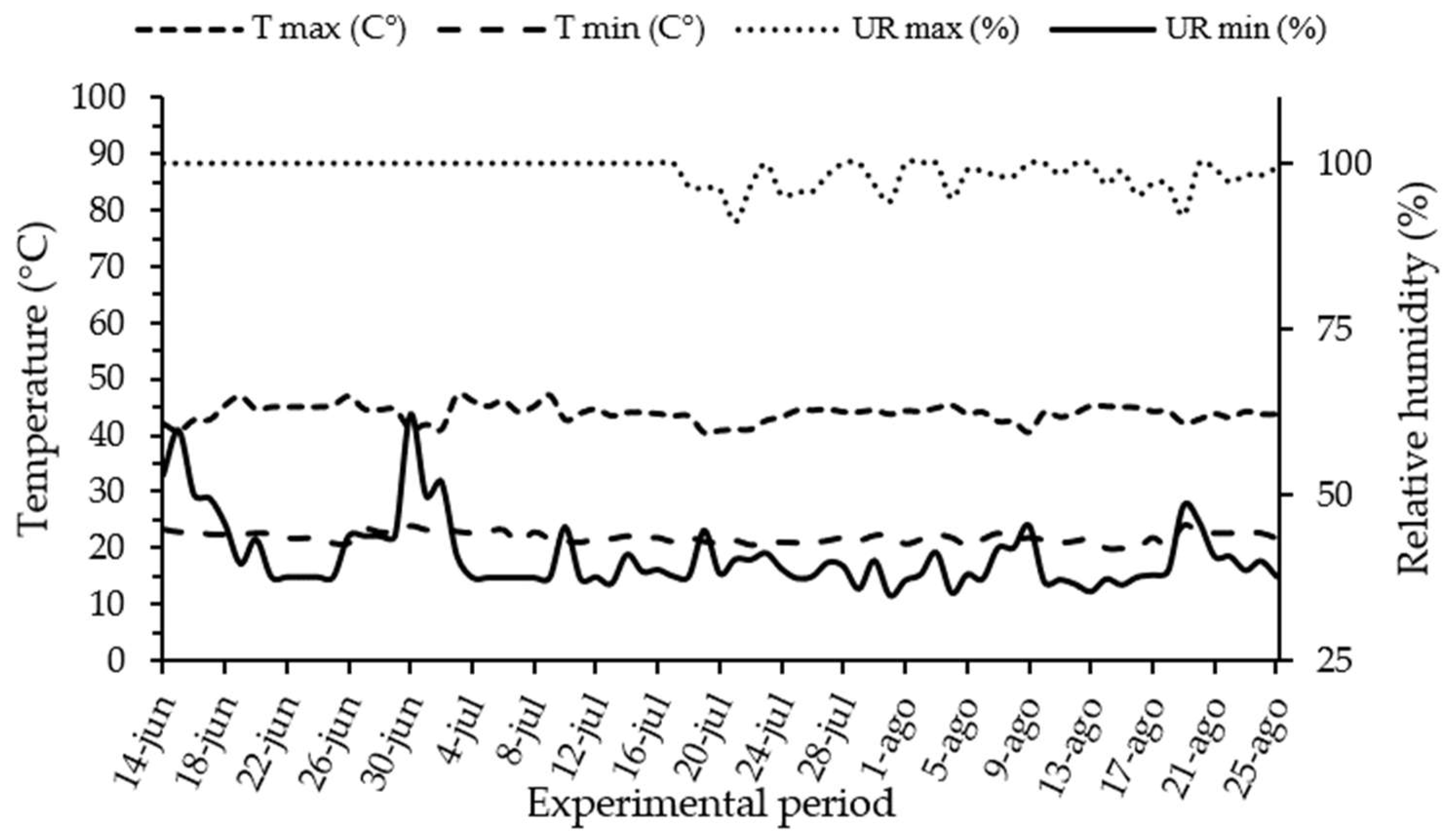
Figure 1.
Temperature and relative humidity data from June to August 2023. Source: Data provided by the authors, 2024.
Figure 1.
Temperature and relative humidity data from June to August 2023. Source: Data provided by the authors, 2024.
Figure 2.
Distribution of the pots and the irrigation system in the greenhouse to grow the cowpea varieties.
