering Response to Temperature and Photoperiod in Safflower. Ind. Crops Prod. 2020, 151, 112474. [Google Scholar] [CrossRef]
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. LED Lighting to Produce High-Quality Ornamental Plants. Plants 2023, 12, 1667. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. Both the Quality and Positioning of the Night Interruption Light are Important for Flowering and Plant Extension Growth. J. Plant Growth Regul. 2019, 39, 583–593. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. Night interruption light quality changes morphogenesis, flowering, and gene expression in Dendranthema grandiflorum. Hortic. Environ. Biotechnol. 2019, 60, 167–173. [Google Scholar] [CrossRef]
- Liu, J.; Van Iersel, M.W. Photosynthetic physiology of blue, green, and red Light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J. Effects of Light Quality on Growth and Phytonutrient Accumulation of Herbs under Controlled Environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z.; Fu, Y.; Feng, L.; Li, M.; Kang, Q.; Wang, C.; Yuan, M.; Chen, Y.; Tao, Q.; et al. Shade Avoidance 3 Mediates crosstalk between shade and nitrogen in Arabidopsis leaf development. Front. Plant Sci. 2022, 12, 800913. [Google Scholar] [CrossRef]
- Runkle, E.S.; Heins, R.D. Specific functions of red, far red, and blue light in flowering and stem extension of long-day plants. J. Am. Soc. Hortic. Sci. 2001, 126, 275–282. [Google Scholar] [CrossRef]
- Craig, D.S.; Runkle, E.S. A Moderate to High Red to Far-red Light Ratio from Light-emitting Diodes Controls Flowering of Short-day Plants. J. Am. Soc. Hortic. Sci. 2013, 138, 167–172. [Google Scholar] [CrossRef]
- Whitman, C.; Padhye, S.; Runkle, E.S. A high daily light integral can influence photoperiodic flowering responses in long day herbaceous ornamentals. Sci. Hortic. 2022, 295, 110897. [Google Scholar] [CrossRef]
- Chaudhary, A.; Poudyal, S. Influence of daily light integral on irrigation needs and flowering of greenhouse-grown geranium and petunia. Sci. Hortic. 2025, 347, 114188. [Google Scholar] [CrossRef]
- Koksal, N.; Meral, I.; Ahmet, T. Supplemental LED Lighting Increases Pansy Growth. Hortic. Bras. 2015, 33, 428–433. [Google Scholar] [CrossRef]
- Locatelli, S.; Zanin, G.; Sambo, P.; Nicoletto, C. Effects of LED Irradiation and Non-Thermal Plasma Treatment on Horned Pansy During Flowering: Enhancing Yield and Functional Quality of Edible Flowers. Horticulturae 2024, 10, 1274. [Google Scholar] [CrossRef]
- Mortensen, L.M. The effect of photosynthetic active radiation and temperature on growth and flowering of ten flowering pot plant species. Am. J. Plant Sci. 2014, 5, 1907–1917. [Google Scholar] [CrossRef][Green Version]
- Munir, M.; Alhajhoj, M.R.; Aziz, K.A.; Baloch, J.D. Flowering time response of Nasturtium (Tropaeolum majus L.) cultivar ‘Empress of India’ to photoperiod, light integral and temperature using photo-thermal model. Songklanakarin J. Sci. Technol. 2015, 37, 247–254. [Google Scholar][Green Version]
- Nakano, Y.; Takase, T.; Sumitomo, K.; Suzuki, S.; Tsuda-Kawamura, K.; Hisamatsu, T. Delay of flowering at high temperature in Chrysanthemum: Duration of darkness and transitions in lighting determine daily peak heat sensitivity. Hortic. J. 2020, 89, 602–608. [Google Scholar] [CrossRef]
- Greyvenstein, O.; Pemberton, B.; Starman, T.; Niu, G.; Byrne, D. Effect of Two-week High-temperature treatment on flower quality and abscission of Rosa L. ‘Belinda’s Dream’ and ‘RADrazz’ (KnockOut) under controlled growing environments. HortScience 2014, 49, 701–705. [Google Scholar] [CrossRef]
- Warner, M.R.; Erwin, J.E. Prolonged high-temperature exposure differentially reduces growth and flowering of 12 Viola x wittrockiana Gams. cvs. Sci. Hortic. 2006, 108, 295–302. [Google Scholar] [CrossRef]
