Variations in Isoflavone During Soybean Maturation and Their Thermal Process-Dependent Conversion
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Thermal Processing
2.4. Measurement of Physiochemical Characteristics
2.5. HPLC Analysis of Soybean Isoflavones
2.6. Measurements of Antioxidant Activity and Total Phenolics
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Soybean Seeds During Maturation and Steaming
3.2. Total Isoflavone Contents of Soybean Seeds During Maturation and Steaming
3.3. Conversion of 12 Individual Types During Maturation and Steaming of Soybean Seeds
3.4. Antioxidant Activities and Total Phenolic Contents
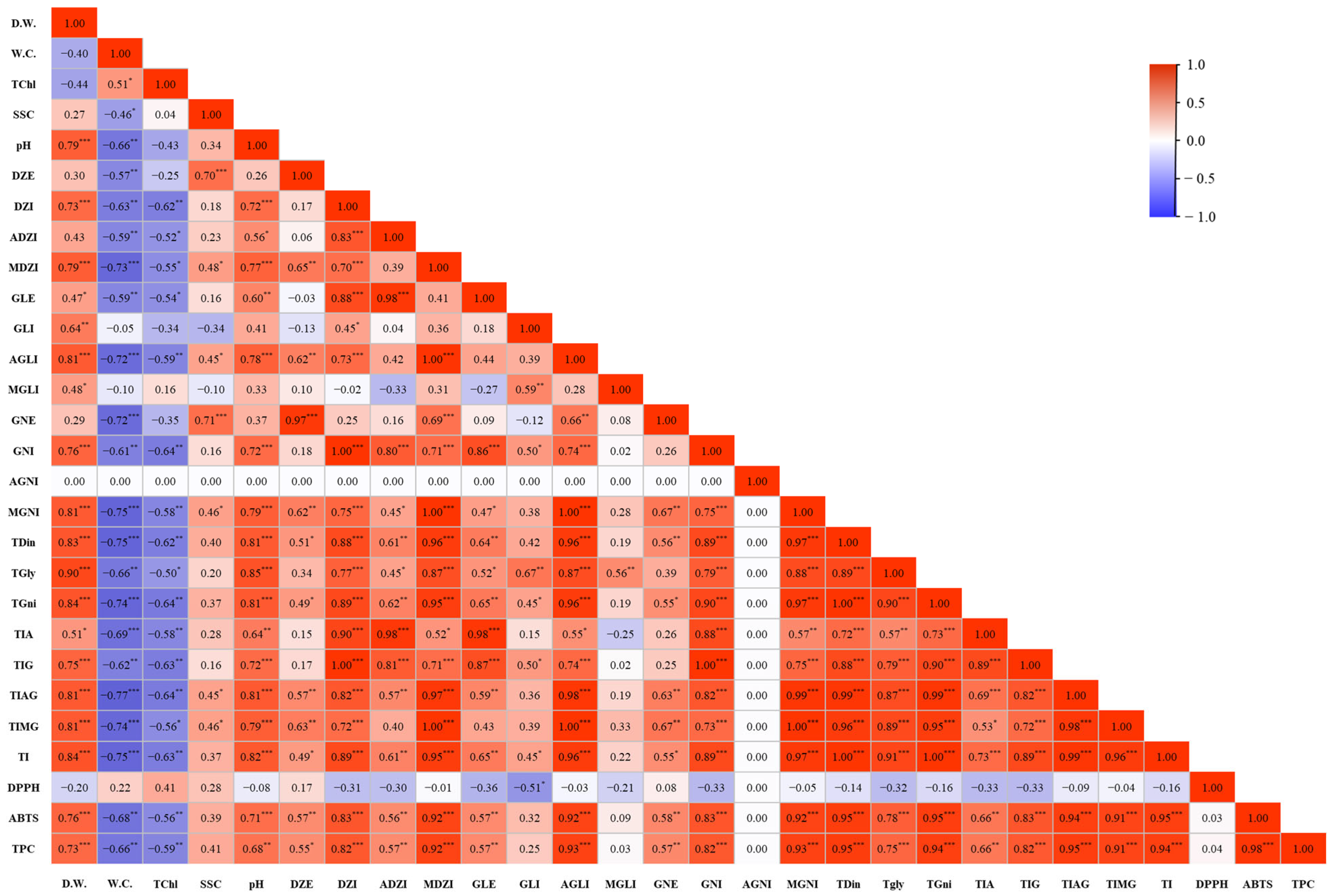
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSC | Soluble solid content |
| TPC | Total phenolic content |
| ADZI | Acetyl daidzin |
| AGLI | Acetyl glycitin |
| AGNI | Acetyl genistin |
| DZE | Daidzein |
| GLE | Glycitein |
| GNE | Genistein |
| MDZI | Malonyl daidzin |
| MGLI | Malonyl glycitin |
| MGNI | Malonyl genistin |
| TI | Total isoflavone |
| TIA | Total isoflavone aglycone |
| TIAG | Total isoflavone acetly-glycosides |
| TIG | Total isoflavone glycosides |
| TIMG | Total isoflavone malonyl-glycosides |
References
- Sakthivelu, G.; Devi, M.A.; Giridhar, P.; Rajasekaran, T.; Ravishankar, G.A.; Nedev, T.; Kosturkova, G. Drought-induced alterations in growth, osmotic potential and in vitro regeneration of soybean cultivars. Gen. Appl. Plant Physiol. 2008, 34, 103–112. [Google Scholar]
