Effects of Single and Split Pre-Harvest Aminoethoxyvinylglycine Applications in Bioactive Compounds and Antioxidant Activity in ‘Baigent’ Apples Under Anti-Hail Nets
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of the Orchard
2.2. Treatments
2.3. Storage Conditions and Analyzed Variables
2.4. Experimental Design and Statistical Analysis
3. Results and Discussion
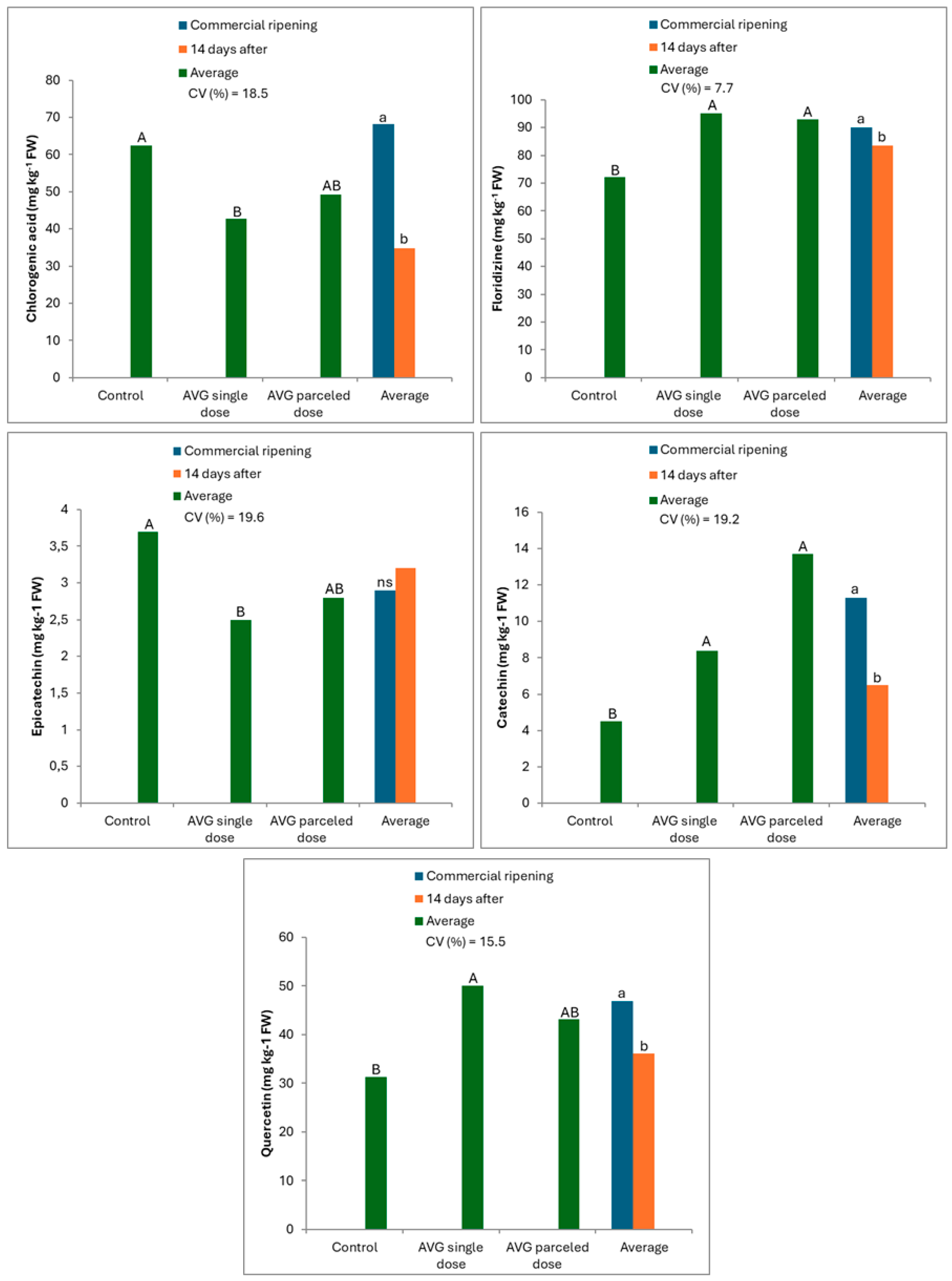
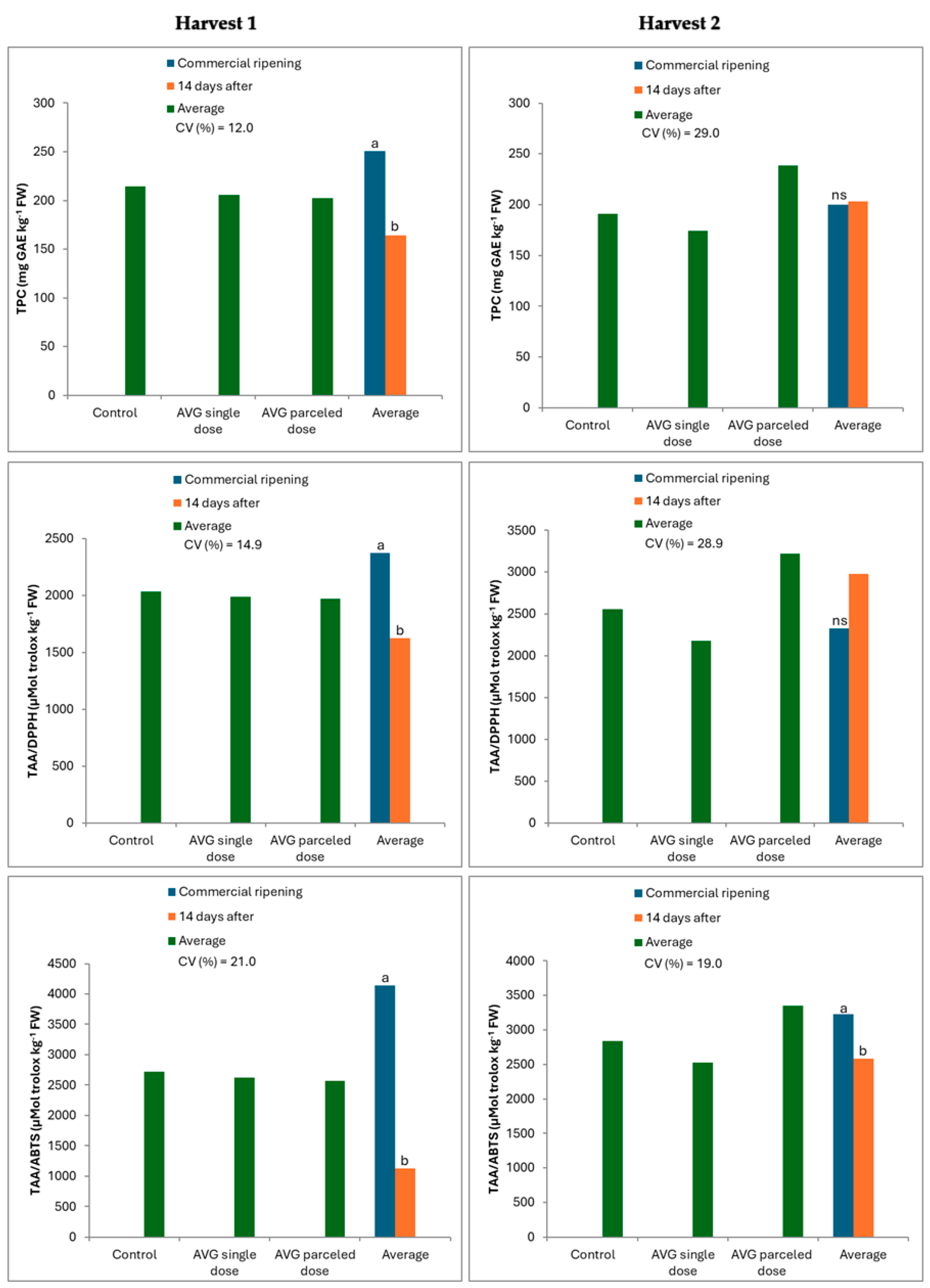
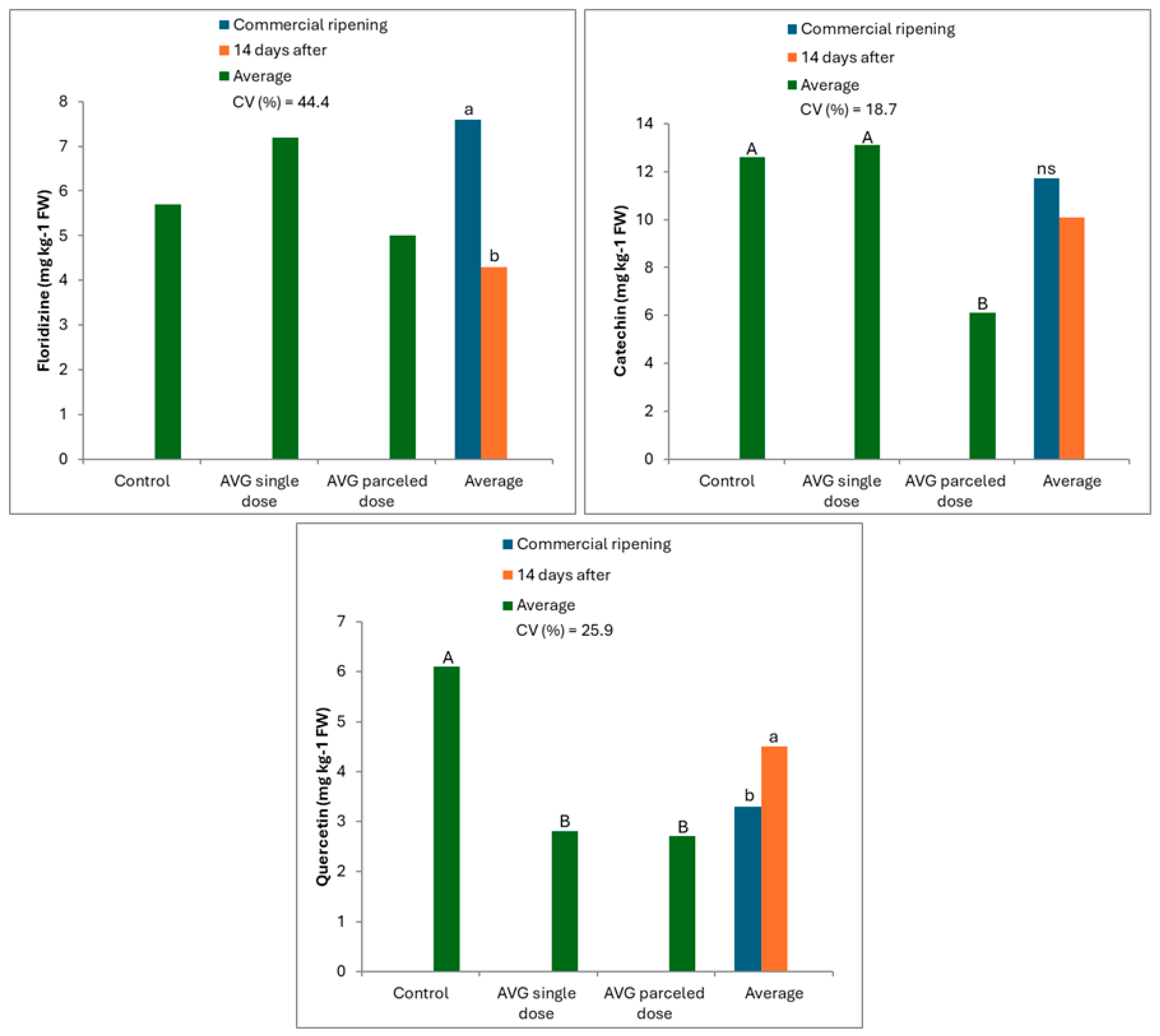
3.1. When Harvesting Fruit
3.2. After Cold Storage (CS)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Islam, M.T.; Sherif, S.M. Effects of Aminoethoxyvinylglycine (AVG) and 1-Methylcyclopropene (1-MCP) on the Pre-Harvest Drop Rate, Fruit Quality, and Stem-End Splitting in ‘Gala’ Apples. Horticulturae 2022, 8, 1100. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, T.; Yu, C.; Tan, W.; Shen, Y.; Duan, L. Aminoethoxyvinylglycine Functional Analogues Prolong the Shelf Life of Apple Fruit. J. Plant Growth Regul. 2024, 43, 314–322. [Google Scholar] [CrossRef]
- Van de Poel, B.; Van Der Straeten, D. 1-Aminocyclopropane-1-Carboxylic Acid (ACC) in Plants: More than Just the Precursor of Ethylene! Front. Plant Sci. 2014, 5, 640. [Google Scholar] [CrossRef] [PubMed]
- Kon, T.M.; Clavet, C.D.; Clarke, G.G. Organic Aminoethoxyvinylglycine Is an Effective Alternative for Reducing Apple Preharvest Drop. HortScience 2023, 58, 733–738. [Google Scholar] [CrossRef]
- Soethe, C.; Steffens, C.A.; Heinzen, A.S.; Hawerroth, F.J.; Santos, E.S.; Amarante, C.V.T.; Duarte, G.C. Maturação de Maçãs ‘Brookfield’ em Função da Forma de Aplicação de Aminoetoxivinilglicina. Rev. Jorn. Pós-Grad. Pesqui.-Congrega 2017, 2017, 2009–2022. [Google Scholar]
- Miah, M.S.; Farcuh, M. Combining the Use of Reflective Groundcovers and Aminoethoxyvinylglycine to Assess Effects on Skin Color, Preharvest Drop, and Quality of ‘Honeycrisp’ Apples in the Mid-Atlantic US. Horticulturae 2024, 10, 179. [Google Scholar] [CrossRef]
