Capsicum chinense Jacq. Response to Pyrolysis-Derived Amendments and Sustainable Fertilizers in Containerized Greenhouse Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Conditions
2.2. Plant Material
2.3. Experimental Design
- Biochar + Cropmax (B_C).
- Wood vinegar + Cropmax (WV_C).
- Cropmax as a standalone application (C).
- Untreated control (Unt.).
2.4. Crop Establishment and Management
2.5. Nutrient Management Regimes and Application Parameters
2.6. Metrics
2.7. Statistical Analysis
3. Results
3.1. Vegetative Growth Parameters
3.1.1. Plant Height
3.1.2. Collar Diameter
3.1.3. Branch Count per Plant
3.1.4. Leaf Count per Plant
3.1.5. Reproductive Development
3.2. Anthocyanin and Chlorophyll Pigment Content
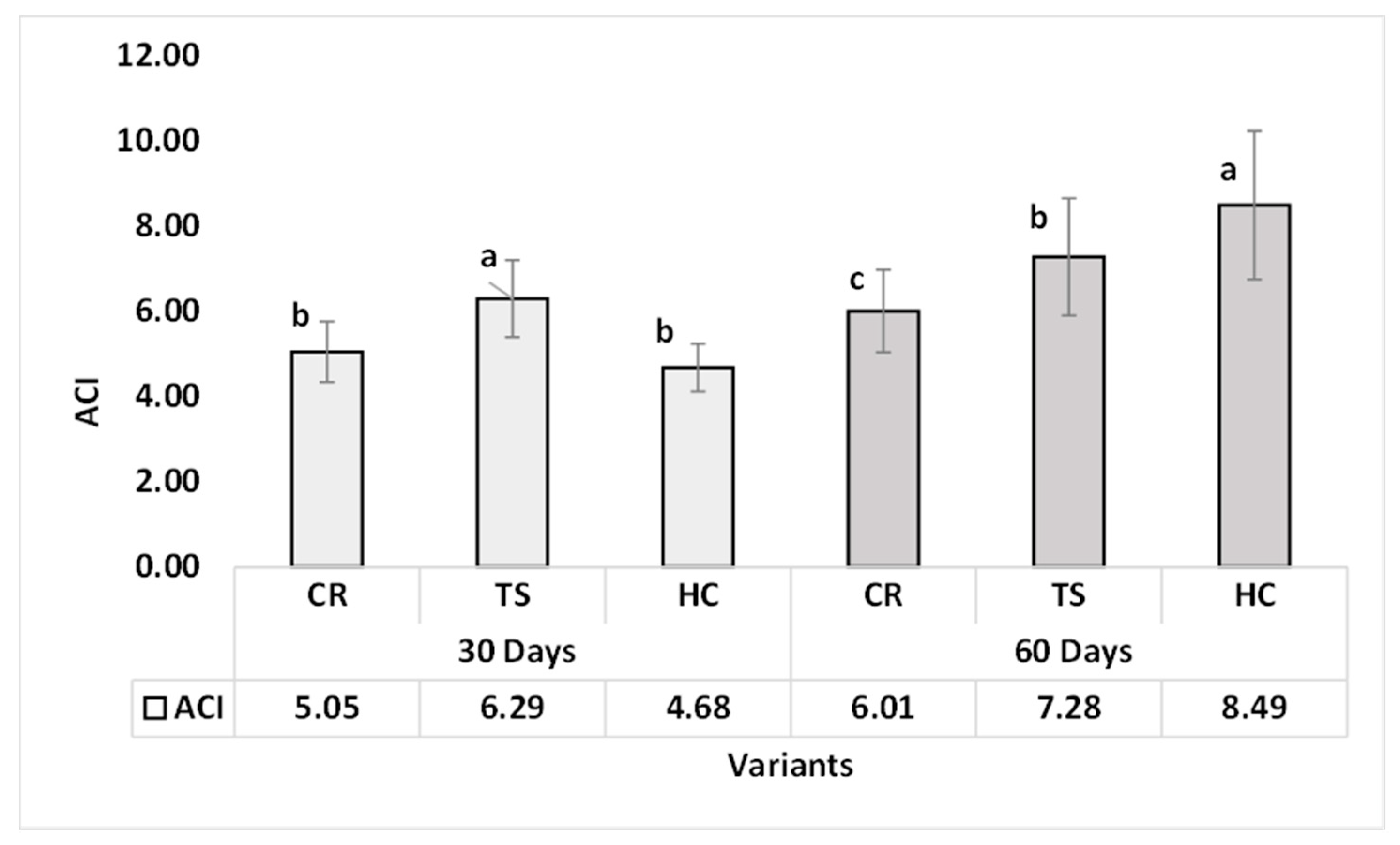
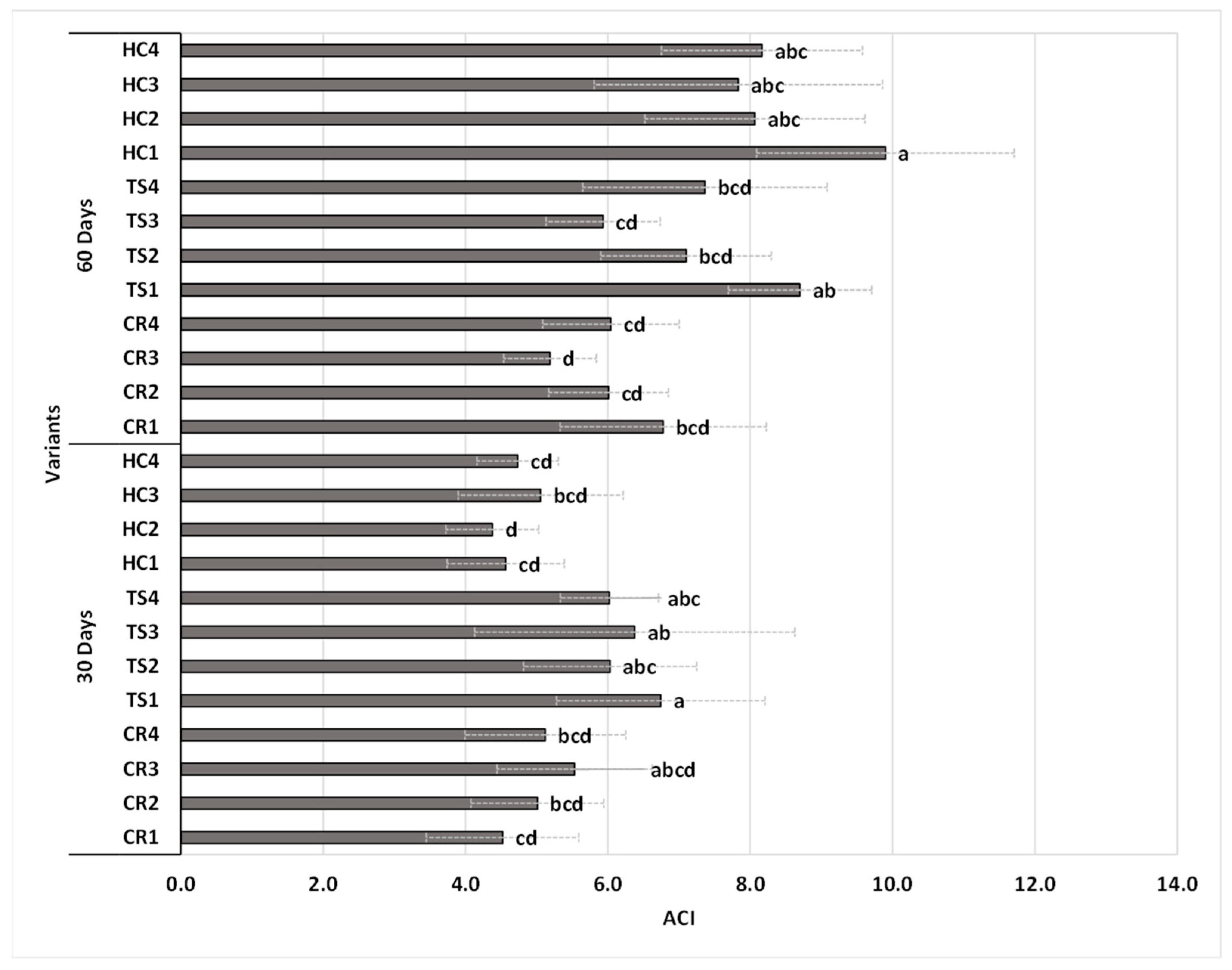
3.2.1. Anthocyanin Pigment Content
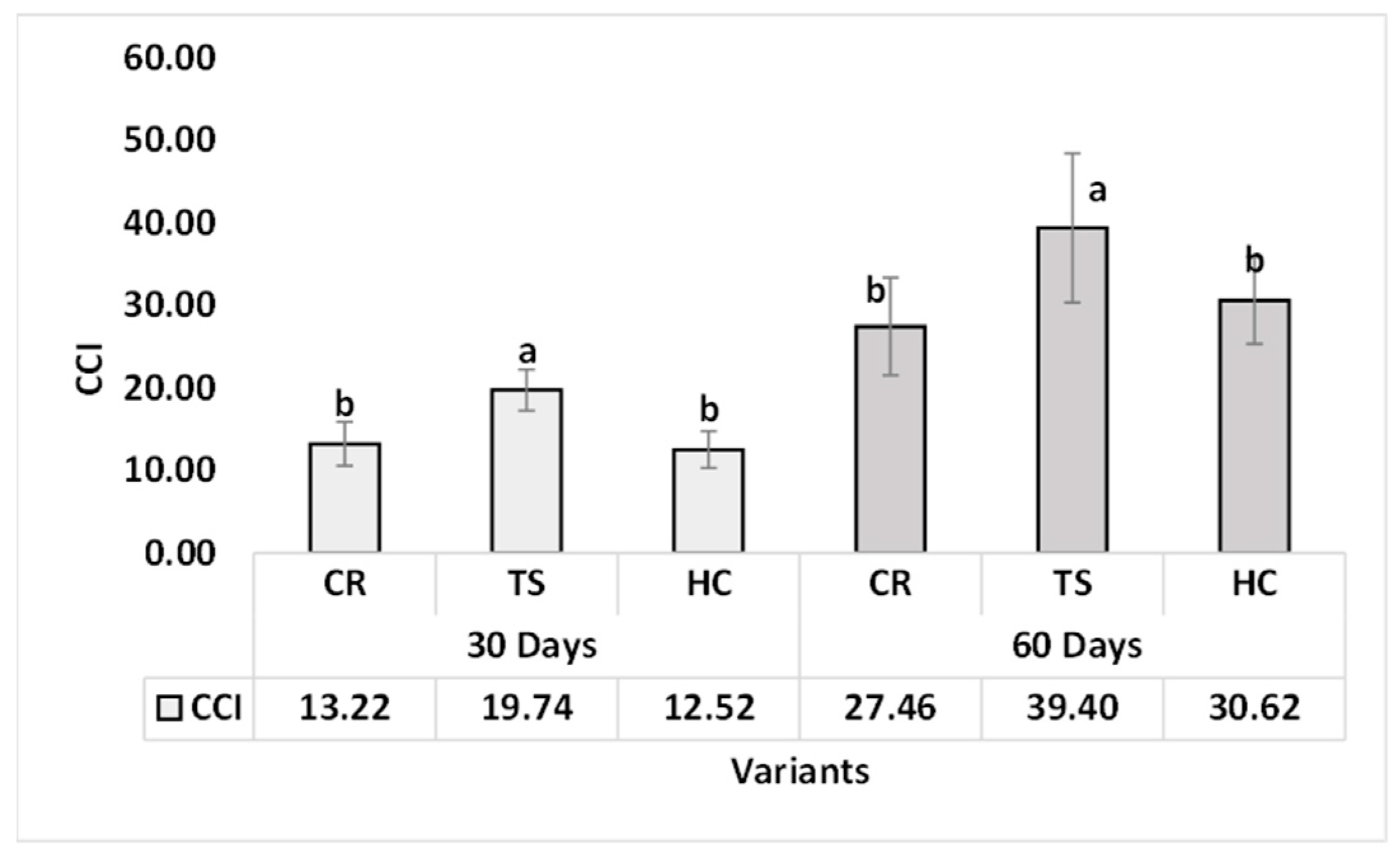
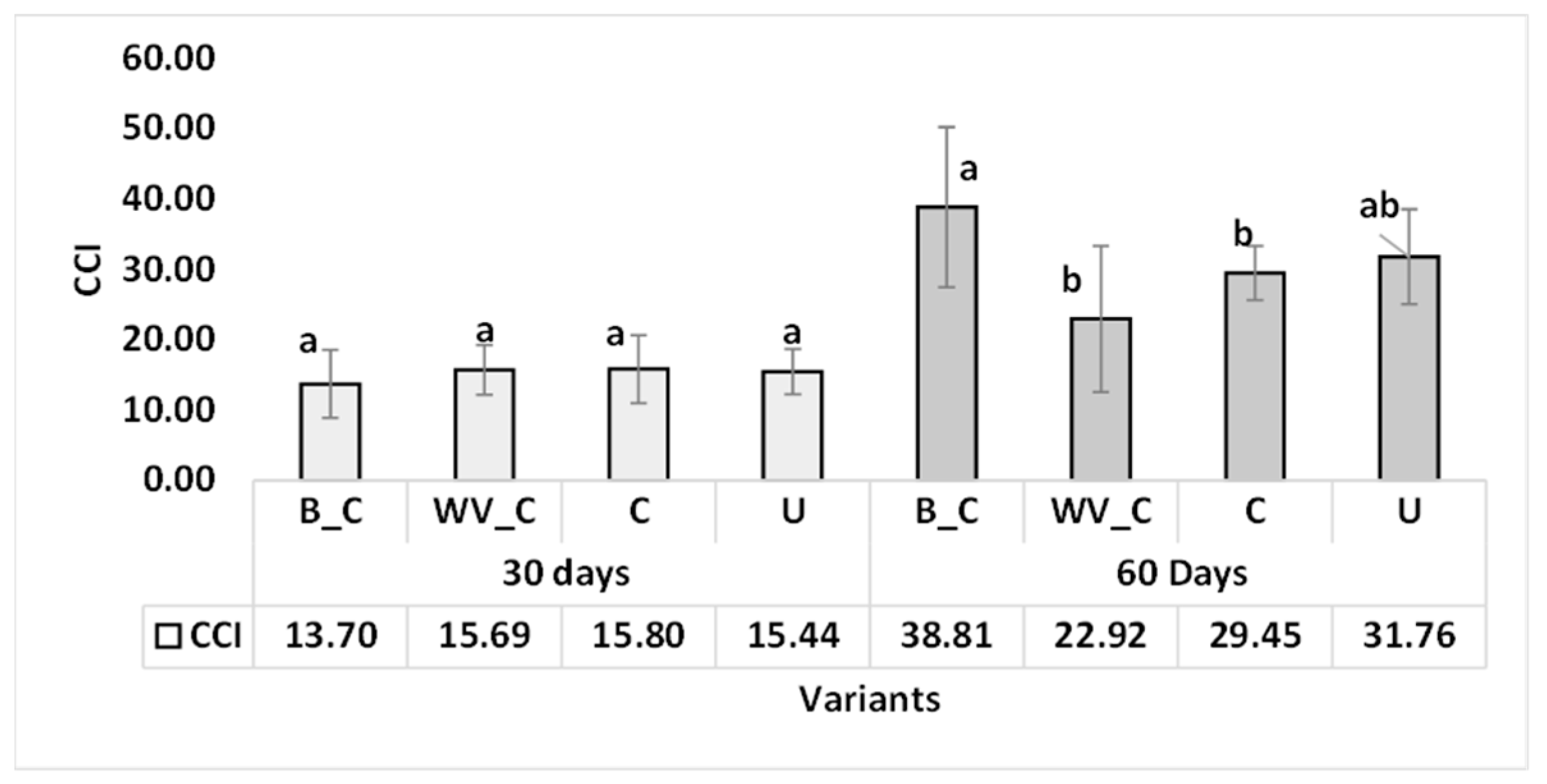
3.2.2. Chlorophyll Pigment Content
3.3. Fruit Yield and Morphometrics
3.4. Seed Metrics and Fruit Pulp Biomass
3.5. Total Dry Matter, Water Content, and Soluble Solids in Chili Pepper Fruits
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çakmakçı, R.; Salık, M.A.; Çakmakçı, S. Assessment and Principles of Environmentally Sustainable Food and Agriculture Systems. Agriculture 2023, 13, 1073. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Lee, D.-J.; Siddique, K.H. Sustainable Agricultural Practices for Food Security and Ecosystem Services. Environ. Sci. Pollut. Res. 2022, 29, 84076–84095. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A. Biological Properties, Bioactive Constituents, and Pharmacokinetics of Some Capsicum spp. and Capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef]
- Alonso-Villegas, R.; González-Amaro, R.M.; Figueroa-Hernández, C.Y.; Rodríguez-Buenfil, I.M. The Genus Capsicum: A Review of Bioactive Properties of Its Polyphenolic and Capsaicinoid Composition. Molecules 2023, 28, 4239. [Google Scholar] [CrossRef]
- Baruah, J.; Lal, M. Capsicum chinense Jacq.: Ethnobotany, Bioactivity and Future Prospects. In Botanical Leads for Drug Discovery; Springer: Singapore, 2020; pp. 349–362. [Google Scholar] [CrossRef]
- Lozada, D.N.; Coon, D.L.; Guzmán, I.; Bosland, P.W. Heat Profiles of ‘Superhot’ and New Mexican Type Chile Peppers (Capsicum spp.). Sci. Hortic. 2021, 283, 110088. [Google Scholar] [CrossRef]
- Saxena, A.; Puranik, N.; Kumari, R.; Verma, S.K. Antimicrobial Activity of Capsaicin and Its Derivatives. In Capsaicinoids: From Natural Sources to Biosynthesis and their Clinical Applications; Springer: Singapore, 2024; pp. 511–528. [Google Scholar] [CrossRef]
- Srivastava, A.; KN, P.; Baliyan, N.; Mangal, M. Capsaicin: Its Sources, Isolation, Quantitative Analysis and Applications. In Capsaicinoids: From Natural Sources to Biosynthesis and their Clinical Applications; Springer: Singapore, 2024; pp. 25–53. [Google Scholar] [CrossRef]
- Floyd, D. The Hot Book of Chilies; Fox Chapel Publishing: Mount Joy, PA, USA, 2019; ISBN 1-62008-377-9. [Google Scholar]
- Bosland, P.W.; Coon, D.; Reeves, G. ‘Trinidad Moruga Scorpion’ Pepper Is the World’s Hottest Measured Chile Pepper at More than Two Million Scoville Heat Units. HortTechnology 2012, 22, 534–538. [Google Scholar] [CrossRef]
- Momo, J.; Islam, K.; Kumar, N.; Ramchiary, N. Molecular Approaches for Breeding Abiotic Stress Tolerance Traits in Capsicum Species. In Genomic Designing for Abiotic Stress Resistant Vegetable Crops; Springer: Berlin/Heidelberg, Germany, 2022; pp. 77–114. [Google Scholar] [CrossRef]
- Gutiérrez, C.L.M.; Medina, D.I.T.; Jaramillo-Flores, M.E. Peppers and Spice Capsicum. In Handbook of Vegetable Preservation and Processing; CRC Press: Boca Raton, FL, USA, 2015; pp. 580–609. [Google Scholar]
- Camposeco-Montejo, N.; Flores-Naveda, A.; Ruiz-Torres, N.; Álvarez-Vázquez, P.; Niño-Medina, G.; Ruelas-Chacón, X.; Torres-Tapia, M.A.; Rodríguez-Salinas, P.; Villanueva-Coronado, V.; García-López, J.I. Agronomic Performance, Capsaicinoids, Polyphenols and Antioxidant Capacity in Genotypes of Habanero Pepper Grown in the Southeast of Coahuila, Mexico. Horticulturae 2021, 7, 372. [Google Scholar] [CrossRef]
- Subhavyuktha, S.; Usha Nandhini Devi, H.; Kumar, K.; Vethamoni, P.I.; Premalatha, N.; Srividhya, S. Employing Empirical Models to Analyze Stability of Yield and Quality Traits in Chili Peppers (Capsicum Species). Crop Sci. 2024, 64, 2977–2997. [Google Scholar] [CrossRef]