- Miadoková, E. Isoflavonoids–an overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009, 2, 211. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Jeong, D. Korean traditional fermented soybean products: Jang. J. Ethn. Foods 2015, 1, 2–7. [Google Scholar] [CrossRef]
- Djanta, M.K.A.; Agoyi, E.E.; Agbahoungba, S.; Quenum, F.J.B.; Chadare, F.J.; Assogbadjo, A.E.; Agbangla, C.; Sinsin, B. Vegetable soybean, edamame: Research, production, utilization and analysis of its adoption in Sub-Saharan Africa. J. Hortic. 2020, 12, 1–12. [Google Scholar]
- Cai, J.S.; Zhu, Y.Y.; Ma, R.H.; Thakur, K.; Zhang, J.G.; Wei, Z.J. Effects of roasting level on physicochemical, sensory, and volatile profiles of soybeans using electronic nose and HS-SPME-GC–MS. Food Chem. 2021, 340, 127880. [Google Scholar] [CrossRef]
- Endo, H.; Ohno, M.; Tanji, K.; Shimada, S.; Kaneko, K. Effect of heat treatment on the lipid peroxide content and aokusami (beany flavor) of soymilk. Food Sci. Techonol. 2007, 10, 328–333. [Google Scholar] [CrossRef][Green Version]
- Qu, S.; Kwon, S.J.; Duan, S.; Lim, Y.J.; Eom, S.H. Isoflavone changes in immature and mature soybeans by thermal processing. Molecules 2021, 26, 7471. [Google Scholar] [CrossRef]
- Muliterno, M.M.; Rodrigues, D.; de Lima, F.S.; Ida, E.I.; Kurozawa, L.E. Conversion/degradation of isoflavones and color alterations during the drying of okara. LWT 2017, 75, 512–519. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, S.K.; Liu, Z. Isoflavone profile in soymilk as affected by soybean variety, grinding, and heat-processing methods. J. Food Sci. 2015, 80, C983–C988. [Google Scholar] [CrossRef]