- Ozturk, B.; Ozkan, Y.; Altuntas, E.; Yidiz, K.; Saracoglu, O. Effect of Aminoethoxyvinylglycine on Biochemical, Physicomechanical and Colour Properties of Cv. Braeburn Apples. Semin. Ciências Agrar. 2013, 34, 1111–1120. [Google Scholar] [CrossRef][Green Version]
- Ozkan, Y.; Altuntas, E.; Ozturk, B.; Yildiz, K.; Saracoglu, O. The Effect of NAA (1-Naphthalene Acetic Acid) and AVG (Aminoethoxyvinylglycine) on Physical, Chemical, Colour and Mechanical Properties of Braeburn Apple. Int. J. Food Eng. 2012, 8, 1–24. [Google Scholar] [CrossRef]
- Condezo-Hoyos, L.; Mohanty, I.P.; Noratto, G.D. Assessing Non-Digestible Compounds in Apple Cultivars and Their Potential as Modulators of Obese Faecal Microbiota in Vitro. Food Chem. 2014, 161, 208–215. [Google Scholar] [CrossRef]
- Achkar, M.T.; Novaes, G.M.; Silva, M.J.D.; Vilegas, W. Propriedade Antioxidante de Compostos Fenólicos: Importância Na Dieta e Na Conservação de Alimentos. Rev. Univ. Val. Rio Verde 2013, 11, 398–406. [Google Scholar] [CrossRef]
- Panzella, L.; Petriccione, M.; Rega, P.; Scortichini, M.; Napolitano, A. A Reappraisal of Traditional Apple Cultivars from Southern Italy as a Rich Source of Phenols with Superior Antioxidant Activity. Food Chem. 2013, 140, 672–679. [Google Scholar] [CrossRef]
- Zheng, H.-Z.; Kim, Y.-I.; Chung, S.-K. A Profile of Physicochemical and Antioxidant Changes During Fruit Growth for the Utilisation of Unripe Apples. Food Chem. 2012, 131, 106–110. [Google Scholar] [CrossRef]
- Hyson, D.A. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed]
- Ceymann, M.; Arrigoni, E.; Schärer, H.; Bozzi Nising, A.; Hurrell, R.F. Identification of Apples Rich in Health-Promoting Flavan-3-Ols and Phenolic Acids by Measuring the Polyphenol Profile. J. Food Compos. Anal. 2012, 26, 128–135. [Google Scholar] [CrossRef]
- Jakobek, L.; García-Villalba, R.; Tomás-Barberán, F.A. Polyphenolic Characterisation of Old Local Apple Varieties from Southeastern European Region. J. Food Compos. Anal. 2013, 31, 199–211. [Google Scholar] [CrossRef]