- Nidheesh, P.; Gopinath, A.; Ranjith, N.; Akre, A.P.; Sreedharan, V.; Kumar, M.S. Potential Role of Biochar in Advanced Oxidation Processes: A Sustainable Approach. Chem. Eng. J. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Zou, R.; Qian, M.; Wang, C.; Mateo, W.; Wang, Y.; Dai, L.; Lin, X.; Zhao, Y.; Huo, E.; Wang, L. Biochar: From by-Products of Agro-Industrial Lignocellulosic Waste to Tailored Carbon-Based Catalysts for Biomass Thermochemical Conversions. Chem. Eng. J. 2022, 441, 135972. [Google Scholar] [CrossRef]
- Acharya, B.S.; Dodla, S.; Wang, J.J.; Pavuluri, K.; Darapuneni, M.; Dattamudi, S.; Maharjan, B.; Kharel, G. Biochar Impacts on Soil Water Dynamics: Knowns, Unknowns, and Research Directions. Biochar 2024, 6, 34. [Google Scholar] [CrossRef]
- Salma, A.; Fryda, L.; Djelal, H. Biochar: A Key Player in Carbon Credits and Climate Mitigation. Resources 2024, 13, 31. [Google Scholar] [CrossRef]
- Dai, Z.; Xiong, X.; Zhu, H.; Xu, H.; Leng, P.; Li, J.; Tang, C.; Xu, J. Association of Biochar Properties with Changes in Soil Bacterial, Fungal and Fauna Communities and Nutrient Cycling Processes. Biochar 2021, 3, 239–254. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of Microbial Communities to Biochar-Amended Soils: A Critical Review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, S.; Wang, R.; Chen, Y.; Siddique, K.H.; Xia, G.; Chi, D. Ameliorative Roles of Biochar-Based Fertilizer on Morpho-Physiological Traits, Nutrient Uptake and Yield in Peanut (Arachis hypogaea L.) under Water Stress. Agric. Water Manag. 2021, 257, 107129. [Google Scholar] [CrossRef]
- Kundu, B.; Kumar, R. Enhancing Crop Resilience to Climate Change through Biochar: A Review. Int. J. Environ. Clim. Change 2024, 14, 170–184. [Google Scholar] [CrossRef]
- Iacomino, G.; Idbella, M.; Staropoli, A.; Nanni, B.; Bertoli, T.; Vinale, F.; Bonanomi, G. Exploring the Potential of Wood Vinegar: Chemical Composition and Biological Effects on Crops and Pests. Agronomy 2024, 14, 114. [Google Scholar] [CrossRef]
- Zhu, K.; Gu, S.; Liu, J.; Luo, T.; Khan, Z.; Zhang, K.; Hu, L. Wood Vinegar as a Complex Growth Regulator Promotes the Growth, Yield, and Quality of Rapeseed. Agronomy 2021, 11, 510. [Google Scholar] [CrossRef]
- Zhou, H.; Shen, Y.; Zhang, N.; Liu, Z.; Bao, L.; Xia, Y. Wood Fiber Biomass Pyrolysis Solution as a Potential Tool for Plant Disease Management: A Review. Heliyon 2024, 10, e25509. [Google Scholar] [CrossRef]
- Ray, A.; Ganguly, S.; Sankar, A. Biocides through Pyrolytic Degradation of Biomass: Potential, Recent Advancements and Future Prospects. In Biopesticides; Woodhead Publishing: Sawston, UK, 2022; pp. 337–352. [Google Scholar] [CrossRef]
- Josephrajkumar, A.; Mani, M.; Anes, K.; Mohan, C. Ecological Engineering in Pest Management in Horticultural and Agricultural Crops. In Trends in Horticultural Entomology; Springer: Singapore, 2022; pp. 123–155. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Guarnieri, M.; Monaci, F.; Loppi, S. Bio-Based Solutions for Agriculture: Foliar Application of Wood Distillate Alone and in Combination with Other Plant-Derived Corroborants Results in Different Effects on Lettuce (Lactuca sativa L.). Biology 2022, 11, 404. [Google Scholar] [CrossRef]
- Filimon, R.M.; Rotaru, L.; Filimon, V.-R. Effects of Exogenous Growth Regulators on Agrobiological, Technological and Physiological Characteristics of an Interspecific Grapevine Cultivar. Biol. Agric. Hortic. 2023, 39, 91–114. [Google Scholar] [CrossRef]
- Jaiswal, V.; Gahlaut, V.; Kumar, N.; Ramchiary, N. Genetics, Genomics and Breeding of Chili Pepper Capsicum frutescens L. and Other Capsicum Species. In Advances in Plant Breeding Strategies: Vegetable Crops—Volume 9: Fruits and Young Shoots; Springer: Cham, Switzerland, 2021; pp. 59–86. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J. Biosynthesis and Metabolic Actions of Simple Phenolic Acids in Plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- He, L.; Geng, K.; Li, B.; Li, S.; Gustave, W.; Wang, J.; Jeyakumar, P.; Zhang, X.; Wang, H. Enhancement of Nutrient Use Efficiency with Biochar and Wood Vinegar: A Promising Strategy for Improving Soil Productivity. J. Sci. Food Agric. 2025, 105, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Idowu, O.; Ndede, E.O.; Kurebito, S.; Tokunari, T.; Jindo, K. Effect of the Interaction between Wood Vinegar and Biochar Feedstock on Tomato Plants. J. Soil Sci. Plant Nutr. 2023, 23, 1599–1610. [Google Scholar] [CrossRef]
- Cojocariu, M.; Marta, A.E.; Jităreanu, C.D.; Chelariu, E.-L.; Căpşună, S.; Cara, I.G.; Amișculesei, P.; Istrate, A.-M.-R.; Chiruță, C. A Study on the Development of Two Ornamental Varieties of Ipomoea batatas Cultivated in Vertical Systems in the Northeastern Region of Europe. Horticulturae 2024, 10, 133. [Google Scholar] [CrossRef]