Figure 2.
Distribution of the pots and the irrigation system in the greenhouse to grow the cowpea varieties.
Figure 3.
Irrigation volume for each salinity throughout the experiment. Source: Data provided by the authors, 2024.
Figure 3.
Irrigation volume for each salinity throughout the experiment. Source: Data provided by the authors, 2024.
Table 1.
Chemical characterization of the irrigation water sources.
Table 1.
Chemical characterization of the irrigation water sources.
| Water | pH | EC | K+ | Na+ | Ca2+ | Mg2+ | Cl− | CO32− | HCO3− | SAR 2 | Hardness |
|---|
| Sources 1 | dS m−1 | --------------------mmolc L−1-------------------- | | mg L−1 |
|---|
| Saline reject | 7.28 | 7.40 | 0.42 | 27.98 | 20.30 | 17.05 | 67.00 | 0.07 | 1.72 | 6.47 | 1867.50 |
| S1 | 7.65 | 0.54 | 0.22 | 3.72 | 0.63 | 0.41 | 2.40 | 0.68 | 3.11 | 5.18 | 51.75 |
| S2 | 7.68 | 3.50 | 0.30 | 13.68 | 8.00 | 6.90 | 26.00 | 0.19 | 1.28 | 5.01 | 745.00 |
| S3 | 7.70 | 5.00 | 0.35 | 18.30 | 13.15 | 9.00 | 41.00 | 0.10 | 2.14 | 5.50 | 1107.50 |
Table 2.
Salt volume added to the soil through irrigation water at each salinity level.
Table 2.
Salt volume added to the soil through irrigation water at each salinity level.
| Salinity | Irrigation Volume | Salts Added via Irrigation |
|---|
| L per Pot | g per Pot |
|---|
| S1 | 46.388 | 14.844 |
| S2 | 30.221 | 67.695 |
| S3 | 23.195 | 74.224 |
Table 3.
Electrical conductivity of the saturation extract (ECse) was standardized at the beginning of the experiment, and the ECse reached 71 DAS, resulting from irrigation water salinity.
Table 3.
Electrical conductivity of the saturation extract (ECse) was standardized at the beginning of the experiment, and the ECse reached 71 DAS, resulting from irrigation water salinity.
| Salinity | ECse of the Soil (dS m−1) |
|---|
| 1 DAS | 71 DAS |
|---|
| S1 | 0.83 | 2.07 |
| S2 | 0.83 | 9.32 |
| S3 | 0.83 | 15.38 |
Table 4.
Summary of the analysis of variance for number of pods per plant (NPP), pod length (PL, cm), average seed weight (ASW, g), number of locules per pod (NLP), empty locules (EL), number of seeds per pod (NSP), seed length (SL, mm), seed width (SW, mm), seed thickness (ST, mm), and 100-seed weight (SW100, g).
Table 4.
Summary of the analysis of variance for number of pods per plant (NPP), pod length (PL, cm), average seed weight (ASW, g), number of locules per pod (NLP), empty locules (EL), number of seeds per pod (NSP), seed length (SL, mm), seed width (SW, mm), seed thickness (ST, mm), and 100-seed weight (SW100, g).