- Inamoto, K.; Goto, T.; Doi, M. Analysis of the relationships between seasonal changes in cut flower yield and quality, and temperature and light intensity, in three rose varieties. Hortic. J. 2024, 93, 101–113. [Google Scholar] [CrossRef]
- Higashiura, M.; Kajihara, S.; Uno, Y.; Yamanaka, M. Effects of temperature and timing/duration of night cooling treatments on flowering time and quality of cut flowers of standard type Carnation (Dianthus caryophyllus). Hortic. J. 2020, 89, 61–68. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; Van Meeteren, U. Flower opening and closure: A review. J. Exp. Bot. 2003, 54, 1801–1812. [Google Scholar] [CrossRef]
- Thines, C.B.; Youn, Y.; Duarte, I.M.; Harmon, G.F. The time of day effects of warm temperature on flowering time involve PIF4 and PIF5. J. Exp. Bot. 2014, 65, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M. The effect of air temperature and thermoperiod on the quality and quantity of Matricaria Chamomilla L. oil. Meded. Landbouwhogesch. Wageninge Ned. 1970, 70, 15. [Google Scholar]
- Kumar, S.; Kumar, S.; Mohapatra, T. Interaction Between Macro- and Micro-Nutrients in Plants. Front. Plant Sci. 2021, 12, 665583. [Google Scholar] [CrossRef]
- Chen, L.; Xu, M.; Cheng, Z.; Yang, L. Effects of Nitrogen Deficiency on the Photosynthesis, Chlorophyll a Fluorescence, Antioxidant System, and Sulfur Compounds in Oryza sativa. Int. J. Mol. Sci. 2023, 25, 10409. [Google Scholar] [CrossRef]
- Chtouki, M.; Naciri, R.; Soulaimani, A.; Zeroual, Y.; Gharous, M.E.; Oukarroum, A. Effect of cadmium and phosphorus interaction on tomato: Chlorophyll A fluorescence, plant growth, and cadmium translocation. Water Air Soil. Pollut. 2021, 232, 3. [Google Scholar] [CrossRef]
- Luo, A.; Zhou, C.; Chen, J. The Associated with Carbon Conversion Rate and Source–Sink Enzyme Activity in Tomato Fruit Subjected to Water Stress and Potassium Application. Front. Plant Sci. 2021, 12, 681145. [Google Scholar] [CrossRef]
- James, E.C.; Marc, W.V.I. Fertilizer Concentration Affects Growth and Flowering of Subirrigated Petunias and Begonias. HortScience 2001, 36, 40–44. [Google Scholar] [CrossRef]
- Mokhtari, N.; Hosein, A. Effect of Brassinosteroid (24-epibrassinolide) on Morphophysiological Parameters and Essential Oils of Calendula Officinalis L. by EC Nutrient Solution. Aust. J. Crop Sci. 2016, 10, 1496–1503. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Petropoulos, S.A.; Prvulovic, D.; Tzortzakis, N. Performance of Hydroponically Cultivated Geranium and Common Verbena under Salinity and High Electrical Conductivity Levels. Agronomy 2021, 11, 1237. [Google Scholar] [CrossRef]
- Kentelky, E.; Morar, I.M. Morphological Responses of Viola Accessions to Nutrient Solution Application and Electrical Conductivity. Plants 2021, 11, 1433. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.I.; Shin, J.H.; Son, J.E. Theoretical and Experimental Analyses of Nutrient Control in Electrical Conductivity-Based Nutrient Recycling Soilless Culture System. Front. Plant Sci. 2021, 12, 656403. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Ecological significance and complexity of N-source preference in plants. Ann. Bot. 2013, 112, 957–963. [Google Scholar] [CrossRef]
- Lin, Y.; Tsay, Y. Influence of differing nitrate and nitrogen availability on flowering control in Arabidopsis. J. Exp. Bot. 2017, 68, 2603–2609. [Google Scholar] [CrossRef]
- Henry, J.B.; McCall, I.; Jackson, B.; Whipker, B.E. Growth Response of Herbaceous Ornamentals to Phosphorus Fertilization. HortScience 2017, 52, 1362–1367. [Google Scholar] [CrossRef]
- James, E.; Marc, V.I. Ebb and Flow Production of Petunias and Begonias as Affected by Fertilizers with Different Phosphorus Content. HortScience 2001, 36, 282–285. [Google Scholar] [CrossRef]