- Ma, J.; Islam, F.; Ayyaz, A.; Fang, R.; Hannan, F.; Farooq, M.A.; Ali, B.; Huang, Q.; Sun, R.; Zhou, W. Wood Vinegar Induces Salinity Tolerance by Alleviating Oxidative Damages and Protecting Photosystem II in Rapeseed Cultivars. Ind. Crops Prod. 2022, 189, 115763. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, J.; Luo, T.; Zhang, K.; Khan, Z.; Zhou, Y.; Cheng, T.; Yuan, B.; Peng, X.; Hu, L. Wood Vinegar Impact on the Growth and Low-Temperature Tolerance of Rapeseed Seedlings. Agronomy 2022, 12, 2453. [Google Scholar] [CrossRef]
- Afsharipour, S.; Mirzaalian Dastjerdi, A.; Seyedi, A. Optimizing Cucumis Sativus Seedling Vigor: The Role of Pistachio Wood Vinegar and Date Palm Compost in Nutrient Mobilization. BMC Plant Biol. 2024, 24, 407. [Google Scholar] [CrossRef]
- Zhang, K.; Khan, Z.; Liu, J.; Luo, T.; Zhu, K.; Hu, L.; Bi, J.; Luo, L. Germination and Growth Performance of Water-Saving and Drought-Resistant Rice Enhanced by Seed Treatment with Wood Vinegar and Biochar under Dry Direct-Seeded System. Agronomy 2022, 12, 1223. [Google Scholar] [CrossRef]
- Siedt, M.; Schäffer, A.; Smith, K.E.; Nabel, M.; Roß-Nickoll, M.; Van Dongen, J.T. Comparing Straw, Compost, and Biochar Regarding Their Suitability as Agricultural Soil Amendments to Affect Soil Structure, Nutrient Leaching, Microbial Communities, and the Fate of Pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E. Biochar and Soil Contributions to Crop Lodging and Yield Performance—A Meta-Analysis. Plant Physiol. Biochem. 2024, 215, 109053. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, M.; Chen, H.; Chen, Y.; Wang, L.; Wang, R. Organic Amendments Promote Saline-Alkali Soil Desalinization and Enhance Maize Growth. Front. Plant Sci. 2023, 14, 1177209. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Gu, W.; Feng, Z.; Xiu, L.; Zhang, W.; Chen, W. Long Term Co-application of Biochar and Fertilizer Could Increase Soybean Yield under Continuous Cropping: Insights from Photosynthetic Physiology. J. Sci. Food Agric. 2024, 104, 3113–3122. [Google Scholar] [CrossRef]
- Vâşcă-Zamfir, D.; Pomohaci, M.C.; Gîdea, M. Researches Regarding the Germination Conditions for the Seeds of Species Used in the Lawn Mixtures. Sci. Papers. Ser. B Hortic. 2021, 65, 690–696. [Google Scholar]
- Nounjan, N.; Theerakulpisut, P. Transgenerational Stress Memory and Transgenerational Effects Caused by Wood Vinegar and Spermidine Are Associated with Early Germination of Rice Seeds under Salt Stress. Plant Growth Regul. 2023, 101, 861–874. [Google Scholar] [CrossRef]
- Ofoe, R.; Gunupuru, L.R.; Wang-Pruski, G.; Fofana, B.; Thomas, R.H.; Abbey, L. Seed Priming with Pyroligneous Acid Mitigates Aluminum Stress, and Promotes Tomato Seed Germination and Seedling Growth. Plant Stress 2022, 4, 100083. [Google Scholar] [CrossRef]
- Rodríguez-López, C.; Urrea-López, R.; García-Valencia, L.E.; Valiente-Banuet, J.I.; Trevino, V.; Díaz de la Garza, R.I. Untargeted Metabolomics Unveils the Edaphic Stress Impact on Habanero Pepper Ripening Fruit. ACS Agric. Sci. Technol. 2023, 3, 33–44. [Google Scholar] [CrossRef]
- Hussain, S.; Nanda, S.; Zhang, J.; Rehmani, M.I.A.; Suleman, M.; Li, G.; Hou, H. Auxin and Cytokinin Interplay during Leaf Morphogenesis and Phyllotaxy. Plants 2021, 10, 1732. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Pugazhendhi, A.; Sindhu, R.; Vinayak, V.; Thanh, N.C.; Brindhadevi, K.; Chi, N.T.L.; Yuan, D. An Assessment of Biochar as a Potential Amendment to Enhance Plant Nutrient Uptake. Environ. Res. 2022, 214, 113909. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; He, L.; Ullah, S.; Quan, Z.; Wei, S.; Iqbal, A.; Munsif, F.; Shah, T.; Xuan, Y.; Luo, Y. Biochar Addition Coupled with Nitrogen Fertilization Impacts on Soil Quality, Crop Productivity, and Nitrogen Uptake under Double-cropping System. Food Energy Secur. 2020, 9, e208. [Google Scholar] [CrossRef]
- Guo, L.; Yu, H.; Kharbach, M.; Zhang, W.; Wang, J.; Niu, W. Biochar Improves Soil-Tomato Plant, Tomato Production, and Economic Benefits under Reduced Nitrogen Application in Northwestern China. Plants 2021, 10, 759. [Google Scholar] [CrossRef]
- Pandian, K.; Vijayakumar, S.; Mustaffa, M.R.A.F.; Subramanian, P.; Chitraputhirapillai, S. Biochar—A Sustainable Soil Conditioner for Improving Soil Health, Crop Production and Environment under Changing Climate: A Review. Front. Soil Sci. 2024, 4, 1376159. [Google Scholar] [CrossRef]