| FV2 | F-Test (Pr > Fc) |
|---|
| NPP | PL | ASW | NLP | EL |
|---|
| Sal | 0.0000 *** | 0.1096 NS | 0.1096 NS | 0.0022 * | 0.0150 * |
| Var | 0.9860 NS | 0.0001 *** | 0.0001 * | 0.0000 *** | 0.0000 * |
| Dose | 0.3866 NS | 0.4207 NS | 0.4207 NS | 0.1323 NS | 0.5681 NS |
| Sal × Var | 0.8038 NS | 0.0511 # | 0.0511 # | 0.2268 NS | 0.0525 # |
| Sal × Dose | 0.3183 NS | 0.3297 NS | 0.3297 NS | 0.0144 * | 0.8039 NS |
| Var × Dose | 0.3163 NS | 0.5468 NS | 0.5468 NS | 0.1759 NS | 0.5841 NS |
| Sal × Var × Dose | 0.1826 NS | 0.0522 # | 0.0522 # | 0.1350 NS | 0.5973 NS |
| Block | 0.1959 NS | 0.5299 NS | 0.5299 NS | 0.0390 * | 0.8389 NS |
| CV (%) | 27.69 | 8.53 | 18.21 | 10.46 | 49.1 |
| FV2 | F-test (Pr > Fc) |
| NSP | SL | SW | ST | SW100 |
| Sal | 0.0001 *** | 0.0284 * | 0.0000 *** | 0.0003 *** | 0.6167 NS |
| Var | 0.7947 NS | 0.0000 *** | 0.0000 *** | 0.0076 ** | 0.6651 NS |
| Dose | 0.2538 NS | 0.7673 NS | 0.9346 NS | 0.4512 NS | 0.0000 *** |
| Sal × Var | 0.0578 # | 0.0613 # | 0.3374 NS | 0.5040 NS | 0.1665 NS |
| Sal × Dose | 0.1226 NS | 0.8516 NS | 0.0769 # | 0.5550 NS | 0.0903 # |
| Var × Dose | 0.3200 NS | 0.0211 * | 0.2885 NS | 0.8319 NS | 0.3884 NS |
| Sal × Var × Dose | 0.0973 # | 0.0067 ** | 0.0104 * | 0.0020 ** | 0.4552 NS |
| Block | 0.4047 NS | 0.4500 NS | 0.7176 NS | 0.4426 NS | 0.8577 NS |
| CV (%) | 20.09 | 5.42 | 5.00 | 5.24 | 15.13 |
Table 5.
Pod length (PL, cm) of cowpea varieties as affected by the interaction between variety (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), irrigation water salinity (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 5.
Pod length (PL, cm) of cowpea varieties as affected by the interaction between variety (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), irrigation water salinity (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Variety | Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| V1 | S1 | 19.12 ± 0.34 Aβa | 19.96 ± 0.30 Aαa | 18.76 ± 0.41 Aβa | 20.02 ± 0.42 Aαa |
| S2 | 20.12 ± 0.54 Aαa | 19.76 ± 0.74 Aαa | 18.56 ± 0.65 Aαa | 20.24 ± 0.67 Aαa |
| S3 | 19.76 ± 0.75 Aαa | 18.92 ± 0.86 Aβa | 20.50 ± 0.53 Aαa | 19.30 ± 0.64 Aαa |
| V2 | S1 | 22.10 ± 1.11 Aαa | 21.66 ± 0.98 Aαa | 21.92 ± 0.88 Aαa | 21.26 ± 0.76 Aαa |
| S2 | 20.98 ± 1.05 Aαa | 20.74 ± 0.74 Aαa | 20.42 ± 1.22 ABαa | 22.02 ± 1.12 Aαa |
| S3 | 20.02 ± 0.63 Aαab | 22.38 ± 0.78 Aαa | 18.86 ± 0.84 Bαb | 18.64 ± 0.56 Bαb |
Table 6.
Average seed weight (ASW, g) of cowpea varieties as affected by the interaction between variety (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), irrigation water salinity (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 6.
Average seed weight (ASW, g) of cowpea varieties as affected by the interaction between variety (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), irrigation water salinity (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Variety | Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| V1 | S1 | 0.240 ± 0.014 Aαa | 0.240 ± 0.025 Aαa | 0.220 ± 0.015 Aαβa | 0.280 ± 0.013 Aαa |
| S2 | 0.220 ± 0.016 Aαa | 0.220 ± 0.018 Aαa | 0.200 ± 0.021 Bβa | 0.200 ± 0.007 Bβa |
| S3 | 0.240 ± 0.014 Aβa | 0.260 ± 0.014 Aαa | 0.280 ± 0.005 Aαa | 0.300 ± 0.009 Aαa |
| V2 | S1 | 0.280 ± 0.020 Aαa | 0.300 ± 0.022 Aαa | 0.280 ± 0.016 Aαa | 0.280 ± 0.026 Aαa |
| S2 | 0.260 ± 0.024 Aαa | 0.260 ± 0.021 Aαa | 0.280 ± 0.012 Aαa | 0.300 ± 0.010 Aαa |
| S3 | 0.320 ± 0.014 Aαa | 0.320 ± 0.021 Aαa | 0.320 ± 0.024 Aαa | 0.240 ± 0.017 Aαb |
Table 7.