- Stewart, C.L.; Lovett-Doust, L. Effect of Phosphorus Treatment on Growth and Yield in the Medicinal Herb Calendula Officinalis L. (Standard Pacific) Under Hydroponic Cultivation. Can. J. Plant Sci. 2003, 83, 611–617. [Google Scholar] [CrossRef]
- Biswas, R.C.; Mannan, M.A.; Kabir, M.Y. Phosphorus increases growth and flower production of gladiolus (Gladiolus grandiflora L.). Food Agri Econ. Rev. 2022, 2, 41–47. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, X.; Yuan, W.; Tang, H.; Luan, M. The Optimal Concentration of KH2PO4 Enhances Nutrient Uptake and Flower Production in Rose Plants via Enhanced Root Growth. Agriculture 2021, 11, 1210. [Google Scholar] [CrossRef]
- Torqueti, S.T.S.; Boldrin, K.V.F.; Nascimento, Â.M.P.D.; Paiva, P.D.O.; Neto, A.E.F.; Luz, I.C.A. Alternative potassium source for the cultivation of ornamental sunflower. Ciênc. Agrotec. 2016, 40, 257–264. [Google Scholar] [CrossRef]
- Owain, M.A. NPK fertilizer effects on growth and flowering of Chinese carnation. Nabatia 2024, 12, 1642. [Google Scholar] [CrossRef]
- Peron, G.; Clizia, F.; Chiara, D.D.; Irene, F.; Stefania, S.; Stefano, D.A. Biostimulation of Calendula Officinalis with a Soy Protein Hydrolysate Induces Flower and Plant Biomass and Flower Count by Reversibly Altering the Floral Metabolome. Ind. Crops Prod. 2024, 214, 118508. [Google Scholar] [CrossRef]
- Zeljković, S.; Nada, P.; Monika, T.K.; Emina, M. Effect of Biostimulant Application on Development of Pansy (Viola Tricolor. Var. Hortensis Dc.) Seedlings. J. Cent. Eur. Agric. 2021, 22, 596–601. [Google Scholar] [CrossRef]
- Andrzejak, R.; Janowska, B.; Rosińska, A.; Skazińska, S.; Borsai, O. Flowering, Quality and Nutritional Status of Tropaeolum majus L. ‘Spitfire’ after Application of Trichoderma spp. Sustainability 2024, 16, 4672. [Google Scholar] [CrossRef]
- Kleiber, T.; Borowiak, K.; Kosiada, T.; Breś, W.; Ławniczak, B. Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species. Open Chem. 2020, 18, 1468–1480. [Google Scholar] [CrossRef]
- Cai, W.; Kunlang, B.; Lingyan, Z.; Jingjin, Z.; Dayi, L.; Hua, B. Energy Consumption of Plant Factory with Artificial Light: Challenges and Opportunities. Renew. Sustain. Energy Rev. 2025, 210, 115235. [Google Scholar] [CrossRef]
- Sumi, M.J.; Jahan, N.; Thamid, S.S.; Tarik, M.E.I.; Hassannejad, S.; Rahimi, M.; Imran, S. LED light effect on growth, pigments, and antioxidants of lettuce (Lactuca sativa L.) baby greens. BMC Plant Biol. 2025, 25, 6621–6628. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, Z.; Liu, W.; Ding, Y.; Zhang, Q.; Wang, S. Side Lighting of Red, Blue and Green Spectral Combinations Altered the Growth, Yield and Quality of Lettuce (Lactuca sativa L. Cv. “Yidali”) in Plant Factory. Plants 2022, 12, 4147. [Google Scholar] [CrossRef]
- Boucher, L.; Nguyen, T.; Brégard, A.; Pepin, S.; Dorais, M. Optimizing Light Use Efficiency and Quality of Indoor Organically Grown Leafy Greens by Using Different Lighting Strategies. Agronomy 2023, 13, 2582. [Google Scholar] [CrossRef]
- Kozai, T.; Nakaoka, H.; Lu, N.; Nguyen, D.T.P.; Hayashi, E. Research and Development Challenges Faced by Plant Factories to Solve Global Problems: From the Perspectives of Civilization and Culture. Horticulturae 2025, 11, 793. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Zhou, Q.; Meng, X.; Peng, J.; Hu, Y. Metabolomics Combined with Transcriptomics Reveals the Formation Mechanism of Different Colored Flowers of Cosmos bipinnata Cav. Agriculture 2025, 15, 255. [Google Scholar] [CrossRef]
- Zhang, S.; Li, T.; Liu, S.; Qi, X.; Yang, Y.; Zhang, J.; Jia, L.; Wang, P.; Mu, X. Integrated Transcriptomics and Metabolomics Reveal Key Genes and Metabolic Pathway in Flower and Fruit Color Formation of Cerasus humilis (Bge.) Sok. Plants 2025, 14, 1103. [Google Scholar] [CrossRef]