- Fallah, N.; Pang, Z.; Lin, Z.; Lin, W.; Mbuya, S.N.; Abubakar, A.Y.; Fabrice, K.M.A.; Zhang, H. Plant Growth and Stress-Regulating Metabolite Response to Biochar Utilization Boost Crop Traits and Soil Health. Front. Plant Sci. 2023, 14, 1271490. [Google Scholar] [CrossRef]
- Haider, F.U.; Khan, I.; Farooq, M.; Cai, L.; Li, Y. Co-Application of Biochar and Plant Growth Regulators Improves Maize Growth and Decreases Cd Accumulation in Cadmium-Contaminated Soil. J. Clean. Prod. 2024, 440, 140515. [Google Scholar] [CrossRef]
- Pang, Z.; Huang, J.; Fallah, N.; Lin, W.; Yuan, Z.; Hu, C. Combining N Fertilization with Biochar Affects Root-Shoot Growth, Rhizosphere Soil Properties and Bacterial Communities under Sugarcane Monocropping. Ind. Crops Prod. 2022, 182, 114899. [Google Scholar] [CrossRef]
- Marin-Recinos, M.F.; Pucker, B. Genetic Factors Explaining Anthocyanin Pigmentation Differences. BMC Plant Biol. 2024, 24, 627. [Google Scholar] [CrossRef]
- Hermanns, A.S.; Zhou, X.; Xu, Q.; Tadmor, Y.; Li, L. Carotenoid Pigment Accumulation in Horticultural Plants. Hortic. Plant J. 2020, 6, 343–360. [Google Scholar] [CrossRef]
- Sunil, L.; Shetty, N.P. Biosynthesis and Regulation of Anthocyanin Pathway Genes. Appl. Microbiol. Biotechnol. 2022, 106, 1783–1798. [Google Scholar] [CrossRef]
- González-Cortés, A.; Robledo-Torres, V.; Luna-García, L.R.; Mendoza-Villarreal, R.; Pérez-Rodríguez, M.Á. Yield and Antioxidant Quality of Habanero Chili Pepper by Supplementing Potassium with Organic Products. Horticulturae 2023, 9, 797. [Google Scholar] [CrossRef]
- Wu, M.; Xu, X.; Hu, X.; Liu, Y.; Cao, H.; Chan, H.; Gong, Z.; Yuan, Y.; Luo, Y.; Feng, B. SlMYB72 Regulates the Metabolism of Chlorophylls, Carotenoids, and Flavonoids in Tomato Fruit. Plant Physiol. 2020, 183, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Variation in Leaf Photosynthetic Capacity within Plant Canopies: Optimization, Structural, and Physiological Constraints and Inefficiencies. Photosynth. Res. 2023, 158, 131–149. [Google Scholar] [CrossRef]
- Hur, G.; Ashraf, M.; Nadeem, M.Y.; Rehman, R.S.; Thwin, H.M.; Shakoor, K.; Seleiman, M.F.; Alotaibi, M.; Yuan, B.-Z. Exogenous Application of Wood Vinegar Improves Rice Yield and Quality by Elevating Photosynthetic Efficiency and Enhancing the Accumulation of Total Soluble Sugars. Plant Physiol. Biochem. 2025, 218, 109306. [Google Scholar] [CrossRef]
- Ray, P.K.; Bharti, P. Biochar: A Quality Enhancer for Fruit Crops. Int. Year Millets 2023, 2, 77–79. [Google Scholar]
- Sharma, S.; Rana, V.S.; Rana, N.; Prasad, H.; Sharma, U.; Patiyal, V. Biochar from Fruit Crops Waste and Its Potential Impact on Fruit Crops. Sci. Hortic. 2022, 299, 111052. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, L.; Wang, M.; Sun, S.; Yang, Y.; Xu, C. Effects of Biochar Application on Tomato Yield and Fruit Quality: A Meta-Analysis. Sustainability 2024, 16, 6397. [Google Scholar] [CrossRef]
- Sharma, R.; Thakur, N. Biochar Applications in Fruit Crop Production: Enhancing Yield, Quality and Sustainability. In Encyclopedia of Agriculture and Allied Sciences; Royal Book Publishing: Salem, India, 2023; Volume 1, pp. 57–70. ISBN 978-93-95423-76-2. [Google Scholar]
- Mhamdi, R. Evaluating the Evolution and Impact of Wood Vinegar Research: A Bibliometric Study. J. Anal. Appl. Pyrolysis 2023, 175, 106190. [Google Scholar] [CrossRef]
- Kocsis, T.; Ringer, M.; Biró, B. Characteristics and Applications of Biochar in Soil–Plant Systems: A Short Review of Benefits and Potential Drawbacks. Appl. Sci. 2022, 12, 4051. [Google Scholar] [CrossRef]
- Haider, F.U.; Coulter, J.A.; Liqun, C.; Hussain, S.; Cheema, S.A.; Jun, W.; Zhang, R. An Overview on Biochar Production, Its Implications, and Mechanisms of Biochar-Induced Amelioration of Soil and Plant Characteristics. Pedosphere 2022, 32, 107–130. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.A.; Hashem, A.; Abd_Allah, E.F. Biochar Production and Characteristics, Its Impacts on Soil Health, Crop Production, and Yield Enhancement: A Review. Plants 2024, 13, 166. [Google Scholar] [CrossRef]
- Ng, C.W.W.; Guo, H.; Ni, J.; Zhang, Q.; Chen, Z. Effects of Soil–Plant-Biochar Interactions on Water Retention and Slope Stability under Various Rainfall Patterns. Landslides 2022, 19, 1379–1390. [Google Scholar] [CrossRef]
- Chahardoli, A.; Jalilian, F.; Memariani, Z.; Farzaei, M.H.; Shokoohinia, Y. Analysis of Organic Acids. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 767–823. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Cerri, M.; Del Buono, D.; Forni, C. Use of Biostimulants as a New Approach for the Improvement of Phytoremediation Performance—A Review. Plants 2022, 11, 1946. [Google Scholar] [CrossRef]