- Hoeck, J.A.; Fehr, W.R.; Murphy, P.A.; Welke, G.A. Influence of genotype and environment on isoflavone contents of soybean. Crop Sci. 2000, 40, 48–51. [Google Scholar] [CrossRef]
- Lin, Y.C.; Saputri, D.S.; Sun, P.W.; Chiu, C.H.; Pan, H.R.; Lau, Y.Y.; Hu, C.C.; Lin, H.Y. Monitoring 12 Isoflavones During Soybean Seed Germination Using LC-MS/MS. J. Food Compos. Anal. 2025, 146, 107909. [Google Scholar] [CrossRef]
- Wu, S.; Wang, M.; Liu, G.; Qin, B.; Xie, C.; Wang, P.; Yang, R. Enhancing aglycone isoflavones in soymilk through soybean germination and incubation. Food Biosci. 2024, 62, 105414. [Google Scholar] [CrossRef]
- Qin, W.; Deng, J.; Guo, J.; Yang, W.; Liu, J. Metabolic reallocation in soybeans under shade stress alters phenylpropanoid profiles with implications for stress adaptation and seed composition. BMC Plant Biol. 2025, 25, 870. [Google Scholar] [CrossRef]
- Huang, R.Y.; Chou, C.C. Heating affects the content and distribution profile of isoflavones in steamed black soybeans and black soybean koji. J. Agric. Food Chem. 2008, 56, 8484–8489. [Google Scholar] [CrossRef]
- Chien, J.T.; Hsieh, H.C.; Kao, T.H.; Chen, B.H. Kinetic model for studying the conversion and degradation of isoflavones during heating. Food Chem. 2005, 91, 425–434. [Google Scholar] [CrossRef]
- Kim, J.A.; Chung, I.M. Change in isoflavone concentration of soybean (Glycine max L.) seeds at different growth stages. J. Sci. Food Agric. 2007, 87, 496–503. [Google Scholar] [CrossRef]