- Stanger, M.C.; Steffens, C.A.; Soethe, C.; Moreira, M.A.; do Amarante, C.V.T. Phenolic Content and Antioxidant Activity During the Development of ‘Brookfield’ and ‘Mishima’ Apples. J. Agric. Food Chem. 2017, 65, 3453–3459. [Google Scholar] [CrossRef]
- Petkovsek, M.M.; Slatnar, A.; Stampar, F.; Veberic, R. The Influence of Organic/Integrated Production on the Content of Phenolic Compounds in Apple Leaves and Fruits in Four Different Varieties over a 2-year Period. J. Sci. Food Agric. 2010, 90, 2366–2378. [Google Scholar] [CrossRef]
- Stanger, M.C.; Steffens, C.A.; Soethe, C.; Moreira, M.A.; do Amarante, C.V.T.; Both, V.; Brackmann, A. Phenolic Compounds Content and Antioxidant Activity of ‘Galaxy’ Apples Stored in Dynamic Controlled Atmosphere and Ultralow Oxygen Conditions. Postharvest Biol. Technol. 2018, 144, 70–76. [Google Scholar] [CrossRef]
- Do Amarante, C.V.T.; Steffens, C.A.; Miqueloto, A.; Zanardi, O.Z.; dos Santos, H.P. Disponibilidade de Luz Em Macieiras “Fuji” Cobertas Com Telas Antigranizo e Seus Efeitos Sobre a Fotossíntese, o Rendimento e a Qualidade Dos Frutos. Rev. Bras. Frutic. 2009, 31, 664–670. [Google Scholar] [CrossRef]
- Mupambi, G.; Anthony, B.M.; Layne, D.R.; Musacchi, S.; Serra, S.; Schmidt, T.; Kalcsits, L.A. The Influence of Protective Netting on Tree Physiology and Fruit Quality of Apple: A Review. Sci. Hortic. 2018, 236, 60–72. [Google Scholar] [CrossRef]
- Soethe, C.; Steffens, C.A.; Hawerroth, F.J.; do Amarante, C.V.T.; Heinzen, A.S.; Stanger, M.C. Postharvest Quality of ‘Baigent’ Apples as a Function of Single and Multiple Preharvest Spray Aminoetoxivinylglycine and Ethephon Applications. Rev. Bras. Frutic. 2019, 41, e-153. [Google Scholar] [CrossRef]