- Harhash, M.M.; Ahamed, M.M.; Mosa, W.F. Mango Performance as Affected by the Soil Application of Zeolite and Biochar under Water Salinity Stresses. Environ. Sci. Pollut. Res. 2022, 29, 87144–87156. [Google Scholar] [CrossRef]
- Duranova, H.; Valkova, V.; Gabriny, L. Chili Peppers (Capsicum spp.): The Spice Not Only for Cuisine Purposes: An Update on Current Knowledge. Phytochem. Rev. 2022, 21, 1379–1413. [Google Scholar] [CrossRef]
- Castañeda, W.; Toro, M.; Solorzano, A.; Zúñiga-Dávila, D. Production and Nutritional Quality of Tomatoes (Solanum lycopersicum var. Cerasiforme) Are Improved in the Presence of Biochar and Inoculation with Arbuscular Mycorrhizae. Am. J. Plant Sci. 2020, 11, 426–436. [Google Scholar] [CrossRef]
- Simiele, M.; Argentino, O.; Baronti, S.; Scippa, G.S.; Chiatante, D.; Terzaghi, M.; Montagnoli, A. Biochar Enhances Plant Growth, Fruit Yield, and Antioxidant Content of Cherry Tomato (Solanum lycopersicum L.) in a Soilless Substrate. Agriculture 2022, 12, 1135. [Google Scholar] [CrossRef]
- Belov, S.V.; Danyleiko, Y.K.; Glinushkin, A.P.; Kalinitchenko, V.P.; Egorov, A.V.; Sidorov, V.A.; Konchekov, E.M.; Gudkov, S.V.; Dorokhov, A.S.; Lobachevsky, Y.P. An Activated Potassium Phosphate Fertilizer Solution for Stimulating the Growth of Agricultural Plants. Front. Phys. 2021, 8, 618320. [Google Scholar] [CrossRef]
- Lopes, J.I.; Arrobas, M.; Raimundo, S.; Gonçalves, A.; Brito, C.; Martins, S.; Pinto, L.; Moutinho-Pereira, J.; Correia, C.M.; Rodrigues, M.Â. Photosynthesis, Yield, Nutrient Availability and Soil Properties after Biochar, Zeolites or Mycorrhizal Inoculum Application to a Mature Rainfed Olive Orchard. Agriculture 2022, 12, 171. [Google Scholar] [CrossRef]
- Wangmo, T.; Dorji, S.; Tobgay, T.; Pelden, T. Effects of Biochar on Yield of Chilli, and Soil Chemical Properties. Asian J. Agric. Ext. Econ. Sociol. 2022, 40, 64–77. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Ma, Y.; Peng, Y.; Fenton, O.; Wang, W.; Zhang, W.; Chen, Q. Unlocking the Potential of Biostimulants Derived from Organic Waste and By-Product Sources: Improving Plant Growth and Tolerance to Abiotic Stresses in Agriculture. Environ. Technol. Innov. 2024, 34, 103571. [Google Scholar] [CrossRef]
- Gao, Y.; Shao, G.; Yang, Z.; Zhang, K.; Lu, J.; Wang, Z.; Wu, S.; Xu, D. Influences of Soil and Biochar Properties and Amount of Biochar and Fertilizer on the Performance of Biochar in Improving Plant Photosynthetic Rate: A Meta-Analysis. Eur. J. Agron. 2021, 130, 126345. [Google Scholar] [CrossRef]
- Huang, L.; Gu, M. Effects of Biochar on Container Substrate Properties and Growth of Plants—A Review. Horticulturae 2019, 5, 14. [Google Scholar] [CrossRef]
- Brtnicky, M.; Dokulilova, T.; Holatko, J.; Pecina, V.; Kintl, A.; Latal, O.; Vyhnanek, T.; Prichystalova, J.; Datta, R. Long-Term Effects of Biochar-Based Organic Amendments on Soil Microbial Parameters. Agronomy 2019, 9, 747. [Google Scholar] [CrossRef]
- Ishfaq, M.; Kiran, A.; ur Rehman, H.; Farooq, M.; Ijaz, N.H.; Nadeem, F.; Azeem, I.; Li, X.; Wakeel, A. Foliar Nutrition: Potential and Challenges under Multifaceted Agriculture. Environ. Exp. Bot. 2022, 200, 104909. [Google Scholar] [CrossRef]
- González-Pernas, F.M.; Grajera-Antolín, C.; García-Cámara, O.; González-Lucas, M.; Martín, M.T.; González-Egido, S.; Aguirre, J.L. Effects of Biochar on Biointensive Horticultural Crops and Its Economic Viability in the Mediterranean Climate. Energies 2022, 15, 3407. [Google Scholar] [CrossRef]
- Ud Din, M.M.; Khan, M.I.; Azam, M.; Ali, M.H.; Qadri, R.; Naveed, M.; Nasir, A. Effect of Biochar and Compost Addition on Mitigating Salinity Stress and Improving Fruit Quality of Tomato. Agronomy 2023, 13, 2197. [Google Scholar] [CrossRef]
- Kannan, S. Foliar Fertilization for Sustainable Crop Production. In Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming; Springer: Dordrecht, The Netherlands, 2010; pp. 371–402. [Google Scholar] [CrossRef]
- Abou-Sreea, A.I.; Azzam, C.R.; Al-Taweel, S.K.; Abdel-Aziz, R.M.; Belal, H.E.; Rady, M.M.; Abdel-Kader, A.A.; Majrashi, A.; Khaled, K.A. Natural Biostimulant Attenuates Salinity Stress Effects in Chili Pepper by Remodeling Antioxidant, Ion, and Phytohormone Balances, and Augments Gene Expression. Plants 2021, 10, 2316. [Google Scholar] [CrossRef]
- Yavaş, İ.; Rahman, M.A.; Hussain, A. Role of Wood Vinegar in Plant Growth Regulation and Abiotic Stress Tolerance: An Overview. In International Research in Agriculture, Forestry and Aquaculture Sciences; Platanus Yayın Grubu: Ankara, Türkiye, 2023; pp. 56–62. [Google Scholar]