- Devi, M.A.; Gondi, M.; Sakthivelu, G.; Giridhar, P.; Rajasekaran, T.; Ravishankar, G.A. Functional attributes of soybean seeds and products, with reference to isoflavone content and antioxidant activity. Food Chem. 2009, 114, 771–776. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Ho, C.T.; Pan, M.H. Bioavailability and health benefits of major isoflavone aglycones and their metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Zhu, D.; Xu, J.; Xu, X.; Liu, J. Bioaccessibility and application of soybean isoflavones: A review. Food Rev. Int. 2023, 39, 5948–5967. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, M.J.; Park, S.H.; Song, E.J.; Nam, Y.D.; Ahn, J.; Jang, Y.J.; Ha, T.Y.; Jung, C.H. Bioavailability of isoflavone metabolites after korean fermented soybean paste (doenjang) ingestion in estrogen-deficient Rats. J. Food Sci. 2018, 83, 2212–2221. [Google Scholar] [CrossRef]
- Lim, Y.J.; Lyu, J.I.; Kwon, S.J.; Eom, S.H. Effects of UV-A radiation on organ-specific accumulation and gene expression of isoflavones and flavonols in soybean sprout. Food Chem. 2021, 339, 128080. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, Y.J.; Duan, S.; Park, T.J.; Eom, S.H. Accumulation of antioxidative phenolics and carotenoids using thermal processing in different stages of Momordica charantia fruit. Molecules 2023, 28, 1500. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Rani, A.; Dixit, A.K.; Bhatnagar, D.; Chauhan, G.S. Relative changes in tocopherols, isoflavones, total phenolic content, and antioxidative activity in soybean seeds at different reproductive stages. J. Agric. Food Chem. 2009, 57, 2705–2710. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; IGITA, K.; KIKUCHI, A.; KUDOU, S.; OKUBO, K. Low Isoflavone Content in Some Early Maturing Cultivars, So-called “Summer-type Soybeans” (Glycine max (L) MERRILL). Jpn. J. Breed 1991, 41, 651–654. [Google Scholar] [CrossRef]