- Algul, B.E.; Al Shoffe, Y.; Park, D.; Cheng, L.; Watkins, C.B. Preharvest 1-Methylcyclopropene and Aminoethoxyvinylglycine Treatment Effects on ‘NY2’ (RubyFrost®) Apple Fruit Quality and Postharvest Watercore Dissipation at Different Temperatures. Postharvest Biol. Technol. 2025, 220, 113301. [Google Scholar] [CrossRef]
- dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; rev. e ampl.; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- Rufino, M.S.M.; Alves, R.E.; Brito, S.E.S.; Morais, S.M.; Sampaio, C.G.; Perez-Jimenez, J.; Saura-Calixto, F. Metodologia Cientifíca: Determinacão da Atividade Antioxidante Total em Frutas Pela Captura do Radical Livre ABTS; Comunicado Técnico; Embrapa: Brasília, Brazil, 2007; Volume 127, pp. 1–4. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/426954/1/Cot128.pdf (accessed on 12 February 2023).
- Larrauri, J.A.; Ruperez, P.; Saura-Calixto, F. Effect of drying temperature on the stabilitity of polyphenols and antioxidant activity of red grape pomace peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [Google Scholar] [CrossRef]
- Roesler, R.; Malta, L.G.; Carrasco, L.C.; Holanda, R.B.; Sousa, C.A.S.; Pastore, G.M. Atividade antioxidante de frutas do cerrado. Ciência Tecnol. Aliment. 2007, 27, 53–60. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. Extraction and determination of total anthocyanin in cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef]
- Soethe, C.; Steffens, C.A.; Hawerroth, F.J.; Moreira, M.A.; Talamini do Amarante, C.V.; Stanger, M.C. Quality of ‘Baigent’ Apples as a Function of Pre-Harvest Application of Aminoethoxyvinylglycine and Ethephon Stored in Controlled Atmosphere. Appl. Food Res. 2022, 2, 100117. [Google Scholar] [CrossRef]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. In Phenolic Compounds—Biological Activity; InTech: Toyama, Japan, 2017. [Google Scholar]
- Ouamnina, A.; Alahyane, A.; Elateri, I.; Boutasknit, A.; Abderrazik, M. Relationship Between Phenolic Compounds and Antioxidant Activity of Some Moroccan Date Palm Fruit Varieties (Phoenix dactylifera L.): A Two-Year Study. Plants 2024, 13, 1119. [Google Scholar] [CrossRef]