Number of pods per plant (NPP) of cowpea under different salinity levels of irrigation water (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1).
Table 7.
Number of pods per plant (NPP) of cowpea under different salinity levels of irrigation water (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1).
| Salinity | NPP |
|---|
| S1 | 8.30 ± 0.31 A |
| S2 | 5.43 ± 0.25 B |
| S3 | 3.18 ± 0.17 C |
Table 8.
Number of locules per pod (NLP) of cowpea as affected by the interaction between irrigation water salinity (S1, S2, S3) and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 8.
Number of locules per pod (NLP) of cowpea as affected by the interaction between irrigation water salinity (S1, S2, S3) and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| S1 | 19.54 Aa | 16.85 Ab | 17.30 Ab | 16.51 Ab |
| S2 | 16.96 Ba | 17.00 Aa | 16.74 Aa | 17.30 Aa |
| S3 | 16.23 Ba | 17.41 Aa | 16.28 Aa | 15.80 Aa |
Table 9.
Number of empty locules (EL) in cowpea pods as a function of the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”) and salinity levels of irrigation water (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1).
Table 9.
Number of empty locules (EL) in cowpea pods as a function of the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”) and salinity levels of irrigation water (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1).
| Salinity | Varieties |
|---|
| V1 | V2 |
|---|
| S1 | 2.97 ± 0.28 Aα | 3.91 ± 0.42 Bα |
| S2 | 2.90 ± 0.29 Aβ | 5.90 ± 0.62 Aα |
| S3 | 4.19 ± 0.46 Aα | 5.33 ± 0.49 ABα |
Table 10.
Number of seeds per pod (NSP) in cowpea as a function of the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), salinity levels of irrigation water (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 10.
Number of seeds per pod (NSP) in cowpea as a function of the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), salinity levels of irrigation water (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Variety | Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| V1 | S1 | 13.76 ± 0.76 Aβa | 13.56 ± 0.46 Aαa | 13.54 ± 0.52 Aαa | 13.28 ± 0.24 Aαa |
| S2 | 13.00 ± 0.83 Aαa | 14.58 ± 0.82 Aαa | 11.94 ± 0.72 Aαa | 14.06 ± 0.67 Aαa |
| S3 | 11.98 ± 0.97 Aαa | 11.32 ± 1.19 Aαa | 13.14 ± 1.02 Aαa | 11.16 ± 1.28 Aαa |
| V2 | S1 | 18.72 ± 3.80 Aαa | 13.10 ± 1.20 Aαb | 14.20 ± 0.57 Aαb | 13.74 ± 0.71 Aαb |
| S2 | 12.32 ± 1.13 Bαa | 11.96 ± 0.71 Aαa | 10.92 ± 1.28 Abαa | 12.96 ± 1.09 Aαa |
| S3 | 11.36 ± 0.73 Bαab | 14.10 ± 0.69 Aαa | 9.68 ± 0.86 Bβb | 10.78 ± 0.86 Aαab |
Table 11.
Seed length (SL, mm) of cowpea as a function of the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), irrigation water salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 11.
Seed length (SL, mm) of cowpea as a function of the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), irrigation water salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Variety | Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| V1 | S1 | 9.60 ± 0.12 Aβa | 8.96 ± 0.20 Aβa | 9.12 ± 0.12 Aβa | 9.72 ± 0.23 Aβa |
| S2 | 9.34 ± 0.26 Aβa | 9.00 ± 0.32 Aβa | 9.04 ± 0.19 Aβa | 9.08 ± 0.18 Bβa |
| S3 | 9.22 ± 0.31 Aβa | 9.70 ± 0.27 Aβa | 9.58 ± 0.23 Aβa | 10.34 ± 0.24 Aαa |
| V2 | S1 | 10.60 ± 0.22 ABαa | 10.62 ± 0.19 Aαa | 10.60 ± 0.33 Aαa | 10.52 ± 0.15 Aαa |
| S2 | 10.42 ± 0.23 Bαa | 10.66 ± 0.31 Aαa | 10.06 ± 0.28 Aαa | 10.76 ± 0.28 Aαa |
| S3 | 11.24 ± 0.20 Aαa | 10.78 ± 0.28 Aαab | 10.82 ± 0.31 Aαab | 10.14 ± 0.20 Aαb |
Table 12.