- Yerramsetty, V.; Mathias, K.; Bunzel, M.; Ismail, B. Detection and structural characterization of thermally generated isoflavone malonylglucoside derivatives. J. Agric. Food Chem 2011, 59, 174–183. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, B.W.; Kim, B.; Kim, H.T.; Ko, J.M.; Baek, I.Y.; Seo, W.T.; Kang, Y.M.; Cho, K.M. Changes in phenolic compounds (isoflavones and phenolic acids) and antioxidant properties in high-protein soybean (Glycine max L. cv. Saedanbaek) for different roasting conditions. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 605–612. [Google Scholar] [CrossRef]
- Yuan, J.P.; Liu, Y.B.; Peng, J.; Wang, J.H.; Liu, X. Changes of isoflavone profile in the hypocotyls and cotyledons of soybeans during dry heating and germination. J. Agric. Food Chem. 2009, 57, 9002–9010. [Google Scholar] [CrossRef]
- Jackson, C.J.; Dini, J.P.; Lavandier, C.; Rupasinghe, H.P.V.; Faulkner, H.; Poysa, V.; Buzzell, D.; DeGrandis, S. Effects of processing on the content and composition of isoflavones during manufacturing of soy beverage and tofu. Process Biochem. 2002, 37, 1117–1123. [Google Scholar] [CrossRef]
- Yu, X.; Meenu, M.; Xu, B.; Yu, H. Impact of processing technologies on isoflavones, phenolic acids, and antioxidant capacities of soymilk prepared from 15 soybean varieties. Food Chem. 2021, 345, 128612. [Google Scholar] [CrossRef]
- Wang, H.J.; Murphy, P.A. Mass balance study of isoflavones during soybean processing. J. Agric. Food Chem. 1996, 44, 2377–2383. [Google Scholar] [CrossRef]