- Awad, M.A.; Wagenmakers, P.S.; Jager, A. Effects of Light on Flavonoid and Chlorogenic Acid Levels in the Skin of ‘Jonagold’ Apples. Sci. Hortic. 2001, 88, 289–298. [Google Scholar] [CrossRef]
- Ozturk, B.; Kucuker, E.; Yıldız, K.; Celik, S.M. Changes of Bioactive Compounds and Ethylene Production of Japanese Plums Treated with Pre-Harvest Aminoethoxyvinylglycine. Int. J. Food Prop. 2015, 18, 2165–2186. [Google Scholar] [CrossRef]
- Ozturk, B.; Kucuker, E.; Karaman, S.; Ozkan, Y. The Effects of Cold Storage and Aminoethoxyvinylglycine (AVG) on Bioactive Compounds of Plum Fruit (Prunus salicina Lindell Cv. ‘Black Amber’). Postharvest Biol. Technol. 2012, 72, 35–41. [Google Scholar] [CrossRef]
- Yildiz, K.; Kilic, K.; Ozkan, Y.; Ozturk, B.; Kucuker, E. The Role of Pre-Harvest Aminoethoxyvinylglycine (AVG) Treatments on Total Phenolics, Antioxidant Capacity and Fruit Quality Attributes of Sweet Cherry Cultivars. Erwerbs-Obstbau 2018, 60, 221–230. [Google Scholar] [CrossRef]
- Xi, Z.; Zhang, Z.; Huo, S.; Luan, L.; Gao, X.; Ma, L.; Fang, Y. Regulating the Secondary Metabolism in Grape Berry Using Exogenous 24-Epibrassinolide for Enhanced Phenolics Content and Antioxidant Capacity. Food Chem. 2013, 141, 3056–3065. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Singh, Z.; Swinny, E.E. Postharvest Application of 1-Methylcyclopropene Modulates Fruit Ripening, Storage Life and Quality of ‘Tegan Blue’ Japanese Plum Kept in Ambient and Cold Storage. Int. J. Food Sci. Technol. 2009, 44, 1272–1280. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Wang, Y.; Yang, X.; Wei, C.; Guan, J. Ethylene Signal Is Involved in the Regulation of Anthocyanin Accumulation in Flesh of Postharvest Plums (Prunus salicina Lindl.). Plants 2023, 12, 893. [Google Scholar] [CrossRef]
- Stroka, M.A.; Ayub, R.A.; da Silva, D.M.; Pessenti, I.L.; Pereira, A.B.; Barbosa, E.A.A. Effect of Anti-Hail Nets with Different Colors on ‘Eva’ Apple Trees Agronomical Responses. Rev. Bras. Frutic. 2021, 43, e-157. [Google Scholar] [CrossRef]