Seed width (SW, mm) of cowpea as a function of the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), irrigation water salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 12.
Seed width (SW, mm) of cowpea as a function of the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), irrigation water salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Variety | Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| V1 | S1 | 7.70 ± 0.14 Aαa | 7.20 ± 0.19 Aβa | 7.24 ± 0.09 Aβa | 7.56 ± 0.15 Aβa |
| S2 | 6.88 ± 0.12 Bαa | 7.04 ± 0.17 Aβa | 6.92 ± 0.08 Aβa | 6.86 ± 0.17 Bβa |
| S3 | 7.06 ± 0.25 Bβa | 7.36 ± 0.26 Aβa | 7.24 ± 0.16 Aβa | 7.48 ± 0.20 Aαa |
| V2 | S1 | 7.98 ± 0.15 Aαa | 7.86 ± 0.11 Aαa | 8.14 ± 0.16 Aαa | 8.08 ± 1.12 Aαa |
| S2 | 7.24 ± 0.23 Bαb | 7.68 ± 0.24 Aαab | 8.00 ± 0.16 Aαa | 7.86 ± 0.15 ABαab |
| S3 | 8.16 ± 0.08 Aαa | 7.90 ± 0.17 Aαab | 7.80 ± 0.11 Aαab | 7.40 ± 0.10 Bαb |
Table 13.
Seed thickness (ST, mm) of cowpea as a function of the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), irrigation water salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 13.
Seed thickness (ST, mm) of cowpea as a function of the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), irrigation water salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Variety | Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| V1 | S1 | 6.18 ± 0.09 Aαa | 6.06 ± 0.09 Aαa | 6.04 ± 0.10 Aαa | 6.10 ± 0.10 Aαa |
| S2 | 5.68 ± 0.12 Bαa | 5.88 ± 0.11 Aαa | 5.90 ± 0.06 Aαa | 5.50 ± 0.17 Bαa |
| S3 | 5.68 ± 0.19 Bαb | 5.92 ± 0.13 Aαab | 6.08 ± 0.09 Aαab | 6.32 ± 0.13 Aαa |
| V2 | S1 | 5.80 ± 0.17 ABαa | 5.96 ± 0.14 Aαa | 5.90 ± 0.15 Aαa | 5.92 ± 0.12 Aαa |
| S2 | 5.42 ± 0.19 Bαa | 5.68 ± 0.15 Aαa | 5.86 ± 0.13 Aαa | 5.76 ± 0.14 Aαa |
| S3 | 6.06 ± 0.17 Aαa | 5.80 ± 0.18 Aαab | 5.80 ± 0.05 Aαab | 5.54 ± 0.15 Aβb |
Table 14.
Hundred-seed weight (SW100, g) of cowpea as a function of the interaction between irrigation water salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1) and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 14.
Hundred-seed weight (SW100, g) of cowpea as a function of the interaction between irrigation water salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1) and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| S1 | 23.006 ± 1.07 Ab | 30.337 ± 1.55 Aa | 24.134 ± 1.64 Ab | 26.406 ± 1.29 Aab |
| S2 | 22.503 ± 1.16 Ab | 27.979 ± 1.78 Aa | 25.317 ± 1.28 Aab | 29.021 ± 1.79 Aa |
| S3 | 23.971 ± 1.99 Ab | 29.784 ± 1.45 Aa | 28.129 ± 1.71 Aab | 25.399 ± 4.03 Aab |
Table 15.
Summary of the analysis of variance for shoot length (SL, cm), root length (RL, cm), stem diameter (SD, mm), shoot fresh mass (SFM, g), shoot dry mass (SDM, g), root fresh mass (RFM, g), root dry mass (RDM, g), and germination percentage (GER, %).
Table 15.