- Wang, H.J.; Murphy, P.A. Isoflavone content in commercial soybean foods. J. Agric. Food Chem. 1994, 42, 1666–1673. [Google Scholar] [CrossRef]
- Yue, X.; Abdallah, A.M.; Xu, Z. Distribution of isoflavones and antioxidant activities of soybean cotyledon, coat and germ. J. Food Process. Preserv. 2010, 34, 795–806. [Google Scholar] [CrossRef]
- Kudou, S.; Fleury, Y.; Welti, D.; Magnolato, D.; Uchida, T.; Kitamura, K.; Okubo, K. Malonyl isoflavone glycosides in soybean seeds (Glycine max Merrill). Agric. Biol. Chem. 1991, 55, 2227–2233. [Google Scholar] [CrossRef]
- Ribeiro, M.L.L.; Mandarino, J.M.G.; Carrao-Panizzi, M.C.; De Oliveira, M.C.N.; Campo, C.B.H.; Nepomuceno, A.L.; Ida, E.I. Isoflavone content and β-glucosidase activity in soybean cultivars of different maturity groups. J. Food Compos. Anal. 2007, 20, 19–24. [Google Scholar] [CrossRef]
- Lee, C.H.; Yang, L.; Xu, J.Z.; Yeung, S.Y.V.; Huang, Y.; Chen, Z.Y. Relative antioxidant activity of soybean isoflavones and their glycosides. Food Chem. 2005, 90, 735–741. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, H. Characterisation and comparison of phenols, flavonoids and isoflavones of soymilk and their correlations with antioxidant activity. Int. J. Food Sci. Technol. 2014, 49, 2290–2298. [Google Scholar] [CrossRef]
- Naim, M.; Gestetner, B.; Bondi, A.; Birk, Y. Antioxidative and antihemolytic activities of soybean isoflavones. J. Agric. Food Chem. 1976, 24, 1174–1177. [Google Scholar] [CrossRef]