- Kucuker, E.; Burhan, O.; Yildiz, K.; Yakup, O. Effect of Aminoethoxyvinylglycine (AVG) on the Quality of Japanese Plum (Prunus salicina Lindell Cv. Fortune) Fruits. Acta Sci. Pol. Hortorum Cultus 2015, 14, 3–17. [Google Scholar]
- He, J.; Feng, Y.; Cheng, Y.; Guan, J. Effects of Preharvest Aminoethoxyvinylglycine (AVG) Treatment on Fruit Ripening, Core Browning and Related Gene Expression in ‘Huangguan’ Pear (Pyrus bretschneideri Rehd.). Horticulturae 2023, 9, 179. [Google Scholar] [CrossRef]
- Whale, S.K.; Singh, Z.; Behboudian, M.H.; Janes, J.; Dhaliwal, S.S. Fruit Quality in ‘Cripp’s Pink’ Apple, Especially Colour, as Affected by Preharvest Sprays of Aminoethoxyvinylglycine and Ethephon. Sci. Hortic. 2008, 115, 342–351. [Google Scholar] [CrossRef]
- Hoang, N.T.T.; Golding, J.B.; Wilkes, M.A. The Effect of Postharvest 1-MCP Treatment and Storage Atmosphere on ‘Cripps Pink’ Apple Phenolics and Antioxidant Activity. Food Chem. 2011, 127, 1249–1256. [Google Scholar] [CrossRef]
- Kucuker, E.; Burhan, O.; Aksit, H.; Nusret, G. Effect of Pre-Harvest Aminoethoxyvinylglycine (AVG) Application on Bioactive Compounds and Fruit Quality of Plum (Prunus salicina Lindell Cv. Black Beauty) at the Time of Harvest and During Cold Storage. J. Anim. Plant Sci. 2015, 25, 763–770. [Google Scholar]
- Gao, Q.; Li, Y.; Li, Y.; Zhang, Z.; Liang, Y. Antioxidant and Prooxidant Activities of Phenolic Acids Commonly Existed in Vegetables and Their Relationship with Structures. Food Sci. Technol. 2022, 42, e07622. [Google Scholar] [CrossRef]
- Plaza, M.; Kariuki, J.; Turner, C. Quantification of Individual Phenolic Compounds’ Contribution to Antioxidant Capacity in Apple: A Novel Analytical Tool Based on Liquid Chromatography with Diode Array, Electrochemical, and Charged Aerosol Detection. J. Agric. Food Chem. 2014, 62, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional Roles of Flavonoids in Photoprotection: New Evidence, Lessons from the Past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive Phenolic Compounds: Production and Extraction by Solid-State Fermentation. A Review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef]
- Li, W.-F.; Mao, J.; Yang, S.-J.; Guo, Z.-G.; Ma, Z.-H.; Dawuda, M.M.; Zuo, C.-W.; Chu, M.-Y.; Chen, B.-H. Anthocyanin Accumulation Correlates with Hormones in the Fruit Skin of ‘Red Delicious’ and Its Four Generation Bud Sport Mutants. BMC Plant Biol. 2018, 18, 363. [Google Scholar] [CrossRef]
- Aglar, E. Effects of Aminoethoxyvinylglycine (AVG) on Fruit Quality and Bioactive Content of Jujube Fruit (Ziziphus jujuba) Harvested at Three Maturity Stages During Cold Storage. Erwerbs-Obstbau 2023, 65, 879–888. [Google Scholar] [CrossRef]
- Rotili, M.C.C.; Coutro, S.; Celant, V.M.; Vorpagel, J.A.; Barp, F.K.; Salibe, A.B.; Braga, G.C. Composição, Atividade Antioxidante e Qualidade Do Maracujáamarelo Durante Armazenamento. Semin. Cienc. Agrar. 2013, 34, 227–240. [Google Scholar] [CrossRef]
- Karaman, S.; Ozturk, B.; Aksit, H.; Erdogdu, T. The Effects of Pre-Harvest Application of Aminoethoxyvinylglycine on the Bioactive Compounds and Fruit Quality of ‘Fortune’ Plum Variety during Cold Storage. Food Sci. Technol. Int. 2013, 19, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Picoli, A.A.; Faria, D.B.; Jomori, M.L.L.; Kluge, R.A. Avaliação de Biorreguladores No Metabolismo Secundário de Beterrabas Inteiras e Minimamente Processadas. Bragantia 2010, 69, 983–988. [Google Scholar] [CrossRef][Green Version]

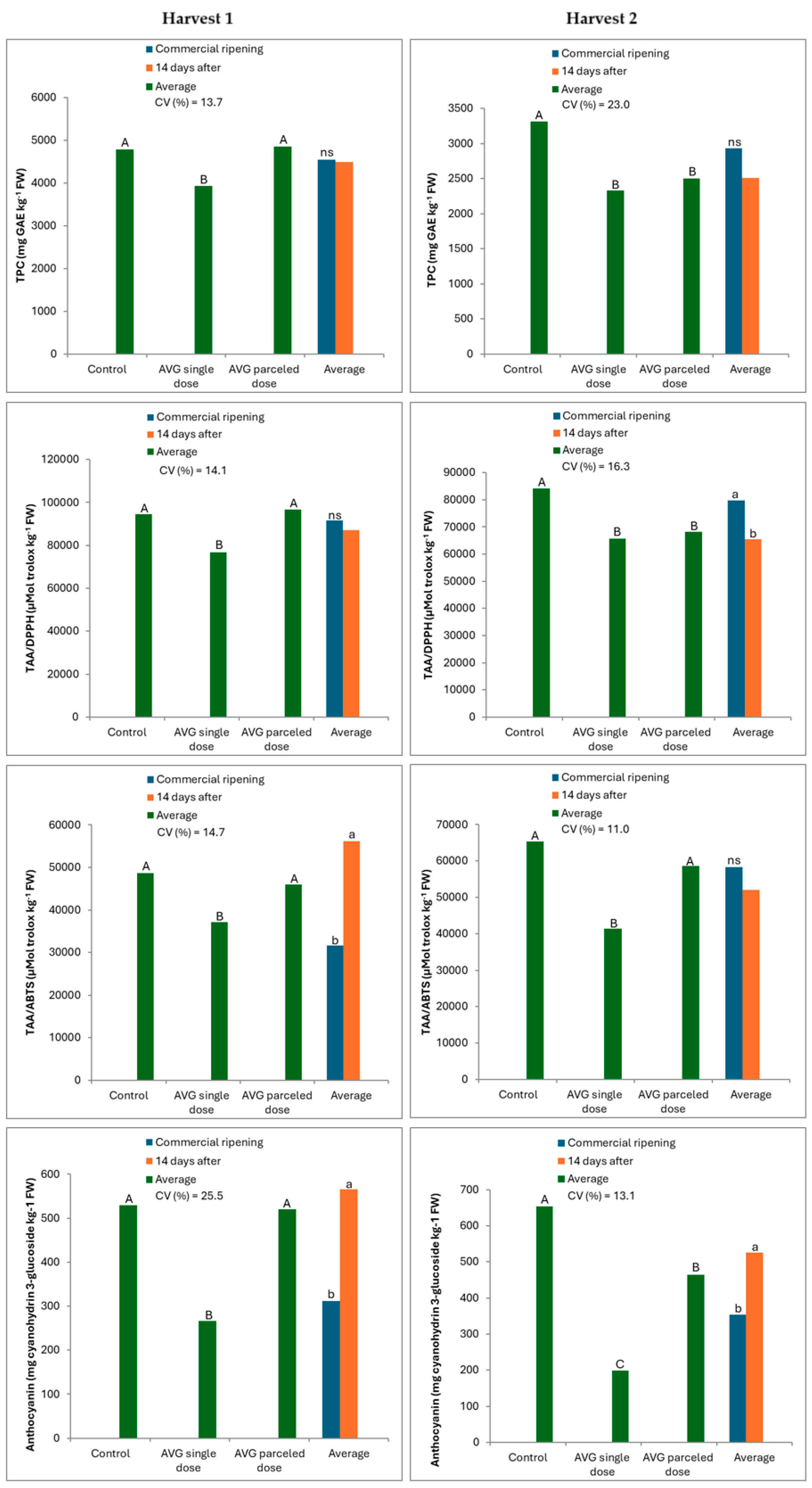

| Correlation | Harvest 1 | Harvest 2 | ||
|---|---|---|---|---|
| Skin | Flesh | Skin | Flesh | |
| TPC × DPPH | 0.99 *** | 0.99 *** | 0.99 *** | 0.95 *** |
| TPC × ABTS | 0.99 *** | 0.81 *** | 0.82 *** | 0.96 *** |
| TPC × ANT | 0.99 *** | - | 0.90 *** | - |
| Treatments | Commercial Ripening | 14 Days After | Average |