Summary of the analysis of variance for shoot length (SL, cm), root length (RL, cm), stem diameter (SD, mm), shoot fresh mass (SFM, g), shoot dry mass (SDM, g), root fresh mass (RFM, g), root dry mass (RDM, g), and germination percentage (GER, %).
| FV | F-Test (Pr > Fc) |
|---|
| SL | RL | SD | SFM | SDM | RFM | RDM | GER |
|---|
| Sal | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.2638 NS | 0.0000 *** | 0.1512 NS |
| Var | 0.0000 *** | 0.0000 *** | 0.5845 NS | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.3111 NS |
| Dose | 0.0000 *** | 0.0031 ** | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.3059 NS |
| Sal × Var | 0.0000 *** | 0.0004 *** | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.0000 *** |
| Sal × Dose | 0.0000 *** | 0.0000 *** | 0.0003 *** | 0.0077 ** | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.0058 ** |
| Var × Dose | 0.0004 *** | 0.0005 *** | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.0006 *** | 0.0001 *** | 0.1545 NS |
| Sal × Var × Dose | 0.0000 *** | 0.0001 *** | 0.0162 * | 0.0000 *** | 0.0000 *** | 0.2335 NS | 0.0000 *** | 0.0122 * |
| CV (%) | 9.57 | 10.26 | 7.56 | 14.96 | 19.61 | 18.03 | 12.74 | 4.19 |
Table 16.
Shoot length (SL, cm) of cowpea seedlings as affected by the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), irrigation water salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 16.
Shoot length (SL, cm) of cowpea seedlings as affected by the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), irrigation water salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Variety | Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| V1 | S1 | 7.78 ± 0.46 Aαa | 7.98 ± 0.21 Aαa | 8.25 ± 0.15 Aαa | 8.43 ± 0.19 Aαa |
| S2 | 8.58 ± 0.38 Aαab | 7.48 ± 0.10 Aαb | 8.80 ± 0.36 Aαa | 8.68 ± 0.18 Aαa |
| S3 | 6.48 ± 0.31 Bαa | 7.10 ± 0.19 Aαa | 6.35 ± 0.23 Bαa | 6.53 ± 0.14 Bαa |
| V2 | S1 | 6.85 ± 0.63 Aβab | 3.45 ± 0.17 Bβc | 6.10 ± 0.39 Aβb | 7.40 ± 0.19 Aβa |
| S2 | 3.53 ± 0.25 Bβa | 3.70 ± 0.24 Bβa | 4.30 ± 0.14 Bβa | 4.48 ± 0.28 Cβa |
| S3 | 3.93 ± 0.21 Bβb | 4.90 ± 0.34 Aβab | 4.93 ± 0.26 Bβab | 6.00 ± 0.53 Bαa |
Table 17.
Root length (RL, cm) of cowpea seedlings as affected by the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 17.
Root length (RL, cm) of cowpea seedlings as affected by the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Variety | Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| V1 | S1 | 17.52 ± 0.46 Aαa | 15.68 ± 0.97 Aαab | 15.74 ± 0.50 Aαab | 14.27 ± 0.66 Aαb |
| S2 | 14.69 ± 0.55 Bαa | 14.03 ± 0.73 ABαa | 13.94 ± 0.68 Aαa | 13.65 ± 0.35 Aαa |
| S3 | 9.35 ± 0.49 Cαb | 12.12 ± 0.55 Bαa | 11.34 ± 0.75 Bαab | 13.52 ± 0.40 Aαa |
| V2 | S1 | 15.85 ± 0.71 Aαa | 8.94 ± 0.19 Aβb | 14.01 ± 1.29 Aαa | 14.43 ± 0.82 Aαa |
| S2 | 8.12 ± 0.65 Bβb | 9.50 ± 0.65 Aβab | 11.42 ± 0.53 Bβa | 11.57 ± 0.74 Bβa |
| S3 | 9.47 ± 0.54 Bαa | 10.14 ± 0.29 Aβa | 10.71 ± 0.34 Bαa | 11.04 ± 0.60 Bβa |
Table 18.
Stem diameter (SD, mm) of cowpea seedlings as affected by the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 18.