- Balanescu, F.; Busuioc, A.C.; Botezatu, A.V.D.; Gosav, S.; Avramescu, S.M.; Furdui, B.; Dinica, R.M. Comparative study of natural antioxidants from Glycine max, Anethum graveolens and Pimpinella anisum seed and sprout extracts obtained by ultrasound-assisted extraction. Separations 2022, 9, 152. [Google Scholar] [CrossRef]
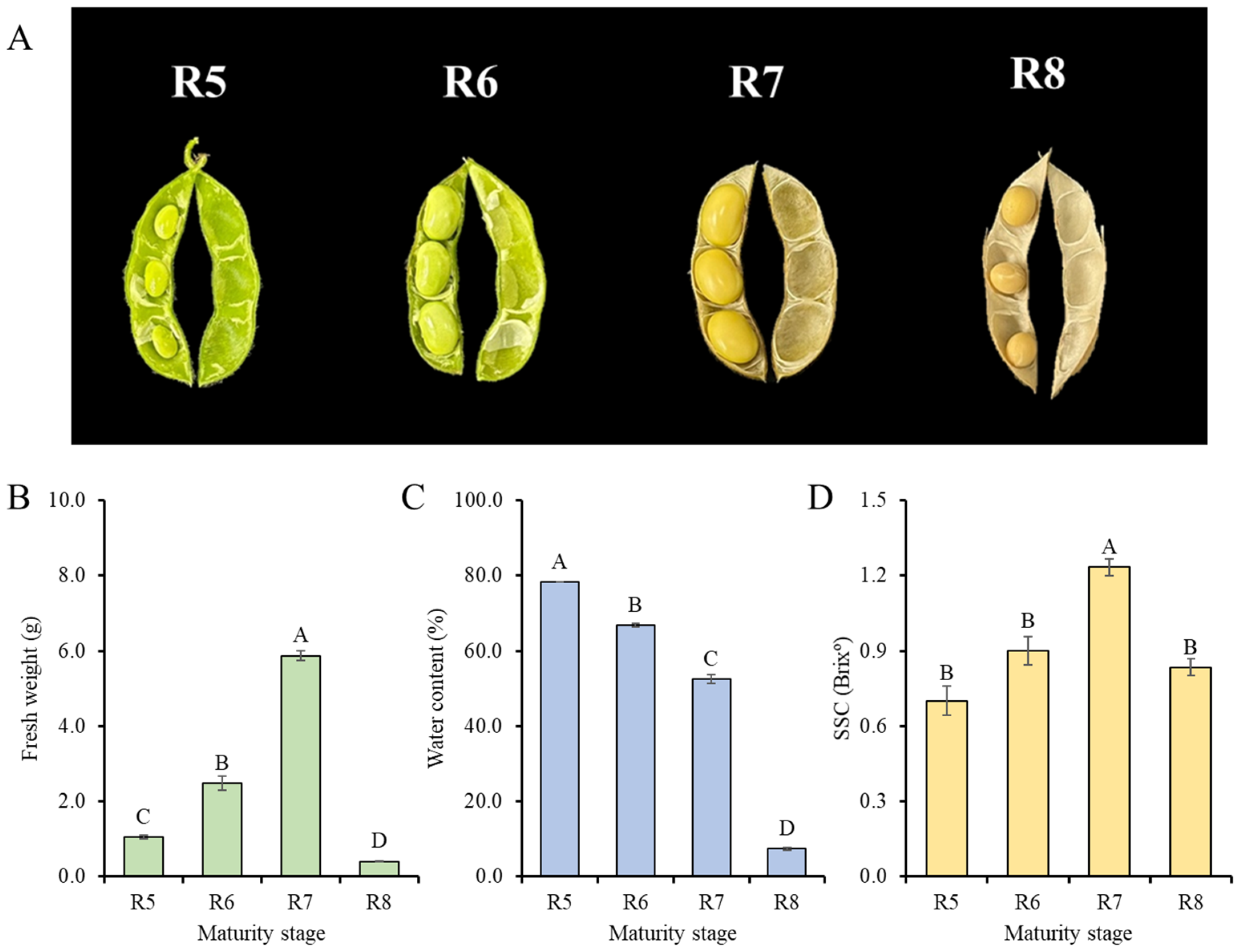

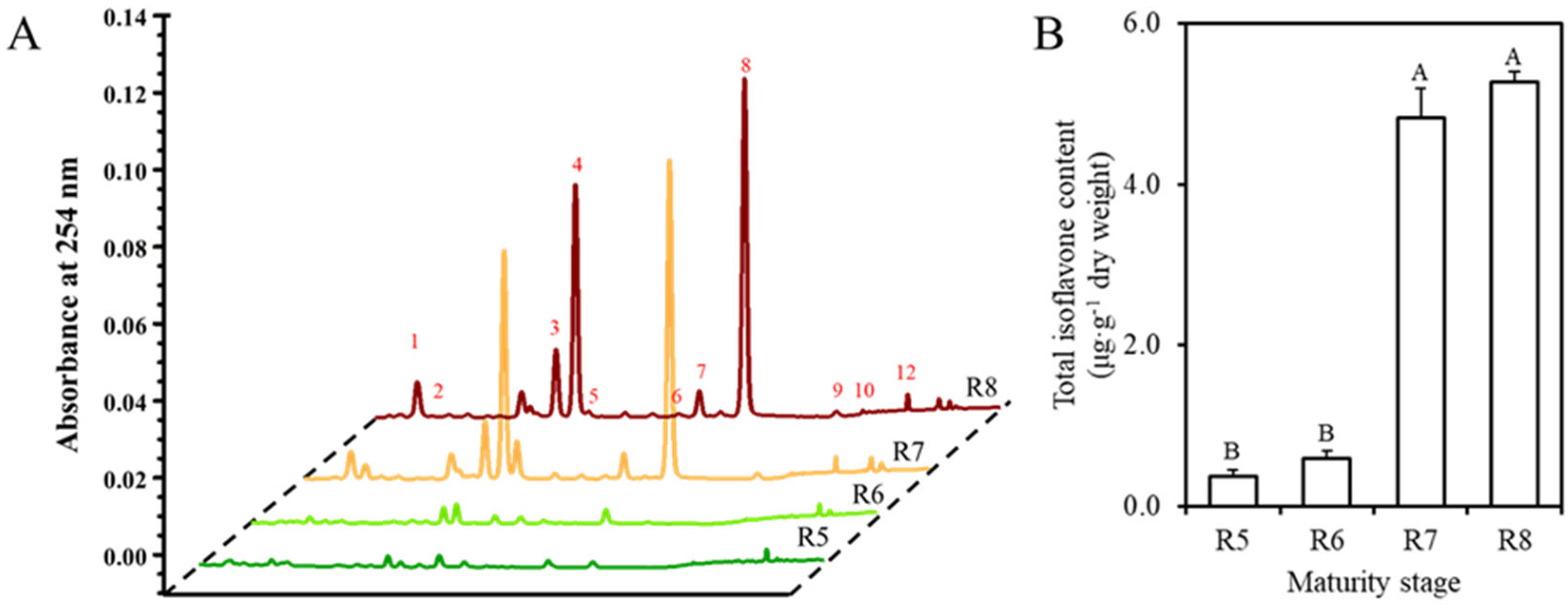


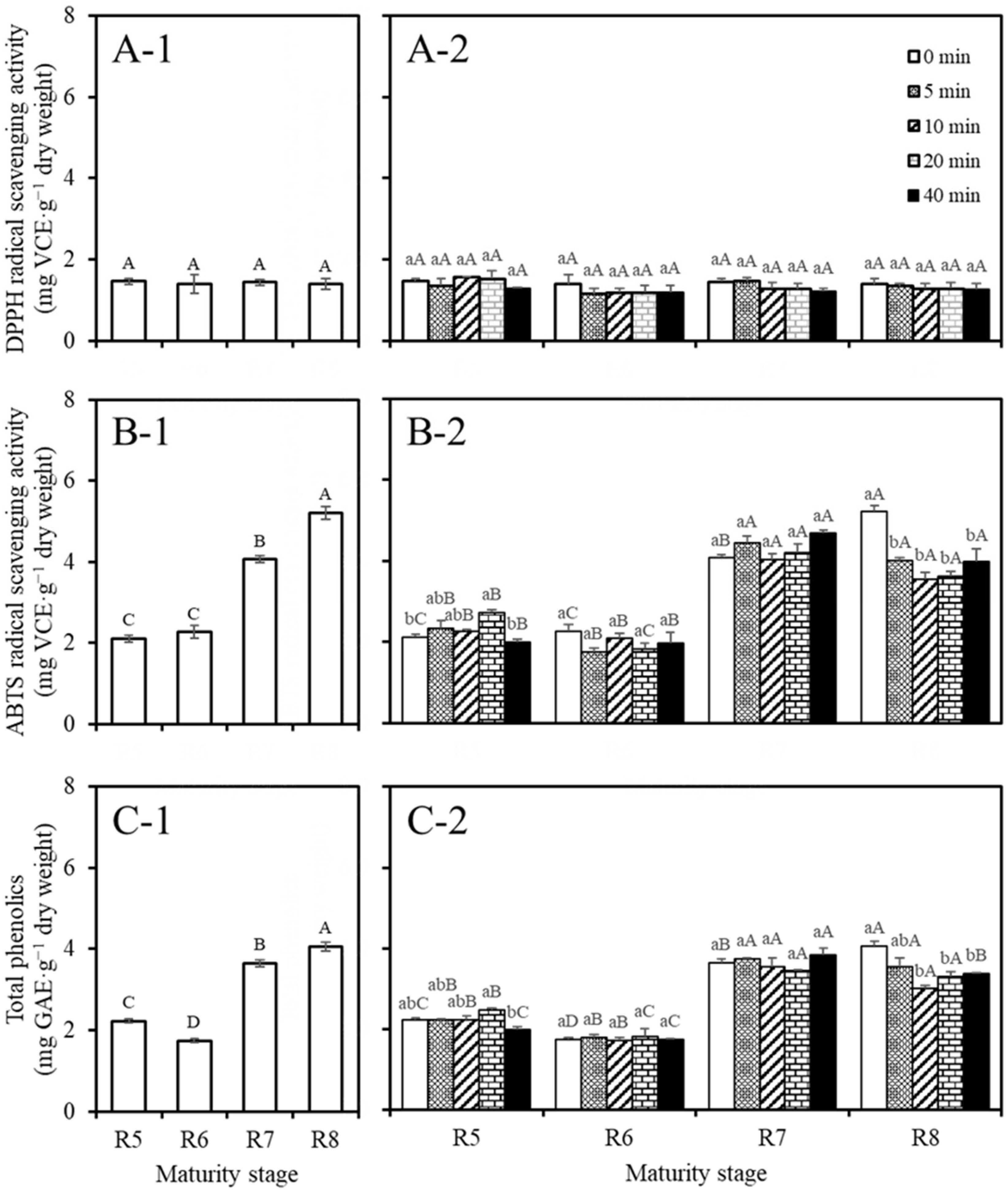
| Stage | Steaming Time (min) | Dry Weight (g/ea) | Water Content (%) | TChl Content (mg/g d.w.) | SSC (Brixº) | pH |
|---|---|---|---|---|---|---|
| R5 | 0 | 0.02 ± 0.00aC | 78.25 ± 0.07aA | 0.15 ± 0.00aB | 0.70 ± 0.06aB | 5.83 ± 0.01dC |
| 5 | 0.02 ± 0.00aD | 78.39 ± 0.29aA | 0.13 ± 0.00bA | 0.63 ± 0.03abAB | 6.25 ± 0.01aB | |
| 10 | 0.02 ± 0.00aD | 76.58 ± 0.58abA | 0.11 ± 0.00cB | 0.57 ± 0.03abB | 6.12 ± 0.01bC | |
| 20 | 0.02 ± 0.00aD | 77.38 ± 0.38abA | 0.12 ± 0.00cA | 0.53 ± 0.03abB | 5.96 ± 0.01cB | |
| 40 | 0.02 ± 0.00aD | 75.46 ± 0.27bA | 0.04 ± 0.00dC | 0.47 ± 0.03bBC | 5.54 ± 0.02eD | |
| R6 | 0 | 0.08 ± 0.01aB | 66.84 ± 0.43aB | 0.18 ± 0.00aA | 0.90 ± 0.06aA | 6.54 ± 0.01bB |
| 5 | 0.06 ± 0.00abC | 65.17 ± 0.40aB | 0.14 ± 0.01bA | 0.47 ± 0.07bB | 5.99 ± 0.03cB | |
| 10 | 0.06 ± 0.00bC | 67.01 ± 0.17aB | 0.14 ± 0.00bA | 0.47 ± 0.03bB | 6.81 ± 0.01aB | |
| 20 | 0.06 ± 0.00bC | 66.38 ± 0.65aB | 0.08 ± 0.01cB | 0.40 ± 0.00bC | 6.02 ± 0.03cB | |
| 40 | 0.05 ± 0.00bC | 67.51 ± 0.20aA | 0.05 ± 0.00dB | 0.40 ± 0.00bC | 6.51 ± 0.04bC | |
| R7 | 0 | 0.15 ± 0.01aA | 52.53 ± 1.18aC | 0.07 ± 0.00aC | 1.23 ± 0.03aB | 7.01 ± 0.03bA |
| 5 | 0.14 ± 0.00aA | 54.78 ± 0.88aC | 0.06 ± 0.00bB | 0.50 ± 0.00bB | 7.15 ± 0.01aA | |