- Leifeld, J.; Walz, I. Pyroligneous Acid Effects on Crop Yield and Soil Organic Matter in Agriculture—A Review. Agronomy 2025, 15, 927. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. The Application of Arbuscular Mycorrhizal Fungi as Microbial Biostimulant, Sustainable Approaches in Modern Agriculture. Plants 2023, 12, 3101. [Google Scholar] [CrossRef]
- Yang, L.; Tang, G.; Xu, W.; Zhang, Y.; Ning, S.; Yu, P.; Zhu, J.; Wu, Q.; Yu, P. Effect of Combined Application of Wood Vinegar Solution and Biochar on Saline Soil Properties and Cotton Stress Tolerance. Plants 2024, 13, 2427. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Tang, Y.; Ding, Y.; Zhao, W.; Wang, Q.; Li, Y.; Wang, Q.; Zhang, P.; Tan, Z.; Rui, Y. Synergistic Effect of Nano-Iron Phosphide and Wood Vinegar on Soybean Production and Grain Quality. Environ. Sci. Nano 2024, 11, 4634–4643. [Google Scholar] [CrossRef]
- Rosa, A.C.R.; Verly, L.B.; Miranda, G.S.; Marques, C.F.P.M.; Vieira, G.F.A.; Carvalho, G.A.; Perin, I.L.B.; Caprini, H.O.G.; da Silva, C.R.; Andrade, J.V. Natural Bio-Stimulants for Seed Growth and Development. In Advances in Seed Quality Evaluation and Improvement; Springer Nature: Singapore, 2025; pp. 105–126. [Google Scholar] [CrossRef]
- Di Sario, L.; Boeri, P.; Matus, J.T.; Pizzio, G.A. Plant Biostimulants to Enhance Abiotic Stress Resilience in Crops. Int. J. Mol. Sci. 2025, 26, 1129. [Google Scholar] [CrossRef] [PubMed]




| Variant | Nutrient Input Design | No of Treatments | Dosage/Concentration | Method of Use | Substance Descriptions | Origin |
|---|---|---|---|---|---|---|
| V1 | Biochar | 1 | 10 g plant−1 | uniform deposition at soil interface, shallowly incorporated at transplanting | bulk density 276 kg m−3, BET 557.76 m2 g−1, 91.3% C, 0.66% N, 0.25% K, pH 8.76; | Gekka Biochar/Explocom GK SRL—Romania |
| Cropmax | 2 | 0.25% | foliar spray at transplanting and 1 month later | pH 7.00; 17 amino acids with reported values (‰, g/L): asparagine 26, glutamine 17, alanine 12, valine 6, isoleucine 4, leucine 5, serine 4, threonine 4, proline 4, glycine 5, phenylalanine 3, tyrosine 3, lysine 3, histidine 1, arginine 1, methionine 1, cystine 1; vitamins and antioxidants including ascorbic acid, tocopherols, and carotenoids (qualitative); macronutrients N 0.20%, P 0.40%, K 0.02%; micronutrients Fe 0.0220%, Mg 0.0550%, Zn 0.0049%, Mn 0.0054%, Cu 0.0035%, B 0.0070%, Ca 0.0010%, Mo 0.0010%, Co 0.0010%, Ni 0.0010%; auxins, cytokinins, and gibberellins (qualitatively confirmed, concentrations proprietary) | Holland Farming B.V.—The Netherlands | |
| V2 | Wood Vinegar | 2 | 0.5% | foliar spray twice during growth | 14 g L−1 C, 3.37 mg dm−3 N, pH 4.24, contains acetic and pyroligneous acids | Gekka Biochar/Explocom GK SRL—Romania |
| Cropmax | 2 | 0.25% | as in V1 | as in V1 | as in V1 | |
| V3 | Cropmax | 2 | 0.25% | foliar spray twice during growth | as in V1 | as in V1 |
| V4 | Untreated (Control) | - | - | - | - | - |
| Variant | Total Height (cm) | Stem Collar Diameter (mm) | No of Branches·Plant−1 | No of Leaves Plant−1 | No of Flowers·Plant−1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30 Days | 60 Days | 30 Days | 60 Days | 30 Days | 60 Days | 30 Days | 60 Days | 30 Days | 60 Days | |
| Cultivar | ||||||||||
| CR | 13.43 ± 1.86 a | 44.17 ± 3.66 a | 4.53 ± 0.74 a | 9.22 ± 1.58 b | - | 4.00 ± 1.04 a | 15.83 ± 1.70 a | 78.50 ± 12.42 a | 0.42 ± 0.79 b | 70.25 ± 24.60 a |
| TS | 9.40 ± 1.64 b | 38.83 ± 6.18 b | 3.73 ± 0.64 b | 10.27 ± 0.96 a | - | 4.17 ± 0.39 a | 16.42 ± 1.00 a | 84.67 ± 18.41 a | 2.00 ± 1.91 a | 79.33 ± 14.37 a |
| HC | 12.79 ± 1.27 a | 42.92 ± 5.81 ab | 4.32 ± 0.80 ab | 8.84 ± 0.95 b | - | 2.67 ± 0.98 b | 13.08 ± 2.15 b | 74.17 ± 13.90 a | 0.00 b | 40.58 ± 15.15 b |
| Fertilization type | ||||||||||
| B_C | 13.13 ± 1.83 a | 46.11 ± 5.81 a | 4.37 ± 0.71 a | 9.40 ± 1.67 a | - | 3.67 ± 1.32 a | 15.11 ± 1.27 a | 88.22 ± 15.99 a | 0.33 ± 0.71 a | 59.89 ± 23.48 a |
| WV_C | 12.42 ± 2.66 ab | 42.67 ± 3.61 ab | 4.19 ± 0.63 a | 9.41 ± 1.44 a | - | 3.78 ± 0.67 a | 15.00 ± 2.50 a | 82.11 ± 15.33 ab | 0.89 ± 1.62 a | 62.00 ± 26.66 a |
| C | 11.37 ± 2.17 ab | 40.33 ± 4.90 b | 4.70 ± 0.91 a | 9.54 ± 0.79 a | - | 3.67 ± 1.00 a | 14.89 ± 2.52 a | 75.78 ± 10.84 ab | 1.22 ± 1.99 a | 63.11 ± 22.00 a |
| Unt. | 10.57 ± 2.28 b | 38.78 ± 5.95 b | 3.52 ± 0.39 b | 9.44 ± 1.45 a | 3.33 ± 1.32 a | 15.44 ± 2.60 a | 70.33 ± 14.71 b | 0.78 ± 1.30 a | 68.56 ± 29.52 a | |