- Sun, Y.; Pinli, H.; Yanan, J.; Zhenzhen, W.; Jiaxing, C.; Yiwei, Z.; Haojing, S. Comprehensive Analysis of Metabolomics and Transcriptomics Reveals Varied Tepal Pigmentation Across Gloriosa Varieties. BMC Plant Biol. 2025, 25, 66. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, W.; Zhang, Y.; Yang, G.; Cao, D.; Li, W. Integrated metabolomic and transcriptomic analysis revealed the transition of functional components in edible flower buds of Hemerocallis citrina Baroni. Food Chem. 2024, 24, 101852. [Google Scholar] [CrossRef] [PubMed]
| Plant Species | Key Compound(s) Reported | Approx. Quantity x | Sources |
|---|---|---|---|
| Calendula | β-carotene Total phenol | 0.85 mg gFW−1, 61.01 mg Catechol gFW−1 | [8] |
| African marigold | Total phenolics Total flavonoids | 48.55 mg GAE gDW−1 132.42 mg QUE gDW−1 | [9] |
| Nasturtium | Total phenolics Total flavonoids | 26.55 mg GAE gDW−1 39.46 ± 2.25 mg QUE gDW–1 | [9] |
| Pansy (Viola × wittrockiana) | Total anthocyanins | 2.42–4.52 mg gFW−1 | [10] |
| Viola (Viola tricolor) | Total phenolics | 0–0.19 mg GAE gFW−1 | [11] |
| Rose (Rosa spp.) | Total phenolics Total flavonoids Anthocyanins | 3.94–18.93 mg GAE gFW−1 0.38–2.06 mg QE gFW−1 0–5.72 mg C3G gFW−1 | [12] |
| Borage (Borago officinalis) | Total carotenoids Total phenolics | 1.81 mg β-carotene gDW−1 15.10 mg gDW−1 | [13] [14] |
| Cornflower (Centaurea cyanus) | Total phenolics Total flavonoids | 19.5–26.6 mg GAE gDW−1 16.9 mg QE gDW−1 | [15] |
| Cosmos (Cosmos sulphureus) | Total carotenoids Total phenol | 18.8 µg gFW−1 86.99 mg Catechol gFW−1 | [8] |
| Plant Species | Research Focus | Main Findings | Application for Edible Flowers in PFALs |
|---|---|---|---|
| Cosmos bipinnatus | Flower color—flavonoid biosynthesis, transcription factors (MYB, bHLH) | Identified key metabolites and regulatory genes controlling anthocyanin accumulation in different flower colors [102]. | Guide lighting and nutrient strategies to enhance desired flower colors. |
| Camellia huana | Flower color and aroma—carotenoid biosynthesis | Revealed genes and metabolic pathways responsible for pigmentation and fragrance formation [103]. | Develop protocols to improve visual and sensory qualities. |
| Gloriosa spp. | Flower color—anthocyanin accumulation, MYB genes | Linked MYB gene expression with anthocyanin content in tepals [104]. | Enable precise environmental control to intensify pigmentation. |
| Hemerocallis citrina | Flower development, metabolites, dynamics, differentially expressed genes (DEGs) | Showed stage-specific metabolite accumulation and gene expression during flowering [105]. | Optimize harvest timing for peak nutritional and sensory quality. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munyanont, M.; Lu, N.; Nguyen, D.T.P.; Takagaki, M. Development of Edible Flower Production and the Prospects of Modern Production Technology. Agronomy 2025, 15, 2159. https://doi.org/10.3390/agronomy15092159
Munyanont M, Lu N, Nguyen DTP, Takagaki M. Development of Edible Flower Production and the Prospects of Modern Production Technology. Agronomy. 2025; 15(9):2159. https://doi.org/10.3390/agronomy15092159
Chicago/Turabian StyleMunyanont, Maitree, Na Lu, Duyen T. P. Nguyen, and Michiko Takagaki. 2025. "Development of Edible Flower Production and the Prospects of Modern Production Technology" Agronomy 15, no. 9: 2159. https://doi.org/10.3390/agronomy15092159
APA StyleMunyanont, M., Lu, N., Nguyen, D. T. P., & Takagaki, M. (2025). Development of Edible Flower Production and the Prospects of Modern Production Technology. Agronomy, 15(9), 2159. https://doi.org/10.3390/agronomy15092159