| 10 | 0.14 ± 0.00aA | 53.50 ± 0.45aC | 0.06 ± 0.00bC | 0.53 ± 0.03bB | 7.15 ± 0.00aA | |
| 20 | 0.14 ± 0.01aA | 54.95 ± 0.46aC | 0.06 ± 0.00bC | 0.43 ± 0.03bBC | 7.11 ± 0.01aA | |
| 40 | 0.14 ± 0.00aA | 55.39 ± 0.49aB | 0.06 ± 0.00bA | 0.57 ± 0.03bB | 6.75 ± 0.01cB | |
| R8 | 0 | 0.08 ± 0.00aB | 7.41 ± 0.29aD | 0.07 ± 0.00aC | 0.83 ± 0.03aB | 6.51 ± 0.02bB |
| 5 | 0.09 ± 0.00aB | 7.61 ± 0.40aD | 0.07 ± 0.00aB | 0.77 ± 0.03aA | 7.14 ± 0.01aA | |
| 10 | 0.08 ± 0.00aB | 10.79 ± 1.03aD | 0.06 ± 0.01bC | 0.77 ± 0.07aA | 7.15 ± 0.01aA | |
| 20 | 0.09 ± 0.00aB | 12.25 ± 0.12aD | 0.06 ± 0.00bC | 0.83 ± 0.03aA | 7.13 ± 0.02aA | |
| 40 | 0.08 ± 0.00aB | 15.46 ± 3.07aC | 0.04 ± 0.00cC | 0.77 ± 0.03aA | 7.08 ± 0.03aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Kim, J.-H.; Eom, S.H. Variations in Isoflavone During Soybean Maturation and Their Thermal Process-Dependent Conversion. Agronomy 2025, 15, 2155. https://doi.org/10.3390/agronomy15092155
Kim JH, Kim J-H, Eom SH. Variations in Isoflavone During Soybean Maturation and Their Thermal Process-Dependent Conversion. Agronomy. 2025; 15(9):2155. https://doi.org/10.3390/agronomy15092155
Chicago/Turabian StyleKim, Ji Hye, Jae-Hee Kim, and Seok Hyun Eom. 2025. "Variations in Isoflavone During Soybean Maturation and Their Thermal Process-Dependent Conversion" Agronomy 15, no. 9: 2155. https://doi.org/10.3390/agronomy15092155
APA StyleKim, J. H., Kim, J.-H., & Eom, S. H. (2025). Variations in Isoflavone During Soybean Maturation and Their Thermal Process-Dependent Conversion. Agronomy, 15(9), 2155. https://doi.org/10.3390/agronomy15092155