| Variant | Total Height (cm) | Stem Collar Diameter (mm) | No of Branches·Plant−1 | No of Leaves·Plant−1 | No of Flowers·Plant−1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30 Days | 60 Days | 30 Days | 60 Days | 30 Days | 60 Days | 30 Days | 60 Days | 30 Days | 60 Days | |
| CR1 | 14.50 ± 0.87 a | 45.00 ± 3.61 ab | 4.78 ± 0.52 ab | 7.92 ± 0.35 c | - | 3.67 ± 1.53 abc | 15.00 ± 1.15 abcd | 79.00 ± 6.93 b | 0.67 ± 1.15 b | 52.67 ± 9.87 ab |
| CR2 | 14.87 ± 0.23 a | 45.67 ± 1.5 ab | 4.53 ± 0.64 abc | 9.33 ± 2.33 abc | - | 4.00 ± 0.00 ab | 15.67 ± 2.89 abc | 80.67 ± 7.23 b | 0.33 ± 0.58 b | 64.33 ± 27.10 ab |
| CR3 | 12.67 ± 1.53 ab | 44.67 ± 2.3 ab | 5.08 ± 0.77 a | 9.72 ± 0.96 abc | - | 4.33 ± 0.58 ab | 16.33 ± 1.53 ab | 80.67 ± 4.16 b | 0.67 ± 1.15 b | 80.00 ± 24.98 a |
| CR4 | 11.67 ± 2.36 b | 41.33 ± 6.0 abc | 3.73 ± 0.50 bc | 9.92 ± 1.89 abc | - | 4.00 ± 1.73 ab | 16.33 ± 1.53 ab | 73.67 ± 26.10 b | 0.00 b | 84.00 ± 30.51 a |
| TS1 | 11.23 ± 1.66 bc | 43.67 ± 8.3 ab | 3.88 ± 0.81 abc | 11.30 ± 0.26 a | - | 4.67 ± 0.58 a | 16.00 ± 1.00 abc | 108.00 ± 9.54 a | 0.33 ± 0.58 b | 83.00 ± 17.58 a |
| TS2 | 9.10 ± 0.95 cd | 42.67 ± 3.2 abc | 3.74 ± 0.46 bc | 10.33 ± 0.63 ab | - | 4.00 ± 0.00 ab | 16.00 ± 1.00 abc | 91.00 ± 7.55 ab | 2.33 ± 2.31 ab | 86.33 ± 7.02 a |
| TS3 | 8.97 ± 1.53 cd | 34.67 ± 2.31 c | 3.98 ± 0.97 abc | 9.25 ± 0.42 abc | - | 4.00 ± 0.00 ab | 16.33 ± 1.15 ab | 68.33 ± 9.07 b | 3.00 ± 2.65 a | 65.33 ± 14.57 ab |
| TS4 | 8.30 ± 1.27 d | 34.33 ± 3.21 c | 3.33 ± 0.25 c | 10.21 ± 1.12 ab | - | 4.00 ± 0.00 ab | 17.33 ± 0.58 a | 71.33 ± 9.45 b | 2.33 ± 1.15 ab | 82.67 ± 12.70 a |
| HC1 | 13.67 ± 1.15 ab | 49.67 ± 4.7 a | 4.45 ± 0.70 abc | 8.97 ± 1.38 bc | - | 2.67 ± 1.15 bc | 14.33 ± 1.53 abcd | 77.67 ± 1.53 b | 0.00 b | 44.00 ± 23.26 b |
| HC2 | 13.30 ± 0.82 ab | 39.67 ± 3.5 bc | 4.30 ± 0.68 abc | 8.56 ± 0.26 bc | - | 3.33 ± 1.15 abc | 13.33 ± 3.06 bcd | 74.67 ± 25.01 b | 0.00 b | 35.33 ± 9.87 b |
| HC3 | 12.47 ± 1.08 ab | 41.67 ± 2.5 abc | 5.04 ± 0.78 a | 9.64 ± 1.09 abc | - | 2.67 ± 1.15 bc | 12.00 ± 1.73 d | 78.33 ± 18.25 b | 0.00 b | 44.00 ± 10.82 b |
| HC4 | 11.73 ± 1.50 b | 40.67 ± 7.0 bc | 3.50 ± 0.40 c | 8.20 ± 0.31 bc | - | 2.00 ± 0.00 c | 12.67 ± 2.52 cd | 66.00 ± 7.00 b | 0.00 b | 39.00 ± 20.66 b |
| Variant | No of Fruits/Plant | Average Fruit Weight (g) | Yield/Plant (g) | Fruit Width (mm) | Fruit Height (mm) | Fruit Form Index (H/W) | Peduncle Weight (g) |
|---|---|---|---|---|---|---|---|
| Cultivar | |||||||
| CR | 16.25 ± 2.26 b | 4.72 ± 1.77 c | 77.85 ± 33.86 b | 25.15 ± 6.10 b | 40.52 ± 7.82 a | 1.68 ± 0.43 a | 0.12 ± 0.05 a |
| TS | 20.33 ± 7.34 a | 8.31 ± 1.46 a | 174.34 ± 87.68 a | 30.28 ± 2.84 a | 36.88 ± 4.24 a | 1.22 ± 0.11 b | 0.16 ± 0.05 a |
| HC | 15.00 ± 3.51 b | 6.86 ± 1.32 b | 104.12 ± 34.1 b | 29.46 ± 5.08 a | 42.42 ± 6.76 a | 1.46 ± 0.22 a | 0.11 ± 0.05 a |
| Fertilization type | |||||||
| B_C | 22.22 ± 7.12 a | 7.92 ± 2.13 a | 183.22 ± 98.0 a | 32.88 ± 5.00 a | 43.43 ± 5.27 a | 1.34 ± 0.21 a | 0.17 ± 0.04 a |
| WV_C | 17.56 ± 2.74 b | 6.61 ± 2.40 ab | 117.33 ± 49.64 b | 26.63 ± 5.14 b | 36.37 ± 4.75 b | 1.42 ± 0.40a | 0.14 ± 0.05 ab |
| C | 15.22 ± 1.99 b | 6.34 ± 1.73 ab | 96.03 ± 26.98 b | 27.12 ± 4.37 b | 41.29 ± 8.91 ab | 1.56 ± 0.43 a | 0.13 ± 0.04 ab |
| Unt. | 13.78 ± 3.63 b | 5.67 ± 1.76 b | 78.48 ± 35.77 b | 26.56 ± 4.28 b | 38.64 ± 5.80 ab | 1.48 ± 0.28 a | 0.09 ± 0.05 b |
| Variant | No of Fruits/Plant | Average Fruit Weight (g) | Yield/Plant (g) | Fruit Width (mm) | Fruit Height (mm) | Fruit Form Index (H/W) | Peduncle Weight (g) |
|---|---|---|---|---|---|---|---|
| CR1 | 17.67 ± 0.58 bc | 6.21 ± 1.52 bc | 109.72 ± 29.93 bcde | 31.04 ± 8.14 ab | 39.28 ± 3.38 b | 1.34 ± 0.39 bcd | 0.16 ± 0.03 ab |
| CR2 | 17.33 ± 2.52 bc | 3.61 ± 1.49 d | 64.019 ± 33.84 de | 20.97 ± 4.26 c | 36.11 ± 7.42 b | 1.78 ± 0.52 ab | 0.07 ± 0.05 cd |
| CR3 | 16.33 ± 2.52 bc | 5.46 ± 1.82 cd | 88.53 ± 28.15 cde | 24.29 ± 6.51 bc | 49.50 ± 10.22 a | 2.07 ± 0.26 a | 0.16 ± 0.06 ab |
| CR4 | 13.67 ± 0.58 bc | 3.62 ± 1.26 d | 49.12 ± 15.86 e | 24.28 ± 2.99 bc | 37.19 ± 5.33 b | 1.53 ± 0.27 bcd | 0.10 ± 0.03 bcd |
| TS1 | 29.33 ± 9.02 a | 10.29 ± 1.39 a | 298.08 ± 86.19 a | 31.95 ± 3.33 ab | 41.05 ± 4.89 ab | 1.29 ± 0.08 cd | 0.20 ± 0.06 a |
| TS2 | 20.00 ± 1.00 b | 8.23 ± 1.38 ab | 164.87 ± 19.35 b | 28.69 ± 4.92 ab | 36.07 ± 4.10 b | 1.27 ± 0.14 d | 0.17 ± 0.06 ab |
| TS3 | 15.67 ± 1.53 bc | 7.55 ± 1.18 bc | 118.14 ± 16.17 bcd | 29.37 ± 4.40 ab | 33.39 ± 3.07 b | 1.14 ± 0.06 d | 0.13 ± 0.04 abc |
| TS4 | 16.33 ± 5.77 bc | 7.08 ± 2.58 bc | 116.25 ± 36.35 bcd | 31.13 ± 3.04 ab | 37.01 ± 7.51 b | 1.18 ± 0.13 d | 0.13 ± 0.05 abc |