|---|---|---|---|
| Chlorogenic acid | |||
| Control | 128.6 Aa | 59.2 Ab | - |
| AVG single dose (125 mg L−1/30 DBEHD *) | 67.3 Ba | 59.4 Aa | - |
| AVG parceled dose (62.5 mg L−1 + 62.5 mg L−1/30 and 20 DBEHD) | 35.4 Ca | 34.6 Ba | - |
| Average | - | - | |
| CV (%) | 17.5 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soethe, C.; Correa, I.d.O.; Amorim, C.; de Souza, N.M.; Hawerroth, F.J.; Moreira, M.A.; Stanger, M.C.; do Amarante, C.V.T.; Steffens, C.A. Effects of Single and Split Pre-Harvest Aminoethoxyvinylglycine Applications in Bioactive Compounds and Antioxidant Activity in ‘Baigent’ Apples Under Anti-Hail Nets. Agronomy 2025, 15, 2152. https://doi.org/10.3390/agronomy15092152
Soethe C, Correa IdO, Amorim C, de Souza NM, Hawerroth FJ, Moreira MA, Stanger MC, do Amarante CVT, Steffens CA. Effects of Single and Split Pre-Harvest Aminoethoxyvinylglycine Applications in Bioactive Compounds and Antioxidant Activity in ‘Baigent’ Apples Under Anti-Hail Nets. Agronomy. 2025; 15(9):2152. https://doi.org/10.3390/agronomy15092152
Chicago/Turabian StyleSoethe, Cristina, Isaac de Oliveira Correa, Catherine Amorim, Natalia Maria de Souza, Fernando José Hawerroth, Marcelo Alves Moreira, Mayara Cristiana Stanger, Cassandro Vidal Talamini do Amarante, and Cristiano André Steffens. 2025. "Effects of Single and Split Pre-Harvest Aminoethoxyvinylglycine Applications in Bioactive Compounds and Antioxidant Activity in ‘Baigent’ Apples Under Anti-Hail Nets" Agronomy 15, no. 9: 2152. https://doi.org/10.3390/agronomy15092152
APA StyleSoethe, C., Correa, I. d. O., Amorim, C., de Souza, N. M., Hawerroth, F. J., Moreira, M. A., Stanger, M. C., do Amarante, C. V. T., & Steffens, C. A. (2025). Effects of Single and Split Pre-Harvest Aminoethoxyvinylglycine Applications in Bioactive Compounds and Antioxidant Activity in ‘Baigent’ Apples Under Anti-Hail Nets. Agronomy, 15(9), 2152. https://doi.org/10.3390/agronomy15092152