Stem diameter (SD, mm) of cowpea seedlings as affected by the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Variety | Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| V1 | S1 | 3.76 ± 0.04 Aβb | 4.14 ± 0.16 Aαab | 4.23 ± 0.14 Aβab | 4.34 ± 0.16 Aβa |
| S2 | 3.72 ± 0.21 Aαb | 4.00 ± 0.13 Aαab | 4.35 ± 0.22 Aαa | 3.73 ± 0.12 Bαb |
| S3 | 3.45 ± 0.23 Aβab | 3.28 ± 0.03 Bαb | 3.91 ± 0.26 Aαa | 3.02 ± 0.04 Cβb |
| V2 | S1 | 4.34 ± 0.10 Aαa | 3.46 ± 0.10 Aβb | 4.71 ± 0.13 Aαa | 4.84 ± 0.18 Aαa |
| S2 | 3.11 ± 0.17 Bβb | 2.73 ± 0.09 Bβb | 3.90 ± 0.09 Bβa | 3.95 ± 0.14 Bαa |
| S3 | 4.02 ± 0.17 Aαa | 3.15 ± 0.02 Aαb | 3.65 ± 0.06 Bαab | 3.69 ± 0.12 Bαa |
Table 19.
Shoot fresh mass (SFM, g) of cowpea seedlings as affected by the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 19.
Shoot fresh mass (SFM, g) of cowpea seedlings as affected by the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Variety | Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| V1 | S1 | 0.673 ± 0.05 ABβb | 0.992 ± 0.08 Aαa | 0.997 ± 0.07 Aαa | 0.848 ± 0.04 Aβab |
| S2 | 0.831 ± 0.08 Aαa | 0.816 ± 0.05 Aαa | 0.954 ± 0.08 Aαa | 0.774 ± 0.03 Aαa |
| S3 | 0.626 ± 0.08 Bαb | 0.607 ± 0.00 Bαb | 0.696 ± 0.04 Bαab | 0.857 ± 0.03 Aαa |
| V2 | S1 | 0.878 ± 0.08 Aαb | 0.341 ± 0.02 Bβc | 0.826 ± 0.03 Aβb | 1.165 ± 0.03 Aαa |
| S2 | 0.266 ± 0.02 Cβb | 0.283 ± 0.02 Bβb | 0.478 ± 0.03 Bβa | 0.478 ± 0.05 Bβa |
| S3 | 0.480 ± 0.07 Bαa | 0.568 ± 0.02 Aαa | 0.594 ± 0.02 Bαa | 0.615 ± 0.07 Bβa |
Table 20.
Shoot dry mass (SDM, g) of cowpea seedlings as affected by the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), salinity levels of irrigation water (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 20.
Shoot dry mass (SDM, g) of cowpea seedlings as affected by the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), salinity levels of irrigation water (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Variety | Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| V1 | S1 | 0.060 ± 0.00 ABβb | 0.098 ± 0.01 Aαa | 0.095 ± 0.00 Aαa | 0.066 ± 0.00 Bβb |
| S2 | 0.079 ± 0.01 Aαa | 0.062 ± 0.00 Bαa | 0.076 ± 0.01 Bαa | 0.058 ± 0.00 Bαa |
| S3 | 0.048 ± 0.01 Bαb | 0.049 ± 0.00 Bαb | 0.052 ± 0.00 Bαb | 0.090 ± 0.00 Aαa |
| V2 | S1 | 0.164 ± 0.01 Aαa | 0.032 ± 0.00 Aβd | 0.058 ± 0.00 Aβc | 0.116 ± 0.02 Aαb |
| S2 | 0.027 ± 0.00 Bβa | 0.030 ± 0.00 Aβa | 0.036 ± 0.00 Bβa | 0.041 ± 0.00 Bαa |
| S3 | 0.035 ± 0.00 Bαa | 0.050 ± 0.00 Aαa | 0.046 ± 0.00 ABαa | 0.045 ± 0.01 Bβa |
Table 21.
Root fresh mass (RFM, g) of cowpea seedlings as affected by the interaction between salinity (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1) and varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”).
Table 21.
Root fresh mass (RFM, g) of cowpea seedlings as affected by the interaction between salinity (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1) and varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”).
| Salinity | Varieties |
|---|
| V1 | V2 |
|---|
| S1 | 0.238 ± 0.02 Bα | 0.218 ± 0.02 Aα |
| S2 | 0.298 ± 0.01 Aα | 0.128 ± 0.01 Bβ |
| S3 | 0.244 ± 0.02 Bα | 0.189 ± 0.01 Aβ |
Table 22.
Root fresh mass (RFM, g) of cowpea seedlings as affected by the interaction between salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1) and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 22.