| HC1 | 19.67 ± 2.08 b | 7.25 ± 1.42 bc | 141.88 ± 10.48 bc | 35.65 ± 3.81 a | 49.97 ± 4.82 a | 1.40 ± 0.01 bcd | 0.14 ± 0.06 abc |
| HC2 | 15.33 ± 2.52 bc | 8.00 ± 0.43 b | 123.12 ± 25.18 bcd | 30.23 ± 2.41 ab | 36.92 ± 7.99 b | 1.22 ± 0.21 d | 0.17 ± 0.01 ab |
| HC3 | 13.67 ± 1.15 bc | 6.01 ± 2.22 bc | 81.42 ± 26.85 cde | 27.71 ± 4.98 bc | 40.99 ± 6.68 ab | 1.48 ± 0.06 bcd | 0.11 ± 0.05 bcd |
| HC4 | 11.33 ± 0.58 c | 6.19 ± 0.04 bc | 70.05 ± 2.29 de | 24.28 ± 3.04 bc | 41.74 ± 2.41 ab | 1.73 ± 0.09 abc | 0.04 ± 0.03 d |
| Variant | No. of Seeds/Fruit | Total Seed Weight (g) | Seedless Fruit Pulp Weight (g) |
|---|---|---|---|
| Cultivar | |||
| CR | 19.00 ± 7.51 c | 0.16 ± 0.05 c | 4.70 ± 1.46 b |
| TS | 47.08 ± 9.88 a | 0.41 ± 0.12 a | 7.61 ± 1.37 a |
| HC | 30.33 ± 12.35 b | 0.28 ± 0.14 b | 4.58 ± 1.44 b |
| Fertilization type | |||
| B_C | 38.33 ± 18.33 a | 0.35 ± 0.21 a | 7.07 ± 1.99 a |
| WV_C | 28.78 ± 13.12 a | 0.27 ± 0.13 a | 5.62 ± 1.58 ab |
| C | 31.56 ± 13.80 a | 0.28 ± 0.12 a | 5.54 ± 1.36 ab |
| Unt. | 29.89 ± 16.10 a | 0.23 ± 0.13 a | 4.31 ± 2.15 b |
| Variant | No of Seeds/Fruit | Total Seed Weight (g) | Seedless Fruit Pulp Weight (g) |
|---|---|---|---|
| CR1 | 20.00 ± 5.00 c | 0.14 ± 0.07 e | 6.28 ± 1.34 bcd |
| CR2 | 16.33 ± 7.81 c | 0.15 ± 0.11 e | 4.43 ± 0.47 def |
| CR3 | 24.33 ± 14.94 c | 0.22 ± 0.18 cde | 4.84 ± 1.46 cde |
| CR4 | 15.33 ± 10.89 c | 0.13 ± 0.10 e | 3.25 ± 1.36 ef |
| TS1 | 49.67 ± 19.03 a | 0.48 ± 0.20 a | 9.39 ± 1.18 a |
| TS2 | 43.00 ± 10.84 ab | 0.41 ± 0.13 abc | 7.46 ± 1.25 b |
| TS3 | 46.33 ± 8.77 a | 0.38 ± 0.10 abcd | 6.81 ± 1.08 bc |
| TS4 | 49.33 ± 14.69 a | 0.38 ± 0.14 abcd | 6.80 ± 1.87 bc |
| HC1 | 45.33 ± 15.56 a | 0.45 ± 0.18 ab | 5.52 ± 1.12 bcd |
| HC2 | 27.00 ± 16.11 bc | 0.25 ± 0.15 bcde | 4.97 ± 1.45 cde |
| HC3 | 24.00 ± 13.89 c | 0.23 ± 0.18 cde | 4.97 ± 1.54 cde |
| HC4 | 25.00 ± 2.83 c | 0.19 ± 0.09 de | 2.87 ± 0.95 f |
| Variant | Total Dry Matter Content (%) | Water Content (%) | Soluble Solids Content (°Brix) |
|---|---|---|---|
| Cultivar | |||
| CR | 7.70 ± 0.69 a | 92.30 ± 0.69 a | 6.50 ± 0.52 b |
| TS | 8.46 ± 2.25 a | 91.54 ± 2.25 a | 7.67 ± 0.94 a |
| HC | 8.23 ± 1.48 a | 91.77 ± 1.48 a | 7.25 ± 0.94 a |
| Fertilization type | |||
| B_C | 9.57 ± 0.80 a | 90.43 ± 0.80 b | 8.08 ± 0.74 a |
| WV_C | 9.50 ± 1.26 a | 90.50 ± 1.26 b | 6.32 ± 0.44 c |
| C | 6.71 ± 0.52 b | 93.29 ± 0.52 a | 6.89 ± 0.47 bc |
| Unt. | 6.75 ± 0.60 b | 93.25 ± 0.60 a | 7.27 ± 1.03 b |
| Variant | Total Dry Matter Content (%) | Water Content (%) | Soluble Solids Content (°Brix) |
|---|---|---|---|
| CR1 | 8.62 ± 0.02 d | 91.38 ± 0.02 c | 7.18 ± 0.30 bc |
| CR2 | 8.12 ± 0.03 d | 91.88 ± 0.03 c | 5.98 ± 0.30 f |
| CR3 | 7.02 ± 0.05 e | 92.98 ± 0.05 b | 6.53 ± 0.25 de |
| CR4 | 7.04 ± 1.04 e | 92.95 ± 1.04 b | 6.28 ± 0.30 ef |
| TS1 | 10.32 ± 0.03 b | 89.69 ± 0.03 e | 8.35 ± 0.25 a |
| TS2 | 10.84 ± 0.02 a | 89.16 ± 0.02 f | 6.35 ± 0.41 ef |
| TS3 | 6.04 ± 0.02 f | 93.96 ± 0.02 a | 7.43 ± 0.26 b |
| TS4 | 6.67 ± 0.02 e | 93.33 ± 0.02 b | 8.55 ± 0.25 a |
| HC1 | 9.79 ± 0.01 c | 90.21 ± 0.01 d | 8.73 ± 0.25 a |
| HC2 | 9.54 ± 0.01 c | 90.46 ± 0.01 d | 6.62 ± 0.46 de |
| HC3 | 7.06 ± 0.02 e | 92.94 ± 0.02 b | 6.70 ± 0.26 cde |
| HC4 | 6.53 ± 0.09 ef | 93.47 ± 0.09 ab | 6.97 ± 0.21 bcd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avasiloaiei, D.I.; Calara, M.; Brezeanu, P.M.; Bălăiță, C.; Brumă, I.S.; Brezeanu, C. Capsicum chinense Jacq. Response to Pyrolysis-Derived Amendments and Sustainable Fertilizers in Containerized Greenhouse Systems. Agronomy 2025, 15, 2125. https://doi.org/10.3390/agronomy15092125
Avasiloaiei DI, Calara M, Brezeanu PM, Bălăiță C, Brumă IS, Brezeanu C. Capsicum chinense Jacq. Response to Pyrolysis-Derived Amendments and Sustainable Fertilizers in Containerized Greenhouse Systems. Agronomy. 2025; 15(9):2125. https://doi.org/10.3390/agronomy15092125
Chicago/Turabian StyleAvasiloaiei, Dan Ioan, Mariana Calara, Petre Marian Brezeanu, Claudia Bălăiță, Ioan Sebastian Brumă, and Creola Brezeanu. 2025. "Capsicum chinense Jacq. Response to Pyrolysis-Derived Amendments and Sustainable Fertilizers in Containerized Greenhouse Systems" Agronomy 15, no. 9: 2125. https://doi.org/10.3390/agronomy15092125
APA StyleAvasiloaiei, D. I., Calara, M., Brezeanu, P. M., Bălăiță, C., Brumă, I. S., & Brezeanu, C. (2025). Capsicum chinense Jacq. Response to Pyrolysis-Derived Amendments and Sustainable Fertilizers in Containerized Greenhouse Systems. Agronomy, 15(9), 2125. https://doi.org/10.3390/agronomy15092125