Root fresh mass (RFM, g) of cowpea seedlings as affected by the interaction between salinity levels (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1) and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| S1 | 0.244 ± 0.02 Aa | 0.125 ± 0.01 Bb | 0.259 ± 0.02 Aa | 0.286 ± 0.01 Aa |
| S2 | 0.160 ± 0.04 Bb | 0.209 ± 0.05 Aab | 0.226 ± 0.02 Aa | 0.257 ± 0.03 Aa |
| S3 | 0.159 ± 0.02 Bc | 0.220 ± 0.01 Aab | 0.216 ± 0.01 Ab | 0.270 ± 0.03 Aa |
Table 23.
Root fresh mass (RFM, g) of cowpea seedlings as affected by the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”) and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 23.
Root fresh mass (RFM, g) of cowpea seedlings as affected by the interaction between varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”) and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Variety | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| V1 | 0.226 ± 0.02 αb | 0.242 ± 0.02 αb | 0.246 ± 0.01 αb | 0.326 ± 0.01 αa |
| V2 | 0.150 ± 0.03 βb | 0.126 ± 0.01 βb | 0.221 ± 0.01 βa | 0.216 ± 0.02 βa |
Table 24.
Root dry mass (RDM, g) of cowpea seedlings as affected by the interaction among varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), salinity levels of irrigation water (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 24.
Root dry mass (RDM, g) of cowpea seedlings as affected by the interaction among varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), salinity levels of irrigation water (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Variety | Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| V1 | S1 | 0.027 ± 0.00 Aαab | 0.025 ± 0.00 Aαb | 0.030 ± 0.00 Aαa | 0.027 ± 0.00 Aαab |
| S2 | 0.024 ± 0.00 Aαa | 0.027 ± 0.00 Aαa | 0.024 ± 0.00 Bαa | 0.027 ± 0.00 Aαa |
| S3 | 0.011 ± 0.00 Aαc | 0.018 ± 0.00 Bαb | 0.014 ± 0.00 Cαab | 0.028 ± 0.00 Aαa |
| V2 | S1 | 0.023 ± 0.00 Aβa | 0.006 ± 0.00 Bβb | 0.021 ± 0.00 Aβa | 0.025 ± 0.00 Aαa |
| S2 | 0.005 ± 0.00 Bβc | 0.008 ± 0.00 Bβbc | 0.011 ± 0.00 Bβab | 0.013 ± 0.00 Bβa |
| S3 | 0.008 ± 0.00 Bαb | 0.015 ± 0.00 Aβa | 0.014 ± 0.00 Bαa | 0.014 ± 0.00 Bβa |
Table 25.
Germination percentage (GER, %) of cowpea seeds as affected by the interaction among varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), salinity levels of irrigation water (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
Table 25.
Germination percentage (GER, %) of cowpea seeds as affected by the interaction among varieties (V1—“Pingo de Ouro”, V2—“Costela de Vaca”), salinity levels of irrigation water (S1—0.54 dS m−1, S2—3.50 dS m−1, S3—5.00 dS m−1), and foliar Mg doses (0, 1, 2, 3 mL L−1).
| Variety | Salinity | Mg Doses (mL L−1) |
|---|
| 0 | 1 | 2 | 3 |
|---|
| V1 | S1 | 97.5 ± 2.50 Aαab | 86.25 ± 5.91 Bβc | 90.0 ± 0.00 Bβbc | 98.75 ± 1.25 Aαa |
| S2 | 98.8 ± 1.25 Aαa | 100.0 ± 0.00 Aαa | 100.0 ± 0.00 Aαa | 100.0 ± 0.00 Aαa |
| S3 | 100 ± 0.00 Aαa | 100.0 ± 0.00 Aαa | 98.25 ± 1.75 Aαa | 94.31 ± 2.78 Aαa |
| V2 | S1 | 100.0 ± 0.00 Aαa | 100.0 ± 0.00 Aαa | 97.5 ± 1.44 Aαa | 100.0 ± 0.00 Aαa |
| S2 | 93.8 ± 1.25 Aαa | 92.5 ± 3.23 Bβa | 100.0 ± 0.00 Aαa | 98.8 ± 1.25 Aαa |
| S3 | 95.0 ± 5.00 Aαa | 100.0 ± 0.00 Aαa | 96.5 ± 2.02 Aαa | 100.0 ± 0.00 Aαa |